Abstract
The molecular mechanism(s) controlling cell migration during vascular morphogenesis in vivo remain largely undefined. To address this within a physiological context, we employed retinaldehyde dehydrogenase-2 (Raldh2) null mouse embryos and demonstrate that retinoic acid (RA) deficiency results in abnormal yolk sac vascular remodeling due to decreased Rac1 activation, increased RhoA activation, and increased focal adhesions. Vinculin was increased in Raldh2−/− yolk sacs, and molecular events important for focal adhesion turnover, FAK phosphorylation (Tyr397) and FAK-paxillin association, were decreased. RA-rescue of vascular remodeling downregulated vinculin and restored FAK phosphorylation (Tyr397) and FAK-paxillin association. Further, vascular rescue with VEGF-A, Indian hedgehog, and bFGF restored FAK phosphorylation (Tyr397) in the endothelium of Raldh2−/− yolk sacs. Our results provide new insights into the regulation of endothelial cell migration during vascular remodeling in vivo by adding the Rac1 and FAK activation pathway as a critical mediator of focal adhesion formation and turnover during vascular remodeling.
Keywords: Raldh2 mutant mice, endothelial cell, migration, yolk sac, vascular remodeling, retinoic acid, focal adhesions, vinculin, FAK, paxillin, Rac1, WAVE2, RhoA
INTRODUCTION
Patterning and remodeling of newly formed blood vessels involves the orchestrated movement of endothelial cells (Lamalice et al., 2007). During mammalian development, de novo blood vessel formation initially occurs extraembryonically within the mesodermal layer of the yolk sac. Mesodermal progenitors cluster at sites of blood vessel formation and undergo differentiation into endothelial and blood cells, forming blood islands (Doetschman et al., 1987). Endothelial cells then lose cell-cell contacts, migrate and re-align to form tubes that fuse to create an interconnecting network of vessels referred to as the primitive capillary plexus. Remodeling of the capillary plexus into a functional hierarchical network of large, medium, and small diameter vessels occurs in a precise and controlled fashion that ensures the orderly movements of endothelial cells (Lamalice et al., 2007). Gene deletion studies in mice have identified multiple growth factors and their receptors that are critical for yolk sac vascular remodeling, including VEGF-A (Carmeliet et al., 1996; Ferrara et al., 1996), ephrinB2 (Adams et al., 1999; Gerety and Anderson, 2002) and angiopoetin-1 (Suri et al., 1996), as well as members of the hedgehog (Byrd et al., 2002), Notch (Krebs et al., 2000; Limbourg et al., 2005) and TGF-β̣ families (Li et al., 1999; Yang et al., 1999). Diffusable vascular growth factors are released from adjacent cells or the ECM and interact with their receptors expressed on endothelial cells to regulate both proliferation and migration necessary for vessel remodeling, however the precise molecular signaling cascades and cellular events that promote endothelial cell migration in vivo are poorly understood.
RhoGTPases, low molecular weight intracellular GTP-binding proteins, are known to transduce extracellular signals from growth factors to the actin cytoskeleton to promote cell migration (Ridley and Hall, 1992; Ridley et al., 1992). RhoA, Rac1, and Cdc42 are among the well-studied Rho-GTPases. Rho proteins are active when GTP-bound and inactive when GDP-bound (Etienne-Manneville and Hall, 2002). Rac1 mediates lamellipodia formation and extension, and hence mediates forward cell migration (Nobes and Hall, 1995). Focal adhesion disassembly, which must occur for cell migration to proceed, requires activation of Rac1 and its effectors such as p21-activated kinase (PAK) (Zhao et al., 2000). Rac1 is ubiquitously expressed in many cell types, however, endothelial-specific deletion of Rac1 results in vascular remodeling defects and early embryonic lethality at 9.5 dpc (days post coitum) (Tan et al., 2008).
These results indicate a central role for Rac1 in coordinating endothelial cell migration during early embryonic vascular development. In contrast to the functional roles of Rac1 during cell migration, Rho inactivation seems to be required for focal adhesion disassembly, and RhoA activation by integrins induces formation of stress fibers and focal adhesions and mediates contraction of the cell rear (Ridley and Hall, 1992; Barry et al., 1997; Clark et al., 1998; Ren et al., 1999; Gallant et al., 2005). With elevated Rho activity, focal adhesions enlarge in size and exhibit increased stability (Ren et al., 2000), thus impeding cell migration.
In addition to Rho-mediated signals, regulation of cell migration involves the formation of focal adhesions and signaling from within these sites. Focal adhesions are points of contact between the cell and the ECM that are enriched in integrins as well as cytoskeletal, adaptor, and signaling proteins including talin, tensin, α-actinin, paxillin, vinculin, zyxin, p130cas, and FAK. For forward cell movement to occur, adhesion sites must form at the front of the cell and dissolve at the rear of the cell (Nobes and Hall, 1999). Targeted gene deletion of the focal adhesion proteins vinculin, paxillin and FAK in mice (Xu et al., 1998a; Hagel et al., 2002; Ilic et al., 2003) results in early embryonic lethality due to mesodermal, vascular, and cardiac defects, thus demonstrating the critical roles these proteins have during early embryonic vascular development.
Vinculin is a major component of focal adhesions and cell-cell junctions, and others have demonstrated that modulation of vinculin levels can directly alter cell motility. Overexpression of vinculin by transfection into 3T3 cells inhibited migration (Rodriguez Fernandez et al., 1992), while reduction of vinculin expression in 3T3 cells transfected with an antisense vinculin cDNA construct promoted cell motility (Rodriguez Fernandez et al., 1993). Consistent with this finding is the observation that cancer cells that lack vinculin are invasive and have high metastatic potential (Lifschitz-Mercer et al., 1997). A role for vinculin signaling in the inhibition of parietal endoderm migration, the initial migratory cell type in the mouse embryo, has been demonstrated using vinculin-deficient F9 teratocarcinoma stem cells induced to form parietal endoderm (Mills et al., 2005). Parietal endoderm derived from vinculin-deficient F9 stem cells migrated approximately two-fold further than parietal endoderm derived from wild type cells. Vinculin−/− F9 stems cells also displayed decreased adhesion. Recruitment of vinculin to focal adhesion sites has been shown to have a role in strengthening the adhesion between cells and the ECM, while reduction of adhesion and increased migration occur with downregulation of vinculin (Coll et al., 1995). Vinculin itself is not essential for focal adhesion formation in that the number of focal adhesions in vinculin-null cells were similar to WT cells (Coll et al., 1995; Mills et al., 2005), however, a role for vinculin in suppressing focal adhesion turnover, and hence motility (Saunders et al., 2006) has been demonstrated in vinculin null mouse embryo fibroblasts wherein expression of vinculin in these cells resulted in larger focal adhesions with increased stability and slow turnover.
The focal adhesion protein FAK is also required for the turnover of focal adhesions. Fibroblasts from FAK−/− mice have decreased spreading, migrate slower than FAK+/+ fibroblasts, and have increased number of focal adhesions (Ilic et al., 1995). These data indicate that FAK is not required for focal adhesion assembly, but plays an essential role in focal adhesion turnover and subsequently is important for cell migration. Regulation of focal adhesion turnover and signal transduction from within these sites involves tyrosine-phosphorylation signaling pathways. Specifically, activation of FAK at its autophosphorylation site on tyrosine residue 397 is associated with focal adhesion disassembly and is required to promote cell migration (Sieg et al., 1999; Webb et al., 2004; Hamadi et al., 2005).
Thus from preexisting and predominantly in vitro data, there is evidence that coordinated regulation of cell migration involves the transduction of signals from extracellular growth factors to intracellular mediators of cell shape and adhesion including RhoGTPases and focal adhesion proteins, however, the integration of all these molecular events during the initiation of embryonic vascular remodeling in vivo has not been examined. To further understand and identify the molecular and cellular events required for proper endothelial cell migration during early embryonic vascular remodeling in an in vivo context, we employed retinaldehyde dehydrogenase-2 (Raldh2) null embryos as a murine model of defective yolk sac vascular plexus remodeling. Raldh2, an enzyme in the second step of the Vitamin A metabolic pathway that oxidizes all-trans-retinal into retinoic acid (RA), is the predominant enzyme responsible for the production of RA during early embryogenesis and is essential for embryonic survival (Niederreither et al., 1999). Raldh2−/− mutants lack primary capillary plexus remodeling at 9.5 dpc and undergo embryonic lethality at 10.5 dpc (Lai et al., 2003). In this study, we demonstrate that RhoGTPase signaling during the yolk sac vascular remodeling stage at 9.5 dpc was dysregulated in the absence of RA signaling, with Rac activation decreased and Rho activation increased. Loss of RA signaling resulted in a downstream effect of increased focal adhesion protein expression which correlated with increased vinculin-containing focal adhesions within the yolk sac mesodermal layer of Raldh2−/− mutants, suggesting a defect in focal adhesion formation and turnover. Activation of FAK by phosphorylation on tyrosine residue 397 was decreased in mutant yolk sacs compared to WT. During normal vascular remodeling, the focal adhesion proteins FAK and paxillin were physically associated, and this protein-protein interaction was impaired in Raldh2−/− mutants. In vivo rescue of yolk sac vascular remodeling in Raldh2−/− embryos from pregnant mice that were fed RA reduced vinculin expression down to levels similar to WT littermates. Although total FAK expression was not restored back to WT levels in RA-rescued Raldh2−/− yolk sacs, levels of FAK phosphorylation (Tyr397) and FAK-paxillin association were induced back to WT levels. Furthermore, in this study, the endoderm-derived growth factors VEGF-A, IHH, and bFGF, which were previously shown to rescue Raldh2−/− remodeling defects independently from proliferation defects (Bohnsack et al., 2004), also increased FAK phosphorylation (Tyr397) back to WT levels in the yolk sac vascular endothelium of Raldh2−/− mutants. Thus, these studies provide novel and needed insights into the signaling cascades that regulate endothelial cell migration during vascular remodeling in vivo.
RESULTS
RhoGTPase Signaling is Dysregulated in Raldh2−/− Mutants During the Vascular Remodeling Stage (9.5 dpc) of Yolk Sac Blood Vessel Development
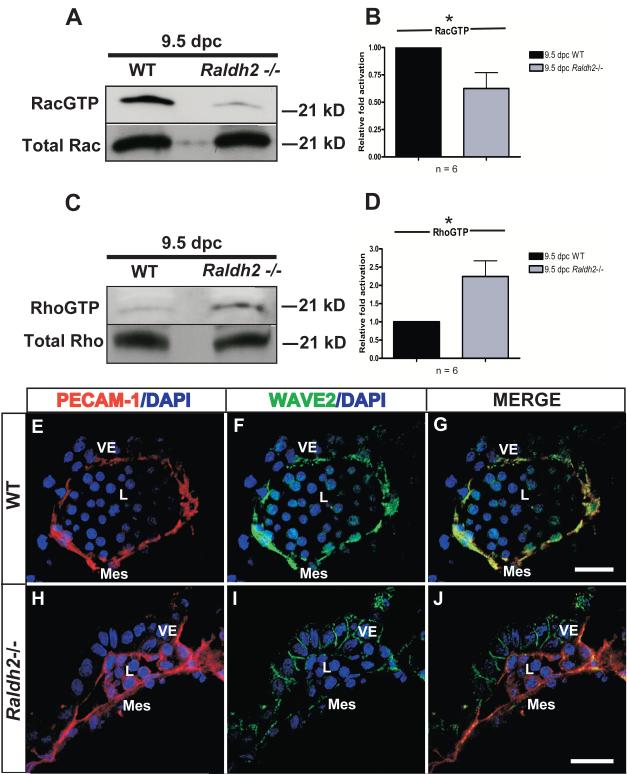
Post-translational signal transduction pathways involving RhoGTPases are known to regulate cell migration. Rac1 activation is required for lamellipodia extension at the leading edge of cells and focal adhesion turnover to promote cell migration (Nobes and Hall, 1999; Zhao et al., 2000), while RhoA activation increases focal adhesion formation and stabilization (Ridley and Hall, 1992) and can impede migration. We sought to determine the activation states of Rac1 and RhoA, two major regulators of the actin cytoskeleton and focal adhesion dynamics, during the vascular remodeling stage of blood vessel development (9.5 dpc). In pooled lysates of 9.5 dpc yolk sacs, Rac1 activation was significantly decreased by 1.6-fold in Raldh2−/− yolk sacs relative to WT (Relative mean fold activation ± SEM: 0.62 ± 0.14; *p=0.025; n = 6) (Figure 1A, B). Conversely, a 2-fold increase in RhoA activation was observed in Raldh2−/− mutants (Relative mean fold activation ± SEM: 2.24 ± 0.43; *p=0.016; n = 6) relative to WT (Figure 1C, D). Increased RhoA activation and decreased activation of Rac1 at the vascular remodeling stage (9.5 dpc) supports an abnormality in cell migration in Raldh2−/− mutants and demonstates a requirement for Rac1 activation during yolk sac vascular remodeling. To further verify Rac1 activation during vascular remodeling and its role in endothelial cell migration at 9.5 dpc, immunostaining for WAVE2, a downstream effector of Rac1 that is predominantly expressed by vascular endothelial cells during early embryonic development, was performed. WAVE-2, a member of the Wiskott-Aldrich syndrome protein (WASP) family of proteins, mediates Rac1-induced membrane ruffling and has been shown to have an important role in directed endothelial cell migration. WAVE2−/− embryos are embryonic lethal at 10 dpc and have defects in both embryonic and yolk sac vessel formation, thus further demonstrating the crucial role of WAVE2 in controlling endothelial cell migration specifically during embryonic vascular remodeling (Yamazaki et al., 2003). Here we demonstrate that in WT yolk sacs wherein increased Rac1 activation occurs, WAVE2 protein expression overlaps with PECAM-1 expression in the endothelial layer of yolk sac blood vessels (Figure 1E-G). In the yolk sac blood vessels of Raldh2−/− mutants wherein there is decreased activation of Rac1, WAVE2 endothelial expression is diminished and relocalizes to the cell-cell borders of the endoderm (Figure 1I). Our results demonstrating the loss of Rac1 activation and endothelial WAVE2 expression in the yolk sac vasculature of Raldh2−/− mutants provide in vivo evidence of a defect in endothelial cell migration manifesting as a morphologic failure in blood vessel formation, and thus reveal an important role for Rac1 activation during yolk sac vascular remodeling.
Figure 1. Increased Rac1 activation and decreased RhoA activation occur during normal vascular remodeling.
(A) Activated and total Rac1 and (C) activated and total RhoA from pulldowns performed on lysates derived from WT vs. Raldh2−/− yolk sacs were determined at 9.5 dpc. RacGTP from WT and Raldh2−/− whole yolk sacs isolated at 9.5 dpc was pulled down utilizing the PAK-1 binding domain bound to glutathione-agarose beads. Similarly, RhoGTP was pulled down with the Rhotekin Rho binding domain bound to glutathione-agarose beads. Levels of activated Rac1 were found to be decreased in Raldh2−/− mutants relative to WT (A). Conversely, RhoA activation was increased in Raldh2−/− mutants relative to WT (C). (B, D) Quantitation of RacGTP (B) and RhoGTP (D) by band densitometry represented by bar graphs denoting fold differences in Rac1 and RhoA activation in 9.5 dpc Raldh2−/− yolk sacs (represented as the mean ± SEM) relative to WT. Data representing the activation for each RhoGTPase were averaged from 6 independent experiments (n = 6) and analyzed by Student’s two-tailed t-test; *p values ≤ 0.05 were considered significant and are denoted by an asterisk (Rac GTP: p = 0.025; RhoGTP: p = 0.016). (E-J) Representative yolk sac sections from WT and Raldh2−/− embryos double-labeled with PECAM-1 (red) and WAVE2 (green) are shown. PECAM-1 (red) marks the endothelial cell layer of yolk sac blood vessels from both WT (E) and Raldh2−/− (H) embryos. (F) WAVE2 (green) is present in the endothelium of the WT yolk sac blood vessel and (G) colocalizes with PECAM-1 (yellow). (I) In contrast, endothelial WAVE2 expression is lost in the Raldh2−/− yolk sac blood vessel and relocalizes to the cell-cell borders of the endoderm layer. Nuclei are stained with DAPI (blue). VE = visceral endoderm; Mes = mesoderm; L = blood vessel lumen; scale bars = 20 μm.
Rac1 Specific Inhibition Abrogates Yolk Sac Blood Vessel Formation
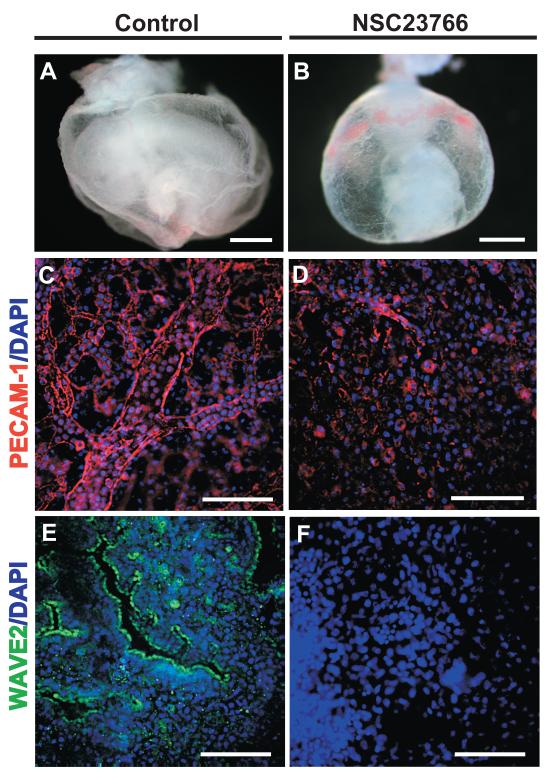
To further verify the role of Rac1 activation in controlling endothelial cell migration during yolk sac vascular remodeling, Rac1 activation was blocked in WT whole embryos cultured from 7.5-9.5 dpc in the presence of the Rac1 GTPase-specific small molecule inhibitor NSC23766. NSC23766 fits into a surface groove of Rac1 and inhibits the binding of Rac1 to its Rac-specific guanine nucleotide exchange factors (GEFs) Trio or Tiam1, subsequently blocking Rac1 activation (Gao et al., 2004). Rac1-specific inhibition can therefore be achieved by treatment with NSC23766 without interfering with the activation of the other closely related RhoGTPases, RhoA and cdc42. Inhibition of Rac1 activation in our studies resulted in lack of vascular remodeling which was observed in NSC23766-treated embryos (Figure 2B) compared to WT control untreated embryos (Figure 2A). Whole-mount staining of yolk sacs with PECAM-1 delineated an arborizing vascular network of small, medium, and large vitelline blood vessels in an untreated embryo (Figure 2C) while loss of PECAM-1 expression and lack of emerging blood vessels was seen in a NSC23766-treated embryo (Figure 2D). Further, WAVE2 expression observed in the yolk sac vascular endothelium of WT control untreated embryos (Figure 2E) was absent with Rac1-specific inhibition in NSC23766-treated embryos that lacked organized blood vessel formation (Figure 2F). Inhibition of large, medium, and small vessel formation in WT embryos upon Rac1-specific inhibition recapitulates the vascular remodeling phenotype seen in Raldh2−/− yolk sacs, thus further demonstrating the importance of Rac1 activation during the process of yolk sac vascular remodeling.
Figure 2. Rac1 specific chemical inhibition recapitulates the yolk sac vascular remodeling defect seen in Raldh2−/− mutants.
(A) A WT control embryo cultured for 48 hrs. from 7.5-9.5 dpc in rat serum exhibits yolk sac vascular remodeling in contrast to (B) a WT embryo cultured in the presence of the Rac1-specific inhibitor NSC23766 (50 μM). (C—F) Whole-mounts of yolk sacs from WT untreated and NSC23766-treated embryos are shown. (C) PECAM-1 (red) delineates a large arborizing vitelline blood vessel and smaller blood vessels in an untreated WT embryo. (D) Diminished PECAM-1 expression correlates with the absence of large, medium, and small vessel formation in the yolk sac of a NSC23766-treated embryo. To verify Rac1 inhibition, WAVE2 (green) expression was compared in untreated and NSC23766-treated embryos. (G) WAVE2 (green) is expressed in the developing yolk sac blood vessels of untreated WT embryos. (H) In contrast, loss of WAVE2 expression was observed in the yolk sac from NSC23766-treated embryos. Nuclei are stained with DAPI (blue). Scale bars = 1000 μm (A, B); 90 μm (C, D, E); 30 μm (F).
Increased Focal Adhesions are Associated with Defective Yolk Sac Capillary Plexus Remodeling in Raldh2−/− mutants
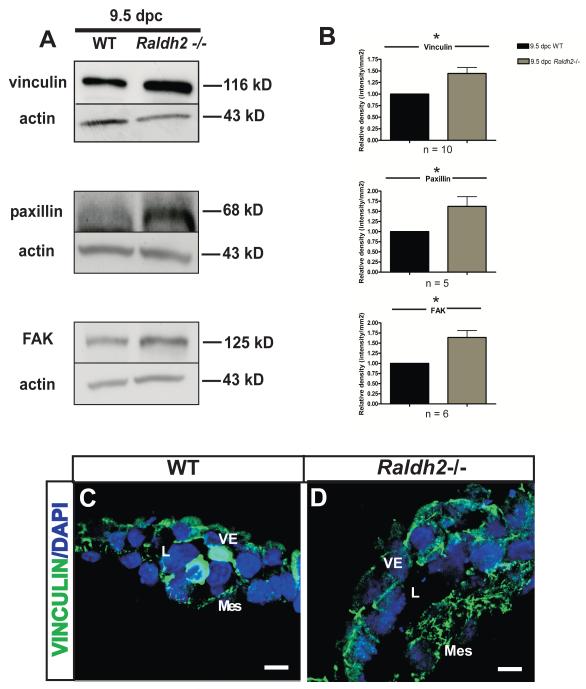
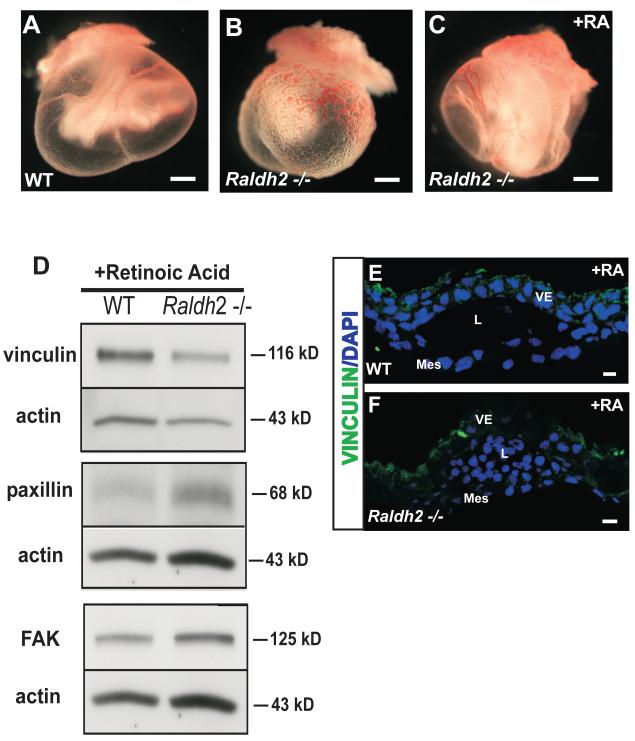
To determine the consequence of decreased Rac1 activation and increased RhoA activation in Raldh2−/− mutants on other intracellular molecular processes that regulate cell migration during vascular remodeling in vivo, we measured the protein expression of major components of focal adhesions that are known to regulate cell migration in vitro. The stage of development again utilized for these experiments was 9.5 dpc at which time the primary capillary plexus is undergoing remodeling into a hierarchy of large, medium, and small vessels. Levels of the focal adhesion proteins vinculin, paxillin, and FAK were measured by Western blot in lysates from 9.5 dpc WT and Raldh2−/− yolk sacs and were all found to be increased in Raldh2−/− yolk sacs compared to WT (Figure 3A). Raldh2−/− yolk sacs had approximately a 1.5-fold increase in the protein levels of vinculin, paxillin, and FAK over that of WT yolk sacs (Relative mean fold increase ± SEM: 1.4 ± 0.13, p=0.0027 for vinculin, n = 10; 1.6 ± 0.24, p=0.032 for paxillin, n = 5; 1.64 ± 0.17, p=0.0036 for FAK, n = 6) (Figure 3B).
Figure 3. Focal adhesions are increased in Raldh2−/− mutants that fail to undergo vascular remodeling.
(A) Representative Western blots are shown that demonstrate increased protein levels of the focal adhesion proteins vinculin, paxillin, and FAK in Raldh2−/− yolk sacs versus WT yolk sacs at 9.5 dpc. Actin was used as the loading control. (B) Quantitation of Western blots for vinculin, paxillin, and FAK is represented by bar graphs indicating the mean band density +/− SEM measured for Raldh2−/− mutants relative to WT for each focal adhesion protein. Vinculin (p = 0.0027), paxillin (p = 0.032), and FAK (p = 0.0036) were significantly increased in Raldh2−/− yolk sacs as determined by a two-tailed student t-test (*p ≤ 0.05 as denoted by an asterisk; n = number of independent Western blot experiments repeated for each focal adhesion protein). Representative fluorescence confocal images are shown which demostrate the localization of vinculin (green) in WT (C) and Raldh2−/− yolk sacs (D). Vinculin expression is increased in the cytoplasm of yolk sac mesodermal cells and endoderm cell-cell borders at 9.5 dpc in Raldh2−/− mutants compared to WT. Nuclei are stained with DAPI (blue). VE = visceral endoderm; Mes = mesoderm; L = blood vessel lumen; scale bars = 10 μm.
To assess localization and extent of focal adhesion formation, 9.5 dpc WT and Raldh2−/− yolk sacs were sectioned and fluorescently immunolabeled with a monoclonal antibody against the focal adhesion marker vinculin. In both WT and Raldh2−/− yolk sacs, vinculin was found to be localized in the cytoplasm of mesodermal cells and the cell-cell junctions of visceral endoderm cells (Figure 3C, D). However, consistent with our Western blot data, we found that vinculin-containing focal adhesions were increased in the mesodermal layer of Raldh2−/− yolk sacs compared to WT by immunofluorescent staining (Figure 3D). The mesodermal layer of Raldh2−/− yolk sacs was noted to be thickened with increased expression of vinculin seen throughout the cytoplasm of mesodermal cells (Figure 3D). The findings of increased focal adhesion protein expression by Western blotting and the presence of increased vinculin-containing focal adhesions observed by fluorescence immunohistochemistry suggest a key modulatory role for vinculin in promoting focal adhesion formation/stabilization and decreased focal adhesion turnover during the process of yolk sac vascular remodeling.
Increased Focal Adhesion Expression in Raldh2−/− Yolk Sacs Occurs Specifically at the Vascular Remodeling Stage (9.5 dpc) and Not Prior
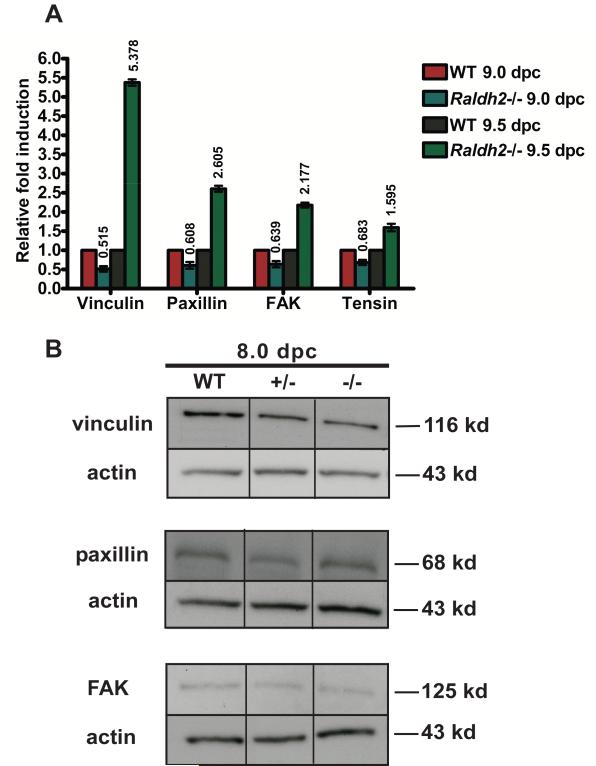
There is existing evidence that blood flow and mechanical forces such as shear stress are important regulators of signaling pathways necessary for remodeling of the capillary plexus (Lucitti et al., 2007), particularly cell signals that promote migration. To precisely define the temporal window of dysregulation of focal adhesion expression during extraembryonic blood vessel formation in Raldh2−/− mutants after the establishment of blood flow, transcript levels of focal adhesion proteins were determined by qRT-PCR in WT and Raldh2−/− yolk sacs at 9.0 (15-20 somite stage) and 9.5 dpc (25-30 somite stage). By 9.0 dpc, blood flow has been established and the initiation of primary capillary plexus remodeling occurs. At 9.5 dpc, a hierarchical network of blood vessels is present that has begun to differentiate into arteries and veins. Relative to corresponding WT controls at 9.5 dpc, transcript levels of the focal adhesion proteins vinculin, paxillin, FAK, and Tensin1 were significantly increased (Relative mean fold increase ± SEM: 5.4 ± 0.084 for vinculin; 2.6 ± 0.081 for paxillin; 2.2 ± 0.064 for FAK; and 1.6 ± 0.098 for tensin) (Figure 4A). Although not increased, focal adhesion protein expression at 9.0 dpc in Raldh2−/− yolk sacs was abnormal as there was an approximately 2-fold decrease in expression of all focal adhesion proteins examined compared to WT. Overall, these results demonstrate lack of appropriate regulation of focal adhesion formation in Raldh2−/− mutant yolk sacs. The increased transcription of focal adhesion proteins specifically at 9.5 dpc in Raldh2−/− yolk sacs is consistent with the increase in protein levels of vinculin, paxillin, and FAK at 9.5 dpc observed in our Western blot experiments and marks 9.5 dpc, and not 9.0 dpc, as the specific temporal stage at which abnormal cell migration is occurring due to increased focal adhesion formation and lack of focal adhesion turnover.
Figure 4. Regulation of focal adhesion formation and turnover is critical during the vascular remodeling stage of blood vessel development (9.5 dpc).
(A) mRNA expression of vinculin, paxillin, FAK, and Tensin1 was assessed by qRT-PCR in yolk sacs harvested at 9.0 dpc (15-20 somite stage) versus 9.5 dpc (25-30 somite stage). Relative to corresponding WT yolk sacs isolated from littermates at either 9.0 dpc or 9.5 dpc, mRNA expression of these genes is increased in Raldh2−/− yolk sacs specifically at 9.5 dpc and not prior at 9.0 dpc. Transcript levels were normalized by using β-actin as the internal control. (B) Protein levels of FAK, paxillin, and vinculin were determined by Western blot in 8.0 dpc WT, Raldh2 heterozygote (+/−), and Raldh2 null (−/−) yolk sacs isolated at the 3-6 somite stage prior to cardiac function and establishment of blood flow. At this stage, protein levels in Raldh2 −/− yolk sacs were not found to be different from WT or Raldh2 +/− yolk sacs. Actin was used as the loading control.
To further assess the role of blood flow establishment on the modulation of focal adhesion protein expression, Western blot analysis was performed for vinculin, paxillin, and FAK on WT and Raldh2−/− yolk sacs at 8.0 dpc (3-6 somite stage), the primary capillary plexus stage of vascular development prior to cardiac function and onset of blood flow. Differential expression of focal adhesion proteins did not occur in Raldh2−/− yolk sacs compared to WT at 8.0 dpc (Figure 4B). Overall, these results delineate 9.5 dpc (25-30 somites) as the stage at which precise temporal molecular regulation of migration pathways controlling focal adhesion formation and turnover must occur in order for yolk sac vascular remodeling to normally progress.
Retinoic Acid Rescues Abnormal Upregulation of Vinculin and Reduces the Number of Focal Adhesions in the Mesoderm of Raldh2−/− Mutants to WT Levels
To confirm the role of RA in the downstream regulation of focal adhesion protein expression during vascular remodeling and determine the distinct migration signaling pathways mediated by RA, in vivo yolk sac vascular rescue studies were conducted. The RA rescue studies were performed by feeding pregnant female Raldh2 heterozygote mice all-trans RA (100 μg of RA/gram of chow) on 7.5 and 8.5 dpc. Dietary supplementation of pregnant female mice during this gestational period promotes remodeling of the yolk sac vasculature in Raldh2−/− mutants that is comparable to that seen in WT embryos at 9.5 dpc without inducing teratogenic effects in WT or heterozygous embryos (Figure 5A-C). Previous data has demonstrated that although remodeling of vitelline blood vessels occurs, the defect in endothelial cell proliferation in Raldh2−/− mutants is not corrected (Lai et al., 2003). After RA treatment, yolk sacs were harvested at 9.5 dpc, genotyped, pooled according to genotype, and lysed for protein extraction. Western blot analysis of RA-rescued yolk sacs at 9.5 dpc revealed that protein levels of vinculin in RA-rescued Raldh2−/− yolk sacs were decreased to levels similar to that of WT yolk sacs, however, paxillin and FAK were not modulated by RA-rescue and remained increased in Raldh2−/− yolk sacs compared to WT (Figure 5D). In contrast to the increased focal adhesions seen in unrescued Raldh2−/− yolk sacs (Figure 3D), fluorescence immunolabeling for vinculin in RA-rescued Raldh2−/− yolk sacs (Figure 5F) showed minimal expression of vinculin in the mesodermal layer similar to WT yolk sacs (Figure 5E). Our finding of decreased protein levels of vinculin, and not FAK or paxillin, in the remodeling vasculature of Raldh2−/− yolk sacs after RA-rescue demonstrates that downregulation of vinculin specifically promotes cell migration and further reveals an important role for vinculin as a modulator of cell migration during vascular remodeling in vivo.
Figure 5. In vivo rescue of Raldh2−/− yolk sac vasculature with RA decreases vinculin expression and the number of focal adhesion sites back to WT levels.
(A-C) Representative images are shown of 9.5 dpc yolk sacs from WT (A) and Raldh2 −/− (B) embryos illustrating morphologic rescue (C) of the yolk sac vascular remodeling defect observed in Raldh2−/− yolk sacs. (D) Representative Western blots demonstrate that increased protein expression of vinculin in untreated Raldh2−/− yolk sacs is reduced to levels similar to WT yolk sacs after in vivo RA-rescue, however increased protein levels of paxillin and FAK are not reverted to WT levels after RA-rescue. (E-F) After RA-rescue, Raldh2−/− yolk sacs (F) at 9.5 dpc express minimal levels of vinculin (green) in the underlying mesodermal layer that is similar to that seen in the mesodermal layer of WT yolk sacs (E). Nuclei are stained with DAPI (blue). VE = visceral endoderm; Mes = mesoderm; L = blood vessel lumen; RA = retinoic acid; scale bars = 1000 μm (A, B, C); 10 μm (E, F).
FAK-Paxillin Association and FAK Phosphorylation (Tyr397) are Required During Yolk Sac Vascular Remodeling
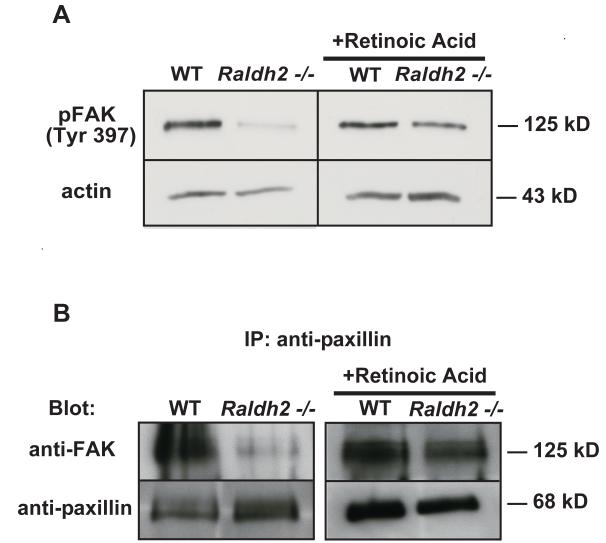
Previous studies have shown that activation of FAK requires phosphorylation of several tyrosine residues but that phosphorylation of tyrosine residue 397, the major tyrosine autophosphorylation site of FAK, is required for efficient disassembly of focal adhesions and subsequently, for cell migration (Hamadi et al., 2005). To further assess the potential mechanism(s) for the defect in focal adhesion turnover, levels of phosphorylated FAK (Tyr397) were determined from protein lysates of yolk sacs from WT and Raldh2−/− embryos. By Western blot, we observed that although levels of total FAK were increased in Raldh2−/− yolk sacs compared to WT (Figure 3A), phosphorylation of FAK (Tyr397) was decreased (Figure 6A). In vivo rescue of yolk sac vascular remodeling in Raldh2−/− embryos from RA-fed pregnant females increased FAK phosphorylation (Tyr397) to WT levels (Figure 6A), thus revealing that FAK phosphorylation (Tyr397) is required during early extraembryonic vascular remodeling in vivo at 9.5 dpc.
Figure 6. FAK activation and association with paxillin are diminished in Raldh2−/− mutants.
(A) Representative Western blots of phosphorylated FAK (Tyr397) in untreated WT and Raldh2−/− yolk sacs and in RA-treated (in vivo) WT and Raldh2−/− yolk sacs at 9.5 dpc demonstrate lower levels of FAK phosphorylation (Tyr397) in Raldh2−/− mutants compared to WT which is increased back to WT levels after in vivo rescue with RA. Actin was used as the loading control. (B) Paxillin was immunoprecipitated from protein lysates from 9.5 dpc WT and Raldh2−/− yolk sacs and representative Western blots show levels of FAK and paxillin. Complex formation between FAK and paxillin occurs in WT yolk sacs during normal vascular remodeling (B, left panel). In contrast, the association between FAK and paxillin was diminished in Raldh2−/− yolk sacs that fail to undergo vascular remodeling (B, left panel). However, after in vivo RA-rescue of yolk sac vascular remodeling in Raldh2−/− mutants, complex formation between FAK and paxillin was observed at a level similar to that detected in WT yolk sacs (B, right panel).
The association of FAK and paxillin has been shown to be important for the initiation of downstream molecular signaling that regulates focal adhesion turnover during renal tubule morphogenesis (Ishibe et al., 2004). With our observation that FAK activation via phosphorylation on Tyr397 was decreased in Raldh2−/− mutant yolk sacs compared to WT, we sought to determine if this finding resulted from an interruption in the association of FAK with paxillin, and therefore, whether or not this protein-protein interaction was important during normal yolk sac vascular remodeling. Paxillin was immunoprecipitated from protein lysates prepared from WT and Raldh2−/− yolk sacs at 9.5 dpc. Western blotting for both FAK and paxillin revealed that these focal adhesion proteins were associated during the vascular remodeling stage of blood vessel development at 9.5 dpc (Figure 6B, left panel). Conversely, we found that complex formation between FAK and paxillin was diminished in Raldh2−/− yolk sacs with unremodeled vasculature (Figure 6B, left panel). However, with in vivo RA-rescue of yolk sac vascular remodeling in Raldh2−/− mutants, the association between FAK and paxillin was observed at a level similar to WT yolk sacs (Figure 6B, right panel). Our results confirm that the protein-protein interaction of FAK and paxillin is important during yolk sac vascular remodeling and may be required for further activation of FAK via phosphorylation on Tyr397.
Ex Vivo Rescue With Endodermal Factors VEGF-A, IHH, and bFGF Induces FAK Phosphorylation (Tyr397) in the Yolk Sac Vascular Endothelium of Raldh2−/− Mutants
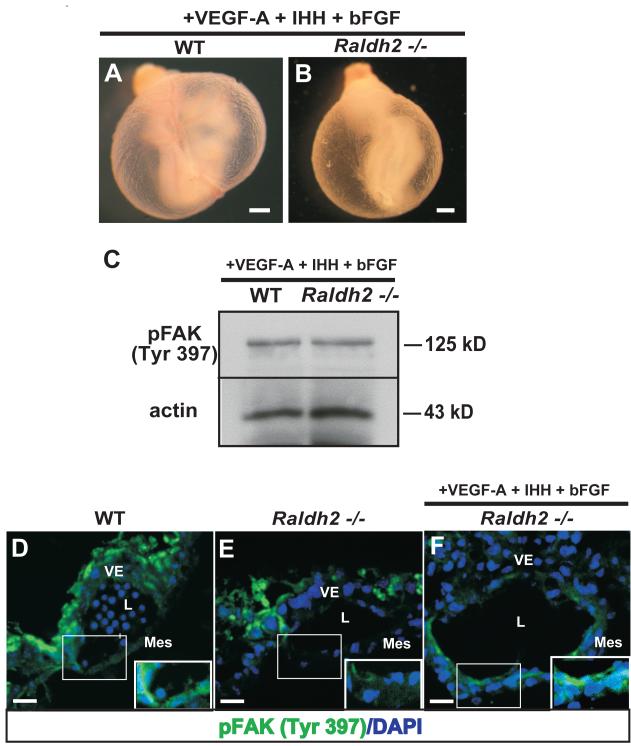
In previous studies from our lab, the endoderm-derived factors VEGF-A, IHH, and bFGF were shown to be indirect targets of RA and were decreased due to loss of visceral endoderm survival in the absence of RA (Bohnsack et al., 2004). When exogenously supplemented in whole mouse embryo culture, these three factors collectively, but not singularly, initiate vascular remodeling (Figure 7B), however loss of endothelial cell proliferative control in Raldh2−/− yolk sacs persists (Bohnsack et al., 2004) suggesting a role for these ligands in regulating endothelial cell migration and not proliferation. Western blot analysis of yolk sac lysates from VEGF-A165-IHH-bFGF-rescued Raldh2−/− yolk sacs demonstrated an increase in FAK phosphorylation on Tyr397 similar to WT levels (Figure 7C). These results were further confirmed by fluorescence immunolabeling of phospho-FAK (Tyr397) in WT, Raldh2−/− yolk sacs, and rescued Raldh2−/− yolk sacs (Figure 7D-F). Phospho-FAK (Tyr397) was localized to the vascular endothelium of WT yolk sacs (Figure 6D) and decreased in the vascular endothelium of Raldh2−/− yolk sacs (Figure 7E). Endothelial expression of phospho-FAK (Tyr397) was present at levels similar to WT in the yolk sac vasculature of Raldh2−/− mutants rescued by exogenous VEGF-A165, IHH, and bFGF (Figure 7F). The finding of phosphorylated FAK (Tyr397) in the vascular endothelium of Raldh2−/− yolk sacs rescued by VEGF-A+IHH+bFGF reveals an in vivo role for phosphorylated FAK (Tyr397) in the process of early yolk sac vascular remodeling at 9.5 dpc and delineates a signaling pathway by which the endoderm factors VEGF-A, IHH, bFGF independently regulate endothelial cell migration.
Figure 7. Endodermal factors VEGF-A, IHH, and bFGF rescue vascular remodeling and FAK activation in the vascular endothelium of Raldh2−/− yolk sacs.
(A-B) Representative images of WT (A) and Raldh2−/− (B) yolk sacs harvested from embryos cultured in the presence of exogenous rh-VEGF-A, human/mouse recombinant IHH, and rh-bFGF demonstate restoration of yolk sac vascular remodeling in Raldh2−/− mutants (B). (C) Levels of phosphorylated FAK (Tyr397) in WT vs Raldh2−/− yolk sacs from embryos cultured in the presence of exogenous VEGF-A, IHH, and bFGF were determined by Western blot. Increased levels of phosphorylated FAK (Tyr397) similar to WT levels were observed in Raldh2−/− mutants after VEGF-A, IHH, and bFGF mediated rescue. Actin was used as the loading control. (D-F) Immunofluorescence for phospho-FAK (Tyr397) (green) was performed on yolk sac sections from WT (D), Raldh2−/− (E), and VEGF-A+IHH+bFGF-rescued Raldh2−/− (F) yolk sacs. Phospho-FAK (Tyr397) (green) was expressed in endothelial cells within the mesodermal layer of WT yolk sacs (inset, D) and was diminished in Raldh2−/− mutants (inset, E). Endothelial expression of phospho-FAK (Tyr397) increased to WT levels in VEGF-A + IHH + bFGF-rescued Raldh2−/− mutants (inset, F). Nuclei are stained with DAPI (blue). VE = visceral endoderm; Mes = mesoderm; L = blood vessel lumen; scale bars = 1000 μm (A, B); 20 μm (D, E, F).
DISCUSSION
In this study, a molecular signaling pathway that regulates endothelial cell migration independently from proliferation involving activation of Rac1, FAK autophosphorylation at tyrosine residue 397, and complex formation between FAK and paxillin was found to be important in the process of early extra-embryonic vascular remodeling. Our finding of increased focal adhesion proteins in unremodeled Raldh2−/− yolk sac blood vessels was correlated with modulation of this specific cell migration pathway in vivo.
RhoGTPases are emerging as critical transducers of growth factor signals to the actin cytoskeleton during early embryonic vascular development (Tan et al., 2008). In our study, we provide further evidence that Rac1 activation is required for normal yolk sac vascular remodeling specifically during early embryonic development at 9.5 dpc. We demonstrate that a decrease or block in Rac1 activation, both seen in Raldh2−/− mutants (Figure 1A) and with the Rac1-specific inhibitor NSC23766 (Figure 2), abrogates yolk sac blood vessel development. We additionally provide evidence that failure of yolk sac vessel remodeling is associated with endothelial loss of the Rac1 effector WAVE2 (Figures 1I and 2F), an important mediator of endothelial cell migration. RA has previously been shown to induce activation of Rac1 to promote neuronal differentiation (Pan et al., 2005; Guleria et al., 2006). Thus, RA has been studied in regards to its role in signaling pathways associated with cell differentiation, however, its role in regulating migration signaling pathways and the contribution of these pathways to vascular remodeling has not been defined. The decrease in Rac1 activation in RA-deficient yolk sacs with unremodeled vasculature at 9.5 dpc compared to WT (Figure 1A, B) was associated with the observation of increased focal adhesions in Raldh2−/− mutant yolk sacs. These results extend the known activities of Rac1 activation to include the regulation of focal adhesion disassembly and turnover during yolk sac vascular remodeling. In addition, we found increased levels of activated RhoA in Raldh2−/− mutants at 9.5 dpc (Figure 1C, D) that is consistent with a defect in migration due to increased stabilization of focal adhesions and enhanced cell-ECM adhesion.
Our findings in the Raldh2−/− mutants of increased expression of vinculin, FAK, and paxillin (Figure 3A, B), key components of focal adhesions that are known to be important regulators of cell migration, and increased number of vinculin-containing focal adhesions in the yolk sac mesoderm layer of these mutants compared to WT (Figure 3C, D) is consistent with a defect in cell migration resulting from lack of focal adhesion turnover. In this study, we found an increase in vinculin-containing focal adhesions at the cell-cell junctions of endodermal cells and in the cytoplasm of cells within the yolk sac mesoderm layer of Raldh2−/− mutants (Figure 3D) associated with lack of remodeling of the primitive capillary plexus. Vinculin is a major component of focal adhesions and cell-cell junctions. The role of vinculin at cell-cell junctions is yet undefined, however our finding that downregulation of vinculin in the underlying mesoderm is necessary for cell migration and blood vessel remodeling in vivo supports previous evidence that vinculin suppresses cell migration by stabilizing focal adhesions (Xu et al., 1998b; Saunders et al., 2006). However, the direct mechanism as to how increased expression of vinculin in the underlying mesodermal layer inhibits the migration of endothelial cells and how this contributes to the overall remodeling of blood vessels is not known. Our data further reveals the importance of regulation of focal adhesion formation and turnover during the vascular remodeling stage of blood vessel development (9.5 dpc) and not prior (Figure 4). The dysregulation of focal adhesion formation and turnover in Raldh2 mutants specifically at 9.5 dpc indicates that molecular regulation of focal adhesion dynamics is critical during the stage of vascular development during which the vitelline blood vessels are actively forming a hierarchical network of branches that are undergoing differentiation into arteries and veins. In contrast, the initiation of vascular remodeling after establishment of blood flow at 9.0 dpc does not appear to be dependent on RA for the regulation of focal adhesion turnover. The decrease in focal adhesion proteins at 9.0 dpc in Raldh2−/− mutants, however suggests that these mutants have an overall defect in focal adhesion formation, thus indicating that regulation of focal adhesion formation is also required at this stage. In addition, the lack of differential expression of focal adhesion proteins in Raldh2−/− mutants compared to WT at 8.0 dpc prior to the establishment of blood flow provides further evidence that signaling pathways that control endothelial cell migration are influenced by blood flow and mechanical forces such as shear stress.
An important cell migratory pathway has previously been described during renal tubule morphogenesis in which the association between FAK and paxillin promotes the phosphorylation of both proteins and mediates both Rac1 activation and focal adhesion turnover downstream (Ishibe et al., 2003; Ishibe et al., 2004). In the context of normal vascular remodeling, we demonstrate that FAK and paxillin associate together and that FAK phosphorylation on Tyr397 is required to promote migration of endothelial cells during extraembryonic blood vessel remodeling at 9.5 dpc (Figure 6B). Conversely, we found that decreased FAK-paxillin association and loss of FAK phosphorylation (Tyr397) occur in Raldh2−/− yolk sacs that lack normal blood vessel remodeling (Figure 6B). We further determined that the interaction of FAK and paxillin is indeed required during vascular remodeling in vivo as RA-rescue of vessel remodeling in the yolk sacs of Raldh2−/− mutants promoted the association of FAK and paxillin. Disruption of FAK-paxillin interaction and an increase in the association of paxillin with vinculin has previously been described in the context of defective cell migration and focal adhesion turnover in vitro (Subauste et al., 2004) indicating a direct role for vinculin in mediating paxillin’s interaction with FAK. The finding that FAK activity is increased in vinculin null embryos is further evidence that increased expression of vinculin may interrupt the interaction of paxillin and FAK by binding to paxillin and preventing FAK phosphorylation. In our studies, however, paxillin and vinculin were not found to be associated in WT or Raldh2−/− yolk sacs (data not shown); therefore the direct, mechanistic role of vinculin in modulating molecular signaling that affects cell migration during vascular remodeling remains to be elucidated.
Previously, we demonstrated that the visceral endoderm-derived factors VEGF-A, IHH, and bFGF added exogenously to Raldh2−/− embryos in culture collectively promote vascular remodeling in the absence of cell proliferative control (Bohnsack et al., 2004). The use of Raldh2−/− mice have provided a unique opportunity to specifically study molecular pathways that affect migration separate from the defect in endothelial cell proliferation. Mounting evidence supports that signals from the endoderm are necessary for proper vascular formation in the underlying mesoderm layer (Risau et al., 1988; Nath et al., 2004). In regards to growth factor-stimulated cell migration, VEGF-A is known to influence endothelial cell migration by modulating cell-cell and cell-ECM adhesion via assembly and disassembly of focal adhesions. VEGF-mediated tyrosine phosphorylation of focal adhesion proteins such as FAK and paxillin occurs in human umbilical vein endothelial cells and is required for the recruitment of these proteins to focal adhesion sites (Abedi and Zachary, 1997). In this study we corroborate our previous findings and further demonstrate that like RA alone, VEGF-A + IHH + bFGF collectively can restore FAK activation in the yolk sac vascular endothelium of Raldh2−/− embryos (Figure 7C, F). These results specify a role for VEGF-A, IHH and bFGF in promoting vascular remodeling via a migration signaling pathway involving Rac1 and FAK activation that is distinct from the TGFβ-fibronectin pathway controlling endothelial cell proliferation (Bohnsack et al., 2004).
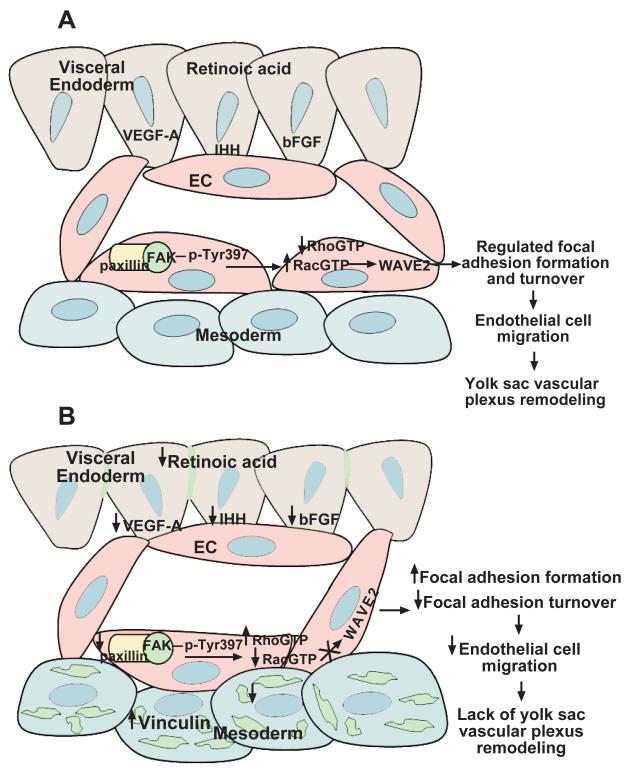
In all, our study identifies a developmentally stage-specific cell migration signaling pathway controlling focal adhesion formation and turnover that is unique from the molecular signaling pathway that regulates endothelial cell proliferation during yolk sac vascular remodeling in vivo at 9.5 dpc. During normal yolk sac vascular remodeling (Figure 8A), RA maintains the integrity of the yolk sac endoderm and its ability to produce the endoderm-derived factors VEGF-A, IHH and bFGF. Our studies demonstrate that these factors act on the underlying mesoderm to promote endothelial cell migration via a specific molecular signaling pathway that involves the association of FAK with paxillin. Upon interaction of FAK and paxillin, FAK is phosphorylated on Tyr397, Rac1 is activated, and WAVE2 is expressed. In the absence of RA signaling (Figure 8B), decreased production of VEGF-A, IHH, and bFGF results. As a consequence, the association of FAK and paxillin is disrupted and there is diminished activation of FAK and Rac1, loss of WAVE2 expression, and increased activation of RhoA. The altered activation states of these signaling proteins results in increased vinculin-containing focal adhesion sites throughout the yolk sac mesoderm that ultimately results in a defect in endothelial cell migration and lack of vascular remodeling.
Figure 8. Working model of the molecular regulation of endothelial cell migration during murine yolk sac vascular remodeling.
(A) For normal vascular remodeling to proceed, RA is required for maintenance of the yolk sac endoderm, the site of production of multiple growth factors such as VEGF-A, IHH and bFGF. These factors act on the underlying mesoderm to promote endothelial cell migration via association of FAK with paxillin, FAK phosphorylation (Tyr397), and activation of Rac1 and its downsteam effector WAVE2. In the absence of RA signaling (B), decreased production of VEGF-A, IHH, and bFGF results due to loss of endoderm integrity. As a consequence, the association of FAK and paxillin is disrupted, activation of FAK and Rac1 is diminished, there is loss of WAVE2 expression, and increased activation of RhoA. The altered activation states of these signaling proteins result in increased vinculin-containing focal adhesion sites throughout the yolk sac mesoderm and ultimately lead to a defect in endothelial cell migration and lack of vascular remodeling.
EXPERIMENTAL PROCEDURES
Mice
The expression of the Raldh2 gene was disrupted in CD1 mice via targeted insertion of the neomycin gene into the coding region as described (Niederreither et al., 1999). Timed matings were performed by checking female mice daily for vaginal plugs; the morning a plug was observed was designated 0.5 dpc. All animal experiments were performed according to animal research protocols approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC).
Genotyping
Heterozygous mice for breeding were genotyped by PCR. Tail clippings were used as the source of DNA and were digested with proteinase K. Tail DNA was extracted with phenol:chloroform:isoamyl alcohol. For genotyping of yolk sacs, the corresponding embryos were digested by incubating in 50 mM NaOH at 95°F for 15 minutes followed by neutralization with 0.5 M Tris-HCL (pH 8.0). For the PCR reaction, primers were used that closely flank the Raldh2 locus as previously described (Niederreither et al., 1999).
Immunofluorescence
9.5 dpc WT and Raldh2−/− conceptuses were isolated and fixed in 4% PFA. For frozen sections, embryos were processed through a sucrose gradient (10% sucrose followed by 20% sucrose overnight) then embedded in M-1 embedding matrix (Shandon Inc., Pittsburgh, PA), flash frozen in 2-methylbutane cooled in liquid nitrogen, and cryosectioned at 10 μm onto Ultrastick® glass slides (Fisher Scientific, Pittsburgh, PA). Tissue sections were blocked with 3% BSA and 0.5% Tween20 in PBS, then incubated with primary antibody overnight. Slides were then washed with 0.2% BSA and 0.5% Tween 20 ten times, then incubated with secondary antibody overnight. For whole mount immunostaining of yolk sacs, embryos were initially dehydrated through a methanol gradient and stored in 100% methanol. Prior to immunostaining, embryos were rehydrated through a methanol gradient and permeabilized with 0.1% Tween in PBS. The primary antibodies used were a monoclonal mouse anti-vinculin (Sigma-Aldrich, St. Louis, MO; dilution 1:50), monoclonal mouse anti-phospho-FAK (Tyr397) (Upstate, Millipore, Billerica, MA; dilution 1:200), monoclonal mouse anti-PECAM-1 (BD Transduction Laboratories, BD Biosciences, San Jose, CA; dilution 1:50), and polyclonal rabbit anti-WAVE2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; dilution 1:50). Alexa Fluor 488 and Alexa Fluor 594 (Molecular Probes-Invitrogen, Carlsbad, CA) were used as the secondary antibodies at a dilution of 1:250-1:400. Nuclei were stained with DAPI (Sigma-Aldrich, St. Louis, MO). Images were obtained using a Zeiss LSM 510 META confocal system or a DeltaVision Spectris Restoration Microscopy System.
Western Blotting and Immunoprecipitation
The expression of focal adhesion proteins vinculin (Upstate, Millipore, Billerica, MA; dilution 1:10,000), paxillin (BD Transduction Laboratories, BD Biosciences, San Jose, CA; dilution 1:500), and FAK (BD Transduction Laboratories, BD Biosciences, San Jose, CA; dilution 1:500) was determined by Western blot analysis. Levels of phosphorylated FAK (Tyr397) were also determined by Western blotting (BD Transduction Laboratories, BD Biosciences, San Jose, CA; dulition 1:1000). Actin (Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:5000) was used as the loading control for all Western Blots. Yolk sacs from wild type or Raldh2−/− yolks sacs were pooled and lysed in RIPA buffer (Upstate, Beverly, MA) containing Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN) and Phosphatase Inhibitor Cocktails I and II (Calbiochem, San Diego, CA). Protein concentration for each sample was determined by BCA assay (Pierce, Rockford, IL) and quantified by a HTS 7000 Plus Bio Assay Reader (Perkin Elmer, Waltham, MA). For Western blotting experiments, 20 μg of protein per sample were loaded on SDS-PAGE gels (Bio-Rad, Hercules, CA) and transferred onto nitrocellulose membranes (Millipore, Billerica, MA). Chemoluminescent detection was performed using the ECL Plus Detection Kit (GE Healthcare Limited, Buckinghamshire, UK). 100-200 μg of protein were used for the coimmunoprecipitation experiments. Paxillin was precipitated with an anti-paxillin monoclonal antibody (BD Transduction Laboratories, BD Biosciences, San Jose, CA) and Trueblot anti-Ig mouse beads (eBioscience, San Diego, CA). Immunoprecipitated paxillin was then detected by Western blot using the same paxillin monoclonal antibody (BD Transduction Laboratories, BD Biosciences, San Jose, CA). Coprecipitation of FAK and vinculin was detected by Western blotting using an anti-FAK monoclonal antibody (BD Transduction Laboratories, BD Biosciences, San Jose, CA) and an anti-vinculin monoclonal antibody (Upstate, Millipore, Billerica, MA). Band densitometry was performed using Quantity One software (Bio-Rad, Hercules, CA).
Reverse Transcription and Real-Time Quantitative PCR
Yolk sacs were dissected and individually placed in RNA Later (Qiagen, Valencia, CA). After genotyping corresponding embryos, WT and Raldh2−/− yolk sacs were pooled separately and total RNA was extracted using the Qiagen RNeasy Mini Kit. RNA was then quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA was synthesized from 1 μg of RNA per sample using Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. qPCR was then performed using primers from Applied Biosystems (vinculin, paxillin, FAK, and tensin1) using the ABI Prism® 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Each reaction was done in triplicate. Fold induction of each gene in the Raldh2−/− yolk sacs relative to WT was calculated. Mouse β–actin was used as the endogenous control.
RhoGTPase Activation Assay
Isolated WT or Raldh2−/− yolk sacs were pooled and rinsed with PBS then lysed with 500 ml of magnesium lysis buffer containing 25 mM HEPES (pH 7.5), 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, 2% glycerol, 10 mg/ml leupeptin, 10 mg/ml aprotinin, Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN) and Phosphatase Inhibitor Cocktails I and II (Calbiochem, San Diego, CA). The tissue was lysed by passage through a 23 gauge syringe needle, then a smaller bore 26-gauge syringe needle. RhoGTPase pull-downs were performed according to manufacturer’s specifications (Upstate, Lake Placid, NY). Western blotting was then performed to detect activated and total RhoGTPase levels. 20 μg of protein were used for determination of total Rac1 and RhoA levels. Band densitometry was performed using Quantity One software (Bio-Rad, Hercules, CA).
In Vivo Retinoic Acid Rescue
On the morning of embryonic day 7.5 and 8.5, maternal diet was supplemented with all-trans retinoic acid (Sigma-Aldrich, St. Louis, MO). 100 μg of RA were added per gram of feed and protected from light exposure. Embryos were dissected at 9.5 dpc and assessed morphologically for the presence of yolk sac vascular remodeling.
Whole Mouse Embryo Culture
At 7.5 dpc, embryos were dissected free from uterine and trophoblastic tissue and Reichart’s membrane was removed. Embryos were cultured in pooled male rat serum for 48 hours in a rolling bottle culture incubator at a speed of 30 rpm (BTC Precision Engineering, Milton Cambridge, England). Embryos were gassed for 3 minutes upon initiation of culture with 5% CO2 and subsequently gassed once daily for 3 minutes with increasing concentrations of CO2 (20% and 40% CO2). The Rac1 specific inhibitor NSC23766 (Calbiochem-EMD4 Biosciences, Gibbstown, NJ) was used at a concentration of 50 μM. For rescue experiments, the following reagents were added to embryos in culture: recombinant human VEGF-A165 (R&D) 50ng/ml diluted in 0.1% BSA□ in PBS, recombinant human FGF basic (R&D) 100ng/ml diluted in 0.1% BSA in PBS and 1mM DTT□, and recombinant human/mouse IHH (R&D) 1 μg/ml diluted in 0.1% BSA in PBS.
Statistics
For Western blots and RhoGTP pulldowns, bands were quantified by densitometry using Quantity One software (Bio-Rad, Hercules, CA) and data were analyzed by Student’s two-tailed t-test. P values less than or equal to 0.05 were considered significant.
ACKNOWLEDGEMENTS
Special thanks to Dr. Anjali Nath and Dr. Joshua Brumberg for critical reading of this manuscript. This study was supported by National Institutes of Health grants (1RO1 HL76260, EB005173, and EB007076) as well as a USDA grant (USDA ARS – 6250-51000) to K.K.H, and a Mentored Clinical Scientist Development Award (KO8 HL081227) and Baylor Child Health Research Center Grant (K12 HD41648) to J.M.E.
Grant information: National Institutes of Health, 1RO1 HL76260, EB005173, and EB007076 to KKH United States Department of Agriculture, USDA ARS – 6250-51000 to KKH National Institutes of Health Mentored Clinical Scientist Development Award, KO8 HL081227 to JME and Baylor Child Health Research Center Grant, K12 HD41648 to JME
ABBREVIATIONS
- dpc
days post coitum
- FAK
Focal adhesion kinase
- IHH
Indian hedgehog
- Raldh2
retinaldehyde dehydrogenase-2
- RA
retinoic acid
- WT
wild-type
REFERENCES
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ST, Flinn HM, Humphries MJ, Critchley DR, Ridley AJ. Requirement for Rho in integrin signalling. Cell Adhes Commun. 1997;4:387–398. doi: 10.3109/15419069709004456. [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Lai L, Dolle P, Hirschi KK. Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev. 2004;18:1345–1358. doi: 10.1101/gad.1184904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll JL, Ben-Ze’ev A, Ezzell RM, Rodriguez Fernandez JL, Baribault H, Oshima RG, Adamson ED. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc Natl Acad Sci U S A. 1995;92:9161–9165. doi: 10.1073/pnas.92.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman TA, Gossler A, Kemler R. Blastocyst-derived embryonic stem cells as a model for embryogenesis. In: Feichtingen W, Kemeter P, editors. Future Aspects in Human In Vitro Fertilization. Springer-Verlag; Berlin: 1987. pp. 187–195. [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Gallant ND, Michael KE, Garcia AJ. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol Biol Cell. 2005;16:4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- Guleria RS, Pan J, Dipette D, Singh US. Hyperglycemia inhibits retinoic acid-induced activation of Rac1, prevents differentiation of cortical neurons, and causes oxidative stress in a rat model of diabetic pregnancy. Diabetes. 2006;55:3326–3334. doi: 10.2337/db06-0169. [DOI] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Ronde P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118:4415–4425. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, McDonagh S, Jin F, Baumbusch C, Gardner DG, Damsky CH. Focal adhesion kinase is required for blood vessel morphogenesis. Circ Res. 2003;92:300–307. doi: 10.1161/01.res.0000055016.36679.23. [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004;16:257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol Cell. 2003;12:1275–1285. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Lai L, Bohnsack BL, Niederreither K, Hirschi KK. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development. 2003;130:6465–6474. doi: 10.1242/dev.00887. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Lifschitz-Mercer B, Czernobilsky B, Feldberg E, Geiger B. Expression of the adherens junction protein vinculin in human basal and squamous cell tumors: relationship to invasiveness and metastatic potential. Hum Pathol. 1997;28:1230–1236. doi: 10.1016/s0046-8177(97)90195-7. [DOI] [PubMed] [Google Scholar]
- Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, LaMonica K, Hong T, Pagliaruli T, Mulrooney J, Grabel L. Roles for Rho/ROCK and vinculin in parietal endoderm migration. Cell Commun Adhes. 2005;12:9–22. doi: 10.1080/15419060500305948. [DOI] [PubMed] [Google Scholar]
- Nath AK, Enciso J, Kuniyasu M, Hao XY, Madri JA, Pinter E. Nitric oxide modulates murine yolk sac vasculogenesis and rescues glucose induced vasculopathy. Development. 2004;131:2485–2496. doi: 10.1242/dev.01131. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Kao YL, Joshi S, Jeetendran S, Dipette D, Singh US. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93:571–583. doi: 10.1111/j.1471-4159.2005.03106.x. [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. Embo J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Risau W, Gautschi-Sova P, Bohlen P. Endothelial cell growth factors in embryonic and adult chick brain are related to human acidic fibroblast growth factor. Embo J. 1988;7:959–962. doi: 10.1002/j.1460-2075.1988.tb02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Fernandez JL, Geiger B, Salomon D, Ben-Ze’ev A. Overexpression of vinculin suppresses cell motility in BALB/c 3T3 cells. Cell Motil Cytoskeleton. 1992;22:127–134. doi: 10.1002/cm.970220206. [DOI] [PubMed] [Google Scholar]
- Rodriguez Fernandez JL, Geiger B, Salomon D, Ben-Ze’ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993;122:1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RM, Holt MR, Jennings L, Sutton DH, Barsukov IL, Bobkov A, Liddington RC, Adamson EA, Dunn GA, Critchley DR. Role of vinculin in regulating focal adhesion turnover. Eur J Cell Biol. 2006;85:487–500. doi: 10.1016/j.ejcb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(Pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–381. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. Faseb J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998a;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Xu W, Coll JL, Adamson ED. Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J Cell Sci. 1998b;111(Pt 11):1535–1544. doi: 10.1242/jcs.111.11.1535. [DOI] [PubMed] [Google Scholar]
- Yamazaki D, Suetsugu S, Miki H, Kataoka Y, Nishikawa S, Fujiwara T, Yoshida N, Takenawa T. WAVE2 is required for directed cell migration and cardiovascular development. Nature. 2003;424:452–456. doi: 10.1038/nature01770. [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]