Abstract
Background
CD56pos NK/NT cells are important innate effectors providing the first line of defense against viral infection. Enhanced NK activity has been shown to protect from HIV-1 infection. However, the role played by these innate effectors in protection against or development of HCV infection is unknown.
Methods
We characterized CD56pos populations in 11 intravenous drug users (IDUs) who remained uninfected despite being repeatedly exposed to HCV. NK profiles in exposed uninfected (EU) individuals were compared to pre-infection samples (median 90 days prior to HCV seroconversion) collected from 14 IDUs who subsequently became infected (EI) and unexposed normal control subjects (NC, n=8).
Results
Flow cytometric analysis of CD56pos populations demonstrated that EUs had a higher proportion of CD56low mature (p=0.0011) NK cells compared to subjects who subsequently became infected. Bead-isolated NKs (>90% purity) from EUs had significantly higher IL-2 induced cytolytic activity against the NK-sensitive cell line K562 at an effector to target ratio of 10:1 (p<0.0001). NKp30, a natural cytotoxicity receptor involved in NK activation, is highest on NK/NT cells in EUs relative to infected subjects. Using the JFH-1 infection system we demonstrate that NKp30high cells in the absence of exogenous stimulation significantly reduce infection of hepatocytes.
Conclusions
We demonstrate that CD56pos populations in EUs are enriched for effector NKs displaying enhanced IL-2 induced cytolytic activity and higher levels of NCR NKp30 activating receptor. In addition NKp30high NK cells are more effective in preventing infection of Huh 7.5 cells than their NKp30low/neg counterparts. For the first time, these data support the hypothesis that NK cells contribute to anti-HCV defense in vivo in the earliest stages of infection, providing innate protection from HCV acquisition.
Hepatitis C virus (HCV), a member of the Flaviviridae family, is known for its high propensity to establish persistent infection (1, 2). The host immune response early in HCV infection is thought to determine subsequent outcome (3), suggesting an important role for innate immunity in viral elimination either directly, preventing establishment of infection, or indirectly, through priming of antigen-specific adaptive immune mechanisms. The observation that a number of intravenous drug users (IDUs) remain healthy with no evidence of infection despite continued long-term exposure to HCV (4) strongly suggests a role for innate immunity in natural protection from HCV infection.
Natural killer (NK) cells are key innate immune effectors that provide the first line of defense against viral infection, shaping subsequent adaptive immunity (5). NK activity is stringently controlled by inhibitory NK receptors (NKRs), which in steady state conditions override signals provided by engagement of activating receptors (6). NKRs include the predominantly inhibitory killer immunoglobulin-like receptors (KIR), C-type lectin-like receptors of the CD94/NKG2 family comprising inhibitory (NKG2A) and activatory (NKG2C/D) isoforms, as well as the natural cytotoxicity receptors (NCRs) such as NKp30, NKp44 and NKp46, orphan receptors that deliver activatory signals (6, 7).
In humans, NKs can be identified by the expression of N-CAM (CD56) and relative expression of this antigen identifies functionally distinct immature/regulatory (CD56bright) and effector (CD56dim) NK subsets. CD56dim NKs carry perforin, and are the main mediators of cytotoxicity (8,). Expression of CD56 and various NKRs is shared by another innate-like effector population, natural T (NT) cells. The functional properties of NTs are similar to NKs, thus, in addition to NKs, NTs are likely to be involved in the first line of defense against viral infection. Of note, the liver, the preferred site of HCV replication, is highly enriched for innate immune effectors, in particular NK and NT cells (9).
The phenotypes and/or functional activities of various populations of these innate effectors have been reported to be impaired in patients with chronic HCV (10–20). Of interest, evidence suggests inheritance of particular KIR genes involved in control of NK activity, may predispose to chronic infection (21, 22). Other studies show that HCV can modulate NK activity, either directly by binding of the HCV envelope-2 (E2) protein to CD81 (23–25) or indirectly by inducing expression of inhibitory ligands for NKs (14, 26, 27). Data on the role of NKs in the setting of acute HCV infection are limited. However, we have demonstrated that reduced IL-2-activated killing (LAK) early in infection was associated with the ultimate development of persistence, suggesting a role for innate NK/NT cells in clearance of HCV in the acute setting (28). A role for these populations in conferring innate protection from HCV acquisition has yet to be established, but, is suggested by an in vitro model where NK cells were key to suppressing HCV infection of human hepatocytes (29).
Enhanced NK activity (30) and has been shown to contribute to protection from HIV-1 infection in exposed individuals. However, the role played by innate CD56pos effector populations in protection against or development of HCV infection is unknown. To address this question, we characterized CD56pos NK and NT cells in pre-infection blood samples from a high-risk long-term exposed IDU cohort in which some individuals remain uninfected despite repeated exposure to HCV (4). We demonstrate relatively increased effector NK cell level as well as enhanced NK cytolytic function, which was associated with an increase in NCR NKp30 expression, in subjects who remain resistant to infection in the face of repeated exposures. We also demonstrate that NKp30high NK cells in the context of the JFH-1 in vitro infection system are more effective in preventing infection of Huh 7.5 cells than their NKp30low/neg counterparts in the absence of exogenous stimulation. Our data offers new insights into mechanisms underlying protection from HCV infection which may have implications for improving immunotherapeutic strategies.
Materials and Methods
Study population
The study group was comprised of 25 intravenous drug users (IDUs), 11 who remained uninfected (EU) despite being repeatedly exposed to HCV, and 14 IDUs who subsequently became infected (EI). The average age of exposed individuals was 25 years, 84% were Caucasian and 60% were female. The age, race and gender distribution did not differ between the groups which subsequently became infected or remained healthy. For the cohort of exposed individuals who subsequently became infected, pre-infection samples (median 90 days prior to HCV seroconversion) were analyzed. All exposed individuals tested negative for HBV/HIV. Eight individuals with no risk factors who tested negative for HCV/HIV served as unexposed normal control subjects (NC). The study protocol was approved by the Institutional Review Boards at the University of Colorado, Aurora, CO; and Johns Hopkins Medical Institutions, Baltimore, MD. Both written and oral consent was obtained before samples were collected.
Sample preparation and storage
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Amersham Biosciences, Piscataway, NJ) density gradient centrifugation and cryopreserved for subsequent analyses.
Flow cytometric analysis
Flow cytometry was performed using a BD FACSCalibur instrument (BD Biosciences, San Jose, CA) compensated with single fluorochromes and analyzed using CellQuest™ software (BD Biosciences). Flurochrome-labeled (FITC/PE/PerCP/APC) monoclonal antibodies (MAbs) specific for CD3/CD56 were obtained from BD Biosciences. Anti-TRAIL-PE MAb was supplied by R&D systems (Minneapolis, MN). Anti-NKp30-PE and NKp44-PE were obtained from Immunotech (Beckman Coulter, Fullerton, CA). PBMCs (2.5 × 105) were stained for cell surface antigen expression at 4°C in the dark for 30 minutes, washed twice in 2 ml phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.01% sodium azide (FACS-wash) and fixed in 200µl of 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Isotype-matched fluorescently-labeled control antibodies were used to determine background levels of staining. Lymphocytes were identified by characteristic forward and side scatter (fsc/ssc) parameters and populations of interest were gated on patterns of CD56/CD3 staining within the lymphocyte population. Results are expressed as % positive of gated population. Intracellular perforin staining was carried out after permeabilization with 0.2% saponin using the δ-G9 antibody from BD.
Cytotoxicity assays
Thawed mononuclear cell suspensions were enriched for NKs using the NK Isolation Kit II from Miltenyi Biotec (Gladbach, Germany) according to the manufacturer’s instructions. Median purity of NKs was >90% in all cases. Following isolation, the NKs were cultured +/− IL-2 (25ng/ml, R&D) for 48 hours at 37°C and 5% CO2. Following culture, carboxy fluorescein succinimidyl ester (CFSE) labeled target cells (K562s) were added to the NKs at effector:target concentrations of 0:1 (negative control) and 10:1 (test) and incubated at 37°C for 4 hours. After incubation, cytotoxicity was measured using the flow-cytometry based Total Cytotoxicity & Apoptosis Detection Kit from Immunochemistry (Bloomington, MN). Immediately before acquisition, 7-aminoactinomycin D (7-AAD) was added to effector:target populations and incubated for 15 minutes on ice. Cells treated with 0.1% Triton-X served as positive controls.
Degranulation assay
Degranulation was determined by flow cytometric analysis of increased CD107a (Lamp, BD) expression on bead-isolated NKs after 4 hour stimulation with PMA (10ng/ml) and Ionomycin (1ug/ml) in the presence of brefeldin A (Sigma-Aldrich) and CD107a. NKs cultured under the same conditions without PMA and Ionomycin served as unstimulated controls.
Cytokine assays
Antibodies for intracellular IFN-γ were supplied by BD Biosciences. Thawed mononuclear cells were stimulated with PMA, (10ng/ml) and ionomycin (1 µg/ml) for 4 hours at 37°C in the presence of brefeldin A. After stimulation cells were stained for surface antigens (as above), fixed for 30 minutes at 4°C in 100µl Fix and Perm Medium A (Caltag, Burlingame, CA), permeabilized using 100µl Fix and Perm Medium B (Caltag) and incubated with anti-cytokine MAb for 1 hour 4°C in the dark. Cell suspensions were then washed in FACS-wash and fixed in 200µl 2% PFA and acquired after 1 hour. Cells cultured under the same conditions in the absence of PMA and ionomycin served as controls.
Hepatocyte cytotoxicity assay
NKs were enriched using magnetic beads and surface stained for CD3, CD56 and NKp30 as described above. NKs (CD3−CD56+) were FACS sorted on expression of NKp30 using a FACS Aria instrument (BD). NKp30high and NKp30low/neg fractions were incubated for 48 hours +/− IL-2 (25ng/ml) at 1×106/ml in 96-well round bottom plates. Huh 7.5 cells (Apath LLC, St. Louis, MO) were seeded at 1.25 × 105 cells/well in 24-well plates. After 24 hours NKs were added at a ratio of 5 NK to 1 Huh 7.5 cell. Cells were infected simultaneously with JFH-1 (National Institute of Infectious Diseases, Tokyo, Japan) at an MOI=.003. Five days post infection; cells were harvested for RNA extraction (RNeasy mini Kit, Qiagen). RNA was transcribed to cDNA using the QuantiTect Reverse Transcription Kit (Qiagen) and HCV transcripts were detected using a 7300 Real Time PCR instrument (Applied Biosystems; Carlsbad, CA). A standard curve was created using JFH-1 plasmid stock (range 1×107 – 1–101). PCR Taqman Master Mix, primers and probes were purchased from Applied Biosystems. Primer and probe sequences were as follows; HCV-forward GCA CAC TCC GCC ATC AAT CAC T; HCV-reverse CAC TCG CAA GCG CCC TAT CA; HCV-probe 6FAM AGG CCT TTC GCA ACC CAA CGC TAC T TAMRA. NKs cultured as above were assessed for the expression of NKp30.
Statistical analyses
Results are expressed as median (range). Non-parametric Mann Whitney U was used to compare differences between patient groups. Significance was defined as a p value of <0.05. The JMP 6.0 (SAS Institute, Inc, Cary NC) statistical software package was used.
Results
The study group was comprised of 25 intravenous drug users (IDUs): 11 remained uninfected (EU) despite being repeatedly exposed to HCV, and 14 subsequently became infected (EI). The average age of exposed individuals was 25 years, and the age, race and gender distributions did not differ between the EU and EI groups. For the first time, this unique cohort of long-term IDUs allowed us to characterize pre-infection innate immune factors that may be protective against HCV acquisition.
CD56 positive cell levels
Flow-cytometric analysis of CD56pos populations in pre-infection blood samples demonstrated that the percentage of total CD56pos lymphocytes did not differ significantly between unexposed normal controls (NC) or exposed individuals, irrespective of subsequent outcome. However, as shown in figure 1, the lymphocyte subset distribution within the overall CD56pos population was altered in EIs, at a time prior to acquisition of HCV. This subgroup of exposed individuals had decreased levels of CD56low effector NKs (median 51.48%, [range 26.12%–81.55%], % of total CD56pos lymphocytes) compared to the EU group (75.20%, [58.60%–80.70%], p=0.0011), which had similar levels to NCs, (67.76%, [43.61%–80.5%]). A higher proportion of NT (CD3+CD56+) cells contributed to the levels of total CD56pos lymphocytes in the EI group which demonstrated lower levels of CD56low NKs (data not shown). These data suggest that decreased effector NK levels predispose to HCV acquisition in exposed individuals.
Figure 1. CD56pos NK cell levels pre-infection in the IDU population.
Flow cytometric analysis demonstrated that exposure to HCV did not result in altered total CD56pos lymphocyte levels (A). However, the lymphocyte subset distribution within the overall CD56pos population was altered in the patient group which subsequently became infected demonstrating lower levels of CD56low mature effector NK cells compared to those that remained uninfected (B). The flow plots shown in panel C demonstrate that total CD56pos cells can be divided into NK and NT cell subsets based on their expression of CD3. NK cell subsets are further characterized by the intensity of CD56 expression.
Impaired NK cytolytic activity but intact IFN-γ production predates acquisition of HCV infection
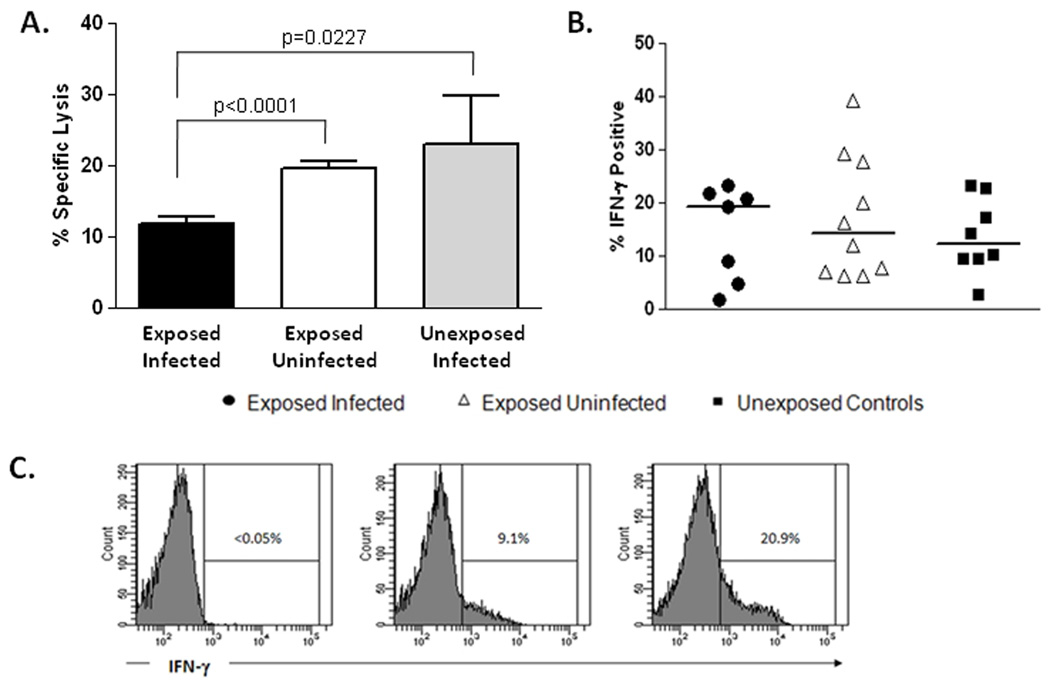
As killing of virally infected cells represents the primary effector function of CD56low NKs, we next tested the cytolytic potential of isolated NKs in our cohorts. This flow-based cytotoxicity assay measures the cytolytic potential of NKs on a per-cell basis (28). As shown in figure 2A,NKs (>90% purity) from HCV-exposed EI individuals had reduced IL-2 induced cytolytic activity against the NK-sensitive cell line K562 at an effector to target ratio of 10:1 compared to EU subjects (p<0.0001) and NCs (p=0.0227). Natural cytotoxicity lysis in the absence of cytokine stimulation, was similar in all groups (data not shown). These data suggest that lower numbers of effector NKs coupled with an impaired ability to exert cytolytic effector function in response to IL-2 predisposes to HCV acquisition in high-risk exposed individuals.
Figure 2. Cytotoxicity and Cytokine production by NK cells.
NK cells were isolated from peripheral blood samples (>90% purity) from HCV-exposed individuals who subsequently became infected (n=12) or remained uninfected (n=11) and normal unexposed controls (n=5). Natural cytotoxicity (no exogenous cytokine added) and lymphokine activated killing activity (LAK, IL-2-induced) were assessed using a flow-cytometry based assay as described in materials and methods. NK cells isolated from patients who subsequently became infected had reduced LAK activity against the NK-sensitive cell line K562 at an effector to target ratio of 10:1 compared to subjects who remained uninfected and unexposed controls (A). Interferon-gamma (IFN-γ) production by NK cells as measured by intracellular flow staining after stimulation by PMA and ionomycin was similar in all groups (B). Representative flow-cytometric histograms are shown for unstimulated NKs (<0.05% positive for IFN-γ), exposed infected (9.1%) and exposed uninfected (20.9%) individuals (C).
In addition to their cytolytic activity, NKs are characterized functionally by their ability to quickly produce interferon gamma (IFN-γ), and in vitro studies suggest that it may be this aspect of their functionality that is important for control of virus replication (31,32). Therefore, we tested the ability of NKs from our cohorts to produce IFN-γ using an intracellular cytokine flow-based assay. As shown in figure 2B the ability to produce IFN-γ is intact for NKs in EIs. These data suggest that IFN-γ production by innate CD56pos NKs does not provide protection from HCV acquisition.
Phenotype of CD56pos lymphocytes
Activation of NKs largely depends on the natural cytotoxicity receptor (NCR) family of molecules and monoclonal antibodies to NCR block NK-mediated lysis of target cells (7). NCRs include NKp46 involved in natural cytotoxicity (33) as well as NKp30 and NKp44 which are expressed on activated NKs (34). Recent studies have highlighted the important role played by NCRs in immune-surveillance of viral infection. Impaired NK function in HIV-1 infected patients has been associated with decreased NCR expression (35). Susceptibility to NK cell lysis of herpes simplex virus (HSV)-infected cells is dependent on NCR and independent of down-regulation of MHC class I molecules or induction of activating NKG2D ligands (36). Envelope proteins from the Dengue and the West Nile virus (two other Flaviviruses) bind NKp44 (37). Human cytomegalovirus (CMV) pp65 protein binds NKp30 thereby inhibiting NK activation and promoting virus survival (38). The role played by NCR in chronic HCV-infection remains controversial with both increases and decreases in expression being reported (39,16). Because we had demonstrated a significant decrease in LAK activity in the patient group that subsequently became infected, we characterized expression of activating NCRs (p30 and p44), previously shown to play a role in determining the cytolytic activity of activated NKs. We included another NK/T cell receptor involved in cell lysis in our analysis TRAIL (tumor necrosis factor (TNF)-related apoptosis-inducing ligand) as HCV core protein has been shown to sensitize hepatocytes to TRAIL-induced apoptosis (40).
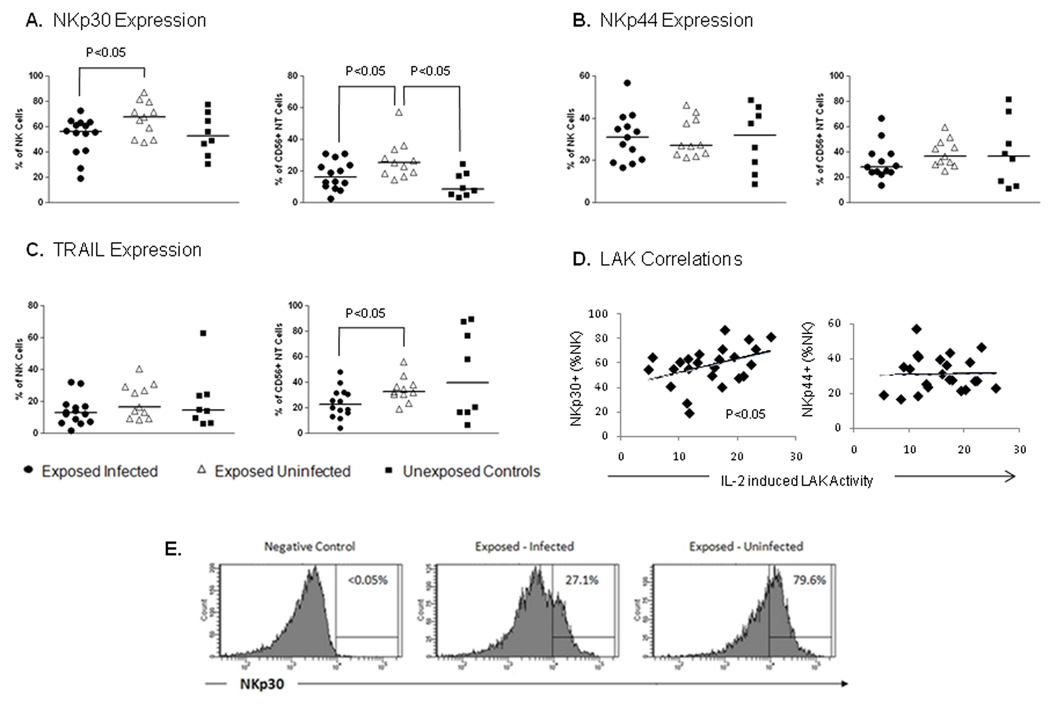
NCR NKp30 expression was significantly upregulated on both total NKs and NTs in the EU patient cohort (figure 3A). Both CD56high and CD56low NK cell subsets express NKp30 at similar levels. There is a trend for increased NKp30 on both subsets (p=0.0666 high; p=0.0627 low). No significant difference in the expression of NCRp44 was demonstrated, although a trend towards reduced NCRp44 on NTs in the EI patient cohort was noted (figure 3B). TRAIL was unchanged on NKs and significantly downregulated on NTs in the EI group (figure 3C). NKp30 was the only cytotoxicity receptor tested to be altered on NKs suggesting that the increase in this receptor may play a role in the enhanced LAK activity in the patient group remaining uninfected. This hypothesis is supported by the correlation shown between LAK activity and NKp30 expression on NKs in the entire exposed cohort (figure 3D). No correlation was seen for expression of NCR NKp44 (Figure 3D) or TRAIL (data not shown) either on NKs or NTs. These data suggest that upregulation of NKp30 may contribute to innate protection against HCV and this receptor may represent a novel target for immune manipulation.
Figure 3. CD56pos NK/NT cell phenotype.
Phenotypic analysis of a range of NK receptors involved in the cytolytic function of NK/NT cells was carried out on gated NK and NT cells. The natural cytotoxicity receptor (NCR) NKp30 was increased on both NK and NT cells populations in the patient group that remained uninfected (A). Expression of another NCR NKp44 did not differ between patient groups; although a trend was observed for lower NKp44 expression on NT cells (p=0.0724) in patients who subsequently became infected (B). TRAIL was significantly down-regulated on NT cells but normal on NK cells in the same patient group (C). Correlation of LAK activity against NK receptor expression in the entire exposed cohort demonstrated a relationship between NKp30 expression on NK cells and LAK activity only and not with the expression of other NK receptors (D). Representative flow-cytometric histograms of NKp30 expression on NK cells (E).
NKp30high NK cells protect against HCV infection in vitro
As NKp30 expression was significantly up-regulated on NKs and correlated with LAK activity in the patient cohort that remained uninfected despite repeated exposure, we tested the functional significance of NKp30 expression in a relevant replicon model. We used the Huh 7.5 JFH-1 in vitro HCV infection system to compare the ability of FACS-sorted NKp30low/neg and NKp30high subsets of NKs to attenuate infection of hepatocytes by HCV. For each of the 4 normal subjects tested un-stimulated NKs expressing high levels of NKp30 were more effective in preventing infection of Huh 7.5 cells than their NKp30low/neg counterparts (p=0.0361 for combined data). IL-2 stimulation of NKs overcomes the lack of NKp30 (figure 4). In a standard degranulation assay, NKp30high NKs demonstrated more efficient degranulation in response to short-term stimulation compared to their NKp30low counterparts (figure 5A). In addition NKp30high NKs express more perforin than NKp30low NKs in the resting state (figure 5B, C). IL-2 is likely to overcome the relatively impaired cytotoxicity of the NKp30low population through upregulation of this receptor on NKs (figure 5D). These data provide further evidence that up-regulation of NKp30 in response to HCV exposure may provide protection from infection.
Figure 4. NKp30high NK cells protect Huh 7.5 cells from HCV infection.
The Huh 7.5 JFH-1 in vitro infection system was used to compare the ability of NKp30low/neg and NKp30high subsets of NK cells to attenuate infection of hepatocytes by HCV. NK cells from four normal donors were used in the assay as described in the materials and methods section. Panel A shows that infection of Huh 7.5 cells at an MOI=0.003 results in robust infection after 5 days, addition of un-stimulated NKs results in a modest reduction in infection and addition of IL-2 stimulated NKs allows only minimal infection. Immunofluorescent staining was carried out using a primary anti-core antibody (Pierce, Rockford, IL) followed by detection with AF-488 labeled secondary (Molecular Probes, Eugene, OR). Panel B shows the quantitative PCR results for the four individual patients. For each of the subjects tested, un-stimulated NKs (black bars) expressing high levels of NKp30 were more effective in preventing infection of Huh 7.5 cells than their NKp30low/neg counterparts (p=0.0361 for combined data). IL-2 stimulation of NK cells overcomes the lack of NKp30 (white bars).
Figure 5. NKp30 expression is enhanced by IL-2 and correlates with perforin expression and degranulation.
NK cells were bead-isolated from four normal control subjects. NKp30high NK cells demonstrate relatively increased degranulation compared to their NKp30low counterparts after short-term stimulation (A). Resting NKs were stained for intracellular perforin. NKp30high NK cells contained higher levels of perforin than their NKp30low counterparts (B). Representative flow histograms showing perforin staining in resting NKp30high/low NK cells (C). Interleukin-2 upregulated the expression of NKp30 on NKs suggesting the underlying mechanism whereby IL-2 stimulation overcomes the lack of NKp30 expression in mediating protection in the Huh 7.5 JFH-1 in vitro infection system (D).
Discussion
HCV infection represents a considerable public health burden. Efforts to develop a vaccine have to date been unsuccessful and treatment of chronic HCV infection remains sub-optimal (41). Understanding the immune correlates that contribute to innate protection from HCV acquisition will aid in the development of novel immune-based treatment strategies. The observation that a number of intravenous drug users (IDUs) remain healthy with no evidence of infection despite continued long-term exposure to HCV (4) strongly suggests a role for innate immunity in natural protection from HCV infection. However, because of logistical difficulties in obtaining samples from high risk individuals prior to HCV infection the hypothesis that innate immune effector populations contribute to natural resistance to HCV infection had not previously been tested.
Support for a role for innate effector populations in protection from viral infection in vivo is provided by studies which have demonstrated enhanced activity of NK (30) and NT (42) cells contribute to protection from HIV-1 infection in high-risk exposed individuals. In vitro studies provide strong evidence that NK cells have a key role in suppressing HCV infection of human hepatocytes (29). Our unique cohort of prospectively collected peripheral blood samples from high-risk intravenous drug users (IDUs) allows us for the first time to address the possible role of these cells in conferring protection from acquisition of HCV infection.
In the present study, we demonstrate that in patients who remain protected from HCV infection, total CD56pos populations are enriched for CD56low effector NKs displaying enhanced IL-2 induced cytolytic activity and higher levels of NKp30 activating NKR. For the first time, these data support the hypothesis that NKs contribute to anti-HCV defense in the earliest stages of infection, providing protection from HCV acquisition. Of note IFN-γ production by NKs was comparable to normal controls suggesting that the cytolytic activity of NKs is more important than cytokine production in mediating protection. This may appear to be contradictory to in vitro studies suggesting that IFN-γ is key for control of viral replication and HCV infection of human hepatocytes cell lines (29,31,32). The contribution of IFN-γ to viral control may vary at different stages of infection. Moreover, there is an association with viral clearance and higher LAK activity in the setting of acute HCV (28). It should be noted that we cannot in the functional assays distinguish the individual contribution of the CD56high/low NK subsets. However, our data presented here in pre-infection suggests that cytotoxicity is important in protection and control early in infection but once chronic infection is established then IFN-γ production by these populations may become more critical for the control of virus.
Our phenotyping panel is not exhaustive and further studies are required to determine the relative contribution of various NKRs to natural protection. These assays are beyond the scope of this study because larger numbers of cells than are available to us would be required. However, the observed up-regulation of NKp30 and its correlation with LAK activity suggests a role in innate protection from HCV infection, although we cannot at this time exclude the involvement of other receptors. Our study demonstrated a significant role for at least one NK receptor (NKp30) in providing innate protection from HCV infection, a larger cohort of patients may identify other NK receptors of importance. Further support for a protective role for NKp30 is provided by the demonstration that NKp30high NKs significantly reduce infection in the JFH-1 in vitro infection system. Of note, this protection was provided without the need for exogenous stimulation by IL-2. This may be of particular importance before induction of adaptive immunity or in the setting of insufficient T cell priming and lack of CD4+ T cell help known to occur in HCV infection (43). In conclusion, our study provides new insights into mechanisms underlying protection from HCV infection which may have implications for improving immunotherapeutic strategies.
Acknowledgment
We would like to thank Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) for kindly providing the JFH-1 plasmid. We thank the Colorado Center for AIDS Research (CFAR) Laboratory Core for access to FACS sorting.
Supported by NIH U19 AI40035 (ALC) and by RO1 DK060590 and U19 A 1066328 (HCV center grant) to HRR
Abbreviations
- EI
Exposed subsequently Infected
- EU
Exposed remaining Uninfected
- IL
Interleukin
- KIR
Killer Immunoglobulin-like Receptor
- LAK
Lymphokine Activated Killing
- NC
Normal unexposed Control
- NCR
Narural Cytotoxicity Receptor
- NK
Natural Killer
- NKRs
Natural Killer cell Receptors
- NT
Natural T cell (CD56+ T cell)
References
- 1.Cohen J. The scientific challenge of hepatitis C. Science. 1999;285:26–30. doi: 10.1126/science.285.5424.26. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 3.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 4.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 5.Biron CA. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 8.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 9.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, et al. The human liver contains multiple populations of NK cells, T cells and CD3+CD56+ natural T cells with distinct cytotoxic activites and Th1, Th2 and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 10.Bonavita MS, Franco A, Paroli M, Santilio I, Benvenuto R, et al. Normalization of depressed natural killer activity after interferon-α therapy is associated with a low frequency of relapse in patients with chronic hepatitis C. Int J Tissue React. 1993;15:11–16. [PubMed] [Google Scholar]
- 11.Corado J, Toro F, Rivera H, Bianco NE, Deibis L, et al. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451–457. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deignan T, Curry MP, Golden-Mason L, Volkov Y, Norris S, et al. Decrease in hepatic CD56+ T cells and Vα24+ natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002;37:101–108. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 13.Pár G, Rukavina D, Podack ER, Horányi M, Szekeres-Barthó J, et al. Decrease in CD3-negative-CD8dim+ and Vδ2/Vγ9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol. 2002;37:514–522. doi: 10.1016/s0168-8278(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 14.Herzer K, Falk CS, Encke J, Eichhorst ST, Ulsenheimer A, et al. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–8309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas M, Gadola S, Meier U, Young NT, Harcourt G, et al. Frequency and phenotype of circulating Vα24/Vβ11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251–2257. doi: 10.1128/JVI.77.3.2251-2257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, et al. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okumura A, Ishikawa T, Maeno T, Sato K, Ayada M, et al. Changes in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol Res. 2005;32:213–217. doi: 10.1016/j.hepres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 20.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, et al. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–1128. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 21.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 22.Rauch A, Laird R, McKinnon E, Telenti A, Furrer H, et al. Swiss HIV Cohort Study. Influence of inhibitory killer immunoglobulin-like receptors and their HLA-C ligands on resolving hepatitis C virus infection. Tissue Antigens. 2007;1(69 Suppl):237–240. doi: 10.1111/j.1399-0039.2006.773_4.x. [DOI] [PubMed] [Google Scholar]
- 23.Crotta S, Stilla A, Wack A, D'Andrea A, Nuti S, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52(2):183–190. doi: 10.1016/j.jhep.2009.11.003. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 26.Nattermann J, Nischalke HD, Hofmeister V, Ahlenstiel G, Zimmermann H, et al. The HLA-A2 restricted T cell epitope HCV core 35–44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443–453. doi: 10.1016/S0002-9440(10)62267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen C, He X, Ma H, Hou N, Wei C, et al. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell Mol Immunol. 2008;5:475–478. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden-Mason L, Castelblanco N, O'Farrelly C, Rosen HR. Phenotypic and Functional Changes of Cytotoxic CD56pos Natural T Cells Determine Outcome of Acute Hepatitis C Virus Infection. J Virol. 2007;81:9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SH, Huang CX, Ye L, Wang X, Song L, et al. Natural killer cells suppress full cycle HCV infection of human hepatocytes. J Viral Hepat. 2008;15:855–864. doi: 10.1111/j.1365-2893.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 31.Ye L, Wang X, Wang S, Wang Y, Song L, et al. CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology. 2009;49:753–762. doi: 10.1002/hep.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Zhang T, Ho C, Orange JS, Douglas SD, et al. Natural killer cells inhibit hepatitis C virus expression. Leukoc Biol. 2004;76:1171–1179. doi: 10.1189/jlb.0604372. [DOI] [PubMed] [Google Scholar]
- 33.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol. 2004;34:2313–2321. doi: 10.1002/eji.200425251. [DOI] [PubMed] [Google Scholar]
- 36.Chisholm SE, Howard K, Gómez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195:1160–1168. doi: 10.1086/512862. [DOI] [PubMed] [Google Scholar]
- 37.Hershkovitz O, Rosental B, Rosenberg LA, Navarro-Sanchez ME, Jivov S, et al. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183:2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 39.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 40.Chou AH, Tsai HF, Wu YY, Hu CY, Hwang LH, et al. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174:2160–2166. doi: 10.4049/jimmunol.174.4.2160. [DOI] [PubMed] [Google Scholar]
- 41.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 42.Montoya CJ, Rugeles MT, Landay AL. Innate immune defenses in HIV-1 infection: prospects for a novel immune therapy. Expert Rev Anti Infect Ther. 2006;4:767–780. doi: 10.1586/14787210.4.5.767. [DOI] [PubMed] [Google Scholar]
- 43.Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]