Abstract
The neuropeptide galanin extensively coexists with norepinephrine in locus coeruleus (LC) neurons. Previous research in this laboratory has demonstrated that unlimited access to activity wheels in the home cage increases mRNA for galanin (GAL) in the LC, and that GAL mediates some of the beneficial effects of exercise on brain function. To assess whether capacity for aerobic exercise modulates this upregulation in galanin mRNA, three heterogeneous rat models were tested: rats selectively bred for 1) high intrinsic (untrained) aerobic capacity (High Capacity Runners, HCR) and 2) low intrinsic aerobic capacity (Low Capacity Runners, LCR) and 3) unselected Sprague-Dawley (SD) rats with and without free access to running wheels for three weeks. Following this exercise protocol, mRNA for tyrosine hydroxylase (TH) and GAL was measured in the LC. The wheel-running distances between the three models were significantly different, and age contributed as a significant covariate. Both selection and wheel access condition significantly affected GAL mRNA expression, but not TH mRNA expression. GAL was elevated in exercising HCR and SD rats compared to sedentary rats while LCR rats did not differ between conditions. Overall running distance significantly correlated with GAL mRNA expression, but not with TH mRNA expression. No strain differences in GAL or TH gene expression were observed in sedentary rats. Thus, intrinsic aerobic running capacity influences GAL gene expression in the LC only insofar as actual running behavior is concerned; aerobic capacity does not influence GAL expression in addition to changes associated with running.
Keywords: galanin, tyrosine hydroxylase, aerobic capacity, running-wheel
1. Introduction
Chronic exercise influences brain function in a variety of beneficial ways [3]. Exercise reduces symptoms of depression and anxiety in humans [9] and animals [8], mitigates the harmful effects of stroke [5], and modulates the severity of seizure effects [11, 23, 29]. Voluntary wheel-running enhances performance on tests of spatial learning [34] and reduces the negative impact of aging on this performance [35]. Many of these effects may be attributed to adaptations in intracellular signaling. Activation of survival and proliferation pathways in the hippocampus, such as PI3-kinase [2], MAPK [30], as well as increased activity of vesicle-related proteins synapsin I [36] and synaptophysin [37], is associated with voluntary running.
The recent finding that regular exercise upregulates galanin (GAL) mRNA expression in the noradrenergic locus coeruleus (LC) [15, 28, 33] suggests that GAL may mediate some of the behavioral effects of exercise. For example, rats with three weeks of free access to cage wheels show significantly reduced seizure behavior to a 0.2 μg intracerebroventricular (ICV) dose of kainic acid, an effect abolished with the GAL receptor antagonist M40 [29]. Since the LC projects to dorsal and ventral aspects of the hippocampus [22] where GAL receptors are found [27], and GAL modulates hippocampal excitability and seizure [23], the exercise-induced upregulation of GAL in the LC could be an important component of hippocampal neuroprotection.
GAL coexists with norepinephrine (NE) in most of the cell bodies of the LC [14, 24]. The LC comprises the primary noradrenergic innervation of the central nervous system and projects to areas throughout the forebrain including neocortex and hippocampus, as well as hypothalamus, thalamus, cerebellum and spinal cord [22, 24, 26]. Receptors for GAL are distributed throughout the brain, with particularly high densities found in the hippocampus and diencephalon, as well as the LC itself [27]; they are G-protein coupled and linked to adenylyl cyclase inhibition [13] and activation of potassium channels [10]. GAL receptor activity is associated with pathways such as Akt, ERK [12], or PKC [38].
The present experiments investigated whether intrinsic (untrained) running capacity influences exercise-induced upregulation of GAL. A rat model system, selectively bred to express greater or lesser intrinsic aerobic capacity [18-20], demonstrates a substantial expansion or reduction in running speed, duration, and maximal oxygen uptake [17]. Thus, the high capacity and low capacity rat strains (HCR and LCR, respectively) differ dramatically in their capacity to run on a treadmill to the point of exhaustion [18, 20]. These differences may be associated with a greater capacity of HCR to deliver and utilize O2 in skeletal muscle [16].
Since age is another characteristic that influences exercise effects in rats [1], the present experiments included and analyzed age as a covariate. Older rats show reduced expression of GAL protein [4] and increased GAL binding site density [21], demonstrating an age-related reduction in GAL signaling. Age also influences how exercise affects other factors, such as brain-derived neurotrophic factor (BDNF) [1].
The present studies thus aimed to further characterize the nature of the relationship between wheel running and GAL in the LC, and address the following questions: 1) Does selection for intrinsic aerobic running capacity influence the exercise-induced upregulation of LC GAL? 2) What effect does wheel running have on mRNA for tyrosine hydroxylase (TH), the rate limiting enzyme in NE production [25]? 3) Does age covary with the effect of exercise on LC GAL (or potentially TH) mRNA upregulation?
2. Method
2.1 Subjects
Previous work details the development of a rat model of aerobic running capacity [18]. Briefly, rats chosen from a genetically heterogeneous N:NIH founder population ran to exhaustion on a treadmill, and the distances were recorded. The 13 lowest running males and 13 lowest running females comprised the beginning of a LCR line, while the 13 highest running males and 13 highest running females started an HCR line [18]. At 10 generations, there was an overall difference in running capacity between HCR and LCR rats of 317% [19]; by 21 generations, the difference reached 450% [20].
A total of 63 male rats, HCR (n=32) and LCR (n=31), from generation 23 of selection, were shipped from the University of Michigan. Phenotype data provided with these rats showed that LCR and HCR groups differed by 650% for intrinsic aerobic running capacity at 11 weeks of age. The mean treadmill running duration for HCR was 61 minutes compare to only 18 minutes for LCR rats. Ages for the LCR and HCR rats ranged from 121 to 217 days. A total of 41 Sprague-Dawley (SD) rats, aged 60 days (n=21) or 321 days (n=20) were supplied by Harlan. For purposes of assignment, rats were designated as young (SD: 60.0±0.00 days, LCR/HCR: 122.74±2.09 days) and old (SD: 321.0±0.00 days, LCR/HCR: 180.4±26.35 days), and were then randomly assigned to exercise (16 HCR, 16 LCR, and 20 SD rats) or sedentary groups (16 HCR, 15 LCR, and 21 SD rats).
Animals were housed in a temperature and humidity-controlled vivarium with lighting maintained on a 12-hour light/dark schedule. Food and water were available ad libitum and animals were weighed weekly throughout the study. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with University of Georgia IACUC approval.
2.2 Exercise Protocol
Rats in the exercise condition were housed individually in polycarbonate cages, provided with unlimited access to a stainless steel cage wheel for 21 days. Previous work has demonstrated that three weeks of cage wheel access is sufficient to induce GAL mRNA upregulation in the LC [33]. Revolutions were recorded with a magnetic counter. Running distances were recorded every other day and calculated by multiplying revolutions by the circumference of the wheel (1.05 m). Three HCR and three LCR rats were excluded from analysis because mean daily revolutions did not exceed 50, which was likely associated with their age and size. Running data from a total of 46 rats (HCR n=13, LCR n=13, SD n=20) were analyzed. Animals in the sedentary condition (HCR n=16, LCR n=15, SD n=21), were housed under identical conditions without a cage wheel.
2.3 In Situ Hybridization
At the start of day 22, approximately 12 hours following the end of the last dark cycle, rats were decapitated and the brains were removed and frozen using dry ice, then stored at -80°C. Coronal sections at the level of LC were cut using a Microm cryostat (Waldorf, Germany) at a thickness of 12 μm. Sections were thaw-mounted onto gelatin-coated glass microscope slides. Anatomical location was verified using a 0.1% thoinin stain. Sections were fixed in 4% (v/v) formaldehyde in 0.12 M sodium phosphate-buffered saline (PBS) solution, rinsed in PBS, and soaked in 0.25% (v/v) acetic anhydride in 0.1 M triethanolamine HCl-0.9% (v/v) NaCl. Sections were dehydrated in a series of ethanol washes, delipidated in chloroform, and rinsed in ethanol.
Oligonucleotide probes (Oligos Etc., Wilsonville, Oregon) for GAL and TH, were labeled at the 3’ end with [35S]-dATP (New England Nuclear, Boston, Massachusetts), terminal deoxynucleotidyl transferase (TdT, 25 units/ml; Roche, Indianapolis, Indiana), and tailing buffer. Column separation was used to remove unbound nucleotide. Sections were hybridized with the radiolabeled probes in solutions containing 25% (v/v) formamide, 72 mM NaCl, 3.2 mM Tris-HCl, .0032 mM EDTA, 0.001% (v/v) sodium pyrophosphate, 0.004% (v/v) sodium dodecyl sulfate, 0.002 mg/ml heparin sulfate, and 2% (v/v) dextran sulfate. Sections were incubated overnight at 37°C, followed by a series of washes in SSC and SSC-50% formamide, water and ethanol, and then were dried.
Hybridized sections were placed into film cassettes and exposed to autoradiographic film (BioMax MR, Eastman Kodak, Rochester, NY) and developed with Kodak GBX developer and Kodak GBX fixer.
2.4 Film Analysis
Autoradiographs were scanned into Adobe Photoshop using a high resolution scanner (Microtek, San Francisco, CA), with a PowerMAC G4 (Apple, Inc., Cupertino, CA) for processing. ImageJ (National Institutes of Health, Bethesda, MD) was used to highlight and quantify grayscale brightness units in the LC.
2.5 Statistical Analysis
A mixed-model, 3-group × 10-time repeated measures analysis of covariance (RM-ANCOVA) adjusted for age (in days at the beginning of wheel-running), with Greenhouse-Geisser correction for sphericity violation, was performed to examine running distance over time. An intraclass correlation coefficient (ICC) was used to describe reliability across days. Two-way ANCOVA adjusted for age was also used to identify any differences in gray scale units for GAL mRNA or TH mRNA among the groups. Bonferroni adjustment was used to correct for multiple follow-up comparisons. Linear regression analysis adjusted for age was used to determine whether relations existed between overall running distance and mRNA for GAL or TH. SPSS 17.0 (Chicago, IL) was used for all statistical analyses.
3. Results
3.1 Running Distance
Daily running distance was highly reliable, ICC(2, 10)=0.97, and increased over time, F(9, 378)=20.77, ε = .212, p<0.001. There was a main effect of strain, F(2, 42)=6.256, p<0.01. HCR rats ran more than LCR rats, p<0.01, but not more than SD rats, p=1.0, which ran more than LCR rats, p=0.013 (figure 1). Significant interactions were identified between the covariate age and running over time, F(9, 378)=10.77, p<0.001, and also between strain and running over time, F(18, 378)=4.25, p<0.01. Follow-up RM-ANCOVA within each strain indicated that running distance did not increase in LCR rats, F(9, 99)=1.38, ε=0.161, p=0.287, but increased linearly in both SD rats, F(9, 162)=12.08, ε=0.145, p<0.001, and HCR rats, F(9, 99)=13.22, ε=0.316, p<0.001.
Figure 1.
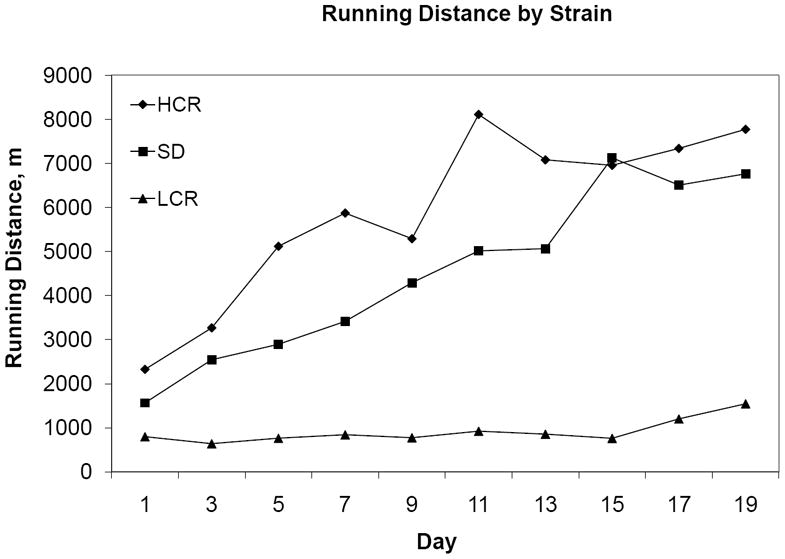
Mean two-day running distance values per group, in meters.
3.2 LC mRNA Expression
3.2.1 GAL
The omnibus F test for our ANCOVA of LC GAL mRNA was statistically significant, F(6, 77)=3.872, p<0.01. Age did not significantly contribute as a covariate to differences in GAL mRNA F(1, 77)=2.959, p=0.089, but there were effects of strain (HCR, LCR, or SD), F(2, 77)=7.273, p<0.01, and exercise condition, F(1, 77)=5.365, p=0.023. The LCs of HCR showed greater GAL mRNA than those of LCR, p<0.01, and SD showed greater LC GAL mRNA than did LCR, p<0.01, but no significant difference was identified between SD and HCR, p=1.0. Strain and exercise condition did not interact to produce an effect on LC GAL mRNA expression, F(2, 77)=1.117, p=0.332.
Overall, voluntary running distance was correlated with GAL mRNA expression, r=0.317, p=0.028. That relation remained after adjustment for age (β=0.40, t=2.25, p=0.03), but it was explained by the strain effect (β=0.41, t=2.39, p=0.022) of lower running distance and lower GAL mRNA in LCR rats compared to SD and HCR rats (p<0.01). For a scatterplot of the data, see figure 2. See also figures 3 and 4.
Figure 2.
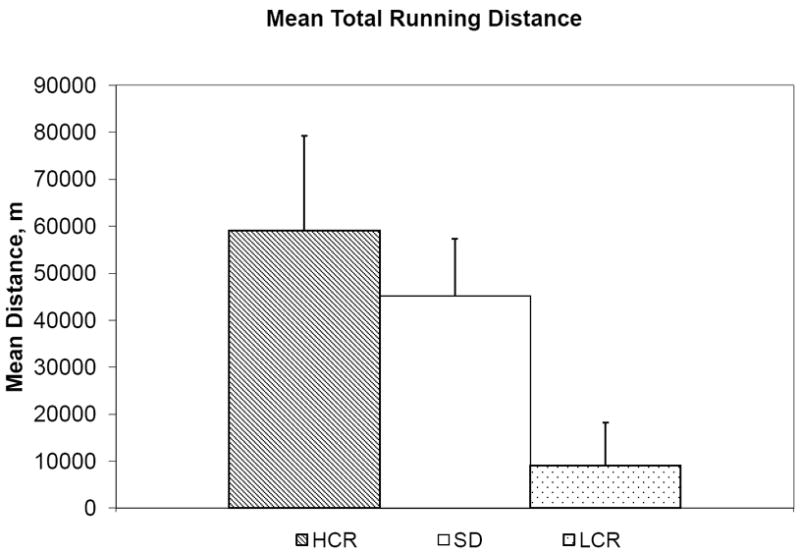
Mean total running distance values per group, in meters.
Figure 3.
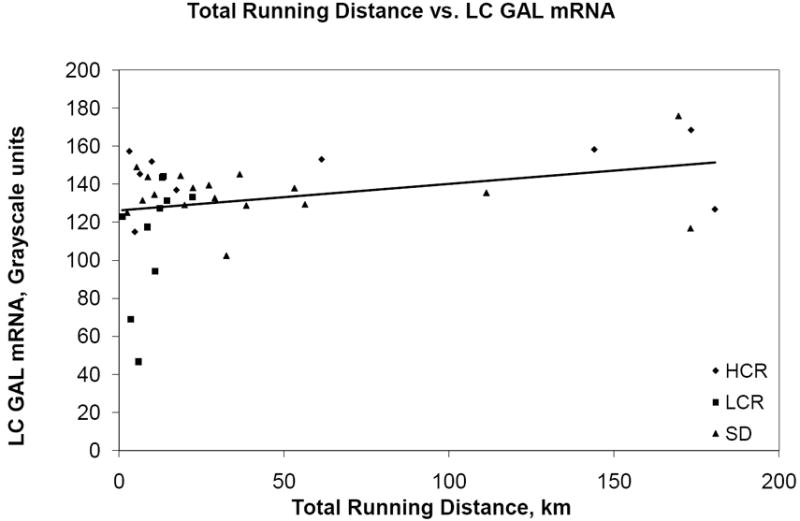
Scatterplot of total running distance (in kilometers) vs. GAL mRNA (grayscale units).
Figure 4.
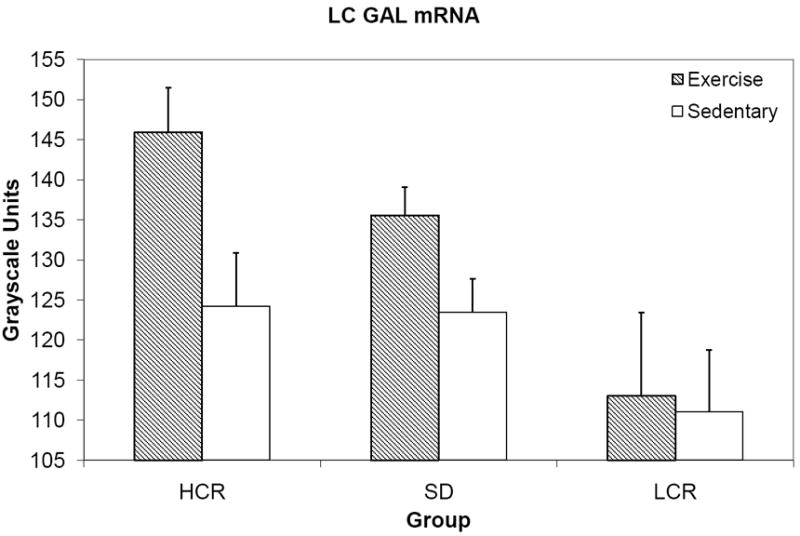
Mean GAL mRNA expression in the LC, grayscale units.
3.2.2 TH
The omnibus F test of TH mRNA was not significant, F(6, 54)=0.659, p=0.683, indicating that strain and exercise condition did not significantly differ or interact with regard to TH mRNA expression in the LC (figure 5).
Figure 5.
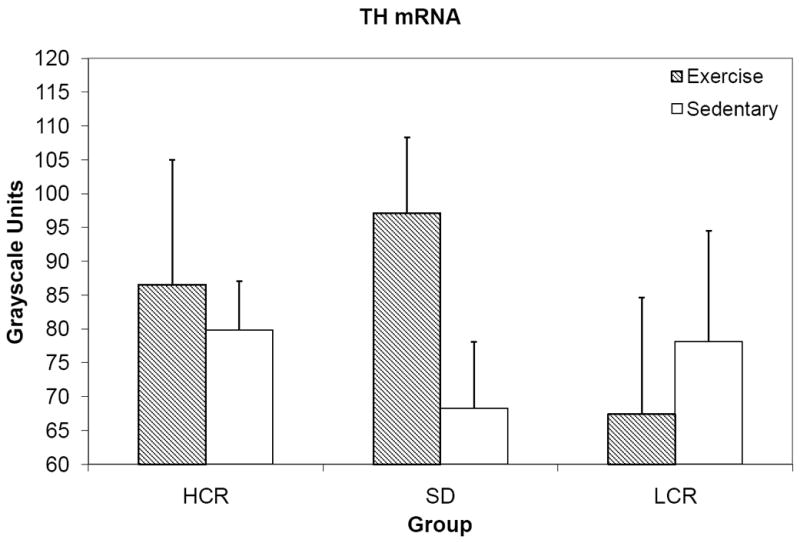
Mean TH mRNA expression in the LC, grayscale units.
Overall running distance and TH mRNA expression were not significantly correlated, r=0.285, p=0.079.
4. Discussion
In the present study, we established that fitness capacity influences upregulation of GAL in the LC. The outbred SD group, presumably of genetically variable composition, had total distance values having fallen in between the HCR and LCR rats, though nearer to HCR than LCR (figures 1 and 3) which is consistent with differences in speed and VO2 max capacities [17]; so it is reasonable to conclude based on the present data that both above-average or average running capacities facilitate an upregulation of GAL in the LC in a similar. However, a below-average aerobic running capacity appears to influence LC GAL regulation in a negative manner (albeit with a nonsignificant interaction), with LCR rats showing little GAL mRNA difference between exercise and sedentary groups (figure 4).
Forced exercise, in the form of short-term treadmill running, has been shown to have an age-related effect on LC TH mRNA expression, with younger rats showing a response to five daily bouts of treadmill running, but not older rats [32]. Though the effects of forced exercise paradigms may be difficult to interpret because they involve stress, both voluntary wheel running and treadmill running have been shown to protect against stress-related depletion in LC NE [6-7], so exercise likely influences NE activity in the LC in some fashion. However, in agreement with previous work [31], wheel running did not produce an effect on TH expression in the LC (figure 5), a finding previously observed in this laboratory with treadmill running [28].
It is important to understand that while exercise enhances GAL activity, age may factor into its upregulation. Indeed, GAL expression changes over a lifespan; septal GAL protein is reduced in older rats [4], while GAL binding sites are increased in piriform and entorhinal corticies, and dentate gyrus in older rats [21]. Other neuronal factors that exhibit a robust increase with exercise, such as BDNF, show a diminished exercise effect with age [1].
The HCR and LCR rats demonstrated a dramatic difference in voluntary wheel-running, with HCR rats running upwards of 2625 m on average per day more than LCR rats. Interestingly, SD running behavior fell closer to the daily running distance of HCR rats. On average HCR rats ran, total distance, more than six times as much as LCR rats (figure 3). This proportion (approximately 650%), is identical to the proportional difference in intrinsic capacity measured at 11 weeks of age in these rats. The RM-ANCOVA within each strain verified what seems apparent in the running data; HCR rats increase their running behavior over three weeks more than the LCR rats, who in the present study did not increase more over time. Also, rats increase their running behavior less over time with age. These findings further highlight the profound effects selection has on aerobic running capacity and help to define how the running behavior of these selected rats relates to the running behavior of the more commonly used SD rat, which is the commercially employed stock for most of the previous exercise research in this laboratory.
Exercise condition and selection did not significantly interact to produce an effect on GAL mRNA expression, but overall running did significantly correlate with GAL mRNA expression. More running did generally correspond with more GAL expression. Considering these relationships, it is then likely that running capacity due to selection affects GAL mRNA expression in the LC only insofar as actual running behavior is increased, without any additional synergistic influence.
5. Conclusion
Selection for aerobic running capacity produces dramatic differences in running behavior, and age contributes to this difference in behavior. Intrinsic aerobic running capacity does influence GAL mRNA expression in the LC, with exercising HCR and SD rats exhibiting higher GAL mRNA than their sedentary counterparts. No such differences between exercising and sedentary groups were seen in the LCR, which may be explained by their relative lack of running since total running distance is related to GAL upregulation in the LC. Overall, the results further suggest that the regulation of GAL in the LC is tightly coupled to exercise.
Footnotes
Data from these experiments were presented at the 39th annual meeting of the Society for Neuroscience, Chicago, IL, October, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlard P, Perreau V, Cotman C. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–20. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Russo-Neustadt A. Exercise activates the phosphatidylinositol 3-kinase pathway. Molecular Brain Research. 2005;135:181–93. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Cotman C, Berchtold N, Christie L. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 4.De Bilbao F, Jazat F, Lamour Y, Senut M. Age-related changes in galanin-immunoreactive cells of the rat medial septal area. The Journal of Comparative Neurology. 1991;313:613–24. doi: 10.1002/cne.903130407. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Li J, Luan X, Ding Y, Lai Q, Rafols J, et al. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124:583–91. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Dishman R, Renner K, White-Welkley J, Burke K, Bunnell B. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Res Bull. 2000;52:337–42. doi: 10.1016/s0361-9230(00)00271-9. [DOI] [PubMed] [Google Scholar]
- 7.Dishman R, Renner K, Youngstedt S, Reigle T, Bunnell B, Burke K, et al. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 8.Duman C, Schlesinger L, Russell D, Duman R. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–58. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn A, Trivedi M, O’Neal H. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33:S587. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- 10.Dunne M, Bullett M, Li G, Wollheim C, Petersen O. Galanin activates nucleotide-dependent K+ channels in insulin-secreting cells via a pertussis toxin-sensitive G-protein. The EMBO Journal. 1989;8:413. doi: 10.1002/j.1460-2075.1989.tb03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott-Hunt C, Marsh B, Bacon A, Pope R, Vanderplank P, Wynick D. Galanin acts as a neuroprotective factor to the hippocampus. Proc Natl Acad Sci U S A. 2004;101:5105. doi: 10.1073/pnas.0304823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott-Hunt C, Pope R, Vanderplank P, Wynick D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J Neurochem. 2007;100:780. doi: 10.1111/j.1471-4159.2006.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He B, Counts S, Perez S, Hohmann J, Koprich J, Lipton J, et al. Ectopic galanin expression and normal galanin receptor 2 and galanin receptor 3 mRNA levels in the forebrain of galanin transgenic mice. Neuroscience. 2005;133:371–80. doi: 10.1016/j.neuroscience.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Holets V, Hokfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 15.Holmes P, Yoo H, Dishman R. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neurosci Lett. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Howlett R, Kirkton S, Gonzalez N, Wagner H, Britton S, Koch L, et al. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol. 2009;106:1819. doi: 10.1152/japplphysiol.00914.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Høydal MA, Wisløff U, Kemi OJ, Ellingsen Ø. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:753–60. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 18.Koch L, Britton S. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Koch L, Britton S. Divergent selection for aerobic capacity in rats as a model for complex disease. Integrative and Comparative Biology. 2005;45:405. doi: 10.1093/icb/45.3.405. [DOI] [PubMed] [Google Scholar]
- 20.Koch L, Britton S. Obesity. Vol. 16. Silver Spring, Md: 2008. Development of Animal Models to Test the Fundamental Basis of Gene-Environment Interactions; p. S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krzywkowski P, Lagny-Pourmir I, Jazat F, Lamour Y, Epelbaum J. The age-related increase in galanin binding sites in the rat brain correlates with behavioral impairment. Neuroscience. 1994;59:599–607. doi: 10.1016/0306-4522(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 22.Loughlin S, Foote S, Bloom F. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 23.Mazarati A, Hohmann J, Bacon A, Liu H, Sankar R, Steiner R, et al. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melander T, Hokfelt T, Rokaeus A, Cuello A, Oertel W, Verhofstad A, et al. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–54. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–7. [PubMed] [Google Scholar]
- 26.Nygren L, Olson L. A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res. 1977;132:85–93. doi: 10.1016/0006-8993(77)90707-7. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409 [PubMed] [Google Scholar]
- 28.O’Neal H, Van Hoomissen J, Holmes P, Dishman R. Prepro-galanin messenger RNA levels are increased in rat locus coeruleus after treadmill exercise training. Neurosci Lett. 2001;299:69–72. doi: 10.1016/s0304-3940(00)01780-8. [DOI] [PubMed] [Google Scholar]
- 29.Reiss J, Dishman R, Boyd H, Robinson J, Holmes P. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: Possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 30.Shen H, Tong L, Balazs R, Cotman C. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 31.Soares J, Holmes P, Renner K, Edwards G, Bunnell B, Dishman R. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci. 1999;113:558–66. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- 32.Tümer N, Demirel H, Serova L, Sabban E, Broxson C, Powers S. Gene expression of catecholamine biosynthetic enzymes following exercise: modulation by age. Neuroscience. 2001;103:703–11. doi: 10.1016/s0306-4522(01)00020-3. [DOI] [PubMed] [Google Scholar]
- 33.Van Hoomissen J, Holmes P, Zellner A, Poudevigne A, Dishman R. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci. 2004;118:1378–90. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- 34.van Praag H, Christie B, Sejnowski T, Gage F. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Praag H, Shubert T, Zhao C, Gage F. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76:356–62. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 37.Vaynman S, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–30. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Hashemi T, Fried S, Clemmons A, Hawes B. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry (Mosc) 1998;37:6711–7. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]


