Abstract
Current cardiovascular therapies are limited by loss of endothelium, restenosis, and thrombosis. The goal of this study is to develop a biomimetic hybrid nanomatrix that combines unique properties of electrospun polycaprolactone (ePCL) nanofibers with self-assembled peptide amphiphiles (PAs). ePCL nanofibers have interconnected nanoporous structures, but they are hampered by lack of surface bioactivity to control cellular behavior. It is hypothesized that PAs can self-assemble onto the surface of ePCL nanofibers and endow them with characteristic properties of native endothelium. PAs, which comprise hydrophobic alkyl tails attached to functional hydrophilic peptide sequences, contained enzyme-mediated degradable sites coupled to either endothelial cell adhesive ligands (YIGSR) or ploylysine (KKKKK) nitric oxide (NO) donors. Two different PAs (PA-YIGSR and PA-KKKKK) were successfully synthesized and mixed in a 90:10 (YK) ratio to obtain PA-YK. PA-YK was reacted with pure NO to develop PA-YK-NO, which was then self-assembled onto ePCL nanofibers to generate a hybrid nanomatrix, ePCL-PA-YK-NO. Uniform coating of self-assembled PA nanofibers on ePCL was confirmed by TEM. Successful NO release from ePCL-PA-YK-NO was observed. ePCL-YK and ePCL-PA-YK-NO showed significantly increased adhesion of human umbilical vein endothelial cells (HUVECs). Also, ePCL-PA-YK-NO showed significantly increased proliferation of HUVECs and reduced smooth muscle cell proliferation. ePCL-PA-YK-NO also displayed significantly reduced platelet adhesion when compared to ePCL, ePCL-PA-YK, and collagen control. These results indicate that this hybrid nanomatrix has great potential applications in cardiovascular implants.
1.0 Introduction
Cardiovascular diseases (CVD) are the leading cause of death in the United States. As of 2006, eighty million Americans suffered from at least one form of CVD. CVDs were responsible for 35.3 % of all deaths in the United States in 2005 [1]. The most common cause of CVDs is atherosclerosis, which involves the deposition of fatty plaque in blood vessels, leading to obstruction and narrowing of the vessels.
Conventional cardiovascular therapies include stent angioplasty and coronary bypass surgery. Over 500,000 coronary bypass grafts are performed every year in the world with artificial grafts or allograft material [1]. Allograft arteries and veins suffer from an array of problems, such as rejection, endothelial cell sloughing and loss of vascular reactivity [2]. There are several artificial vascular grafts available in the market, made from synthetic or natural materials. Most frequently used synthetic grafts are made of Dacron, polyurethane, or extended polytetrafluoroethane (ePTFE). However, synthetic vascular grafts, especially at the small diameter level (<5mm), are prone to thrombosis [3]. Most grafts also suffer from a biomechanical mismatch[4], lack of re-endothelialization, followed by restenosis and eventually, intimal hyperplasia [5]. The lack of a confluent and viable layer of endothelial cells is considered to be one of the major challenges to the clinical success of arterial vascular grafts[6].
Electrospinning has been garnering a lot of attention recently[7–11], due to its ability to fabricate highly interconnected, non-woven fibers with diameters in the nanoscale ranges, which are structurally similar to nanofibrillar extracellular matrix (ECM) proteins[12]. Due to their ability to physically resemble natural ECM protein structure, several studies have been conducted into using electrospun materials as cardiovascular devices such as vascular grafts [13–17]. An important feature of electrospinning is its ability to deposit these nanofibers on a rotating mandrel to form a tubular structure, which is essential for vascular grafts[18, 19], and it is also possible to generate scaffolds with mechanical properties comparable to those of native arteries[17]. However, electrospun nanofibers are hindered by their lack of bioactivity which is crucial for promoting clinical patency of vascular grafts. To address this issue, polymers were electrospun along with natural proteins such as collagen and gelatin [17, 20]. While these techniques served to increase cell adhesion, they were not able to effectively deal with all the issues currently faced by small diameter synthetic vascular grafts, namely, restenosis, thrombosis, and intimal hyperplasia. Thus, the current challenge in cardiovascular devices is to replicate the native endothelial environment on the surfaces of these devices, so that restenosis, thrombosis and intimal hyperplasia can be limited.
Native endothelium consists of a monolayer of endothelial cells that adhere to the underlying nanofibrillar basement membrane and regulate vascular tone by release of soluble factors such as nitric oxide (NO). The local release of NO by endothelial cells plays a critical role in controlling the vascular tone, as it limits smooth muscle cell proliferation and prevents platelet adhesion and activation while simultaneously promoting endothelial cell proliferation [21–24]. NO, therefore, regulates vascular cell homeostasis. Thus, the loss of this endothelium due to injury at the graft implant site leads to restenosis caused by smooth muscle cell proliferation with accompanying ECM production. Therefore, by developing a native endothelium mimicking environment on the surface of vascular grafts, their clinical patency can be improved and their application can be greatly diversified including various cardiovascular prosthetic devices.
This native endothelium mimicking characteristic can be endowed upon these electrospun nanofibers by coating them with peptide amphiphiles (PAs). PAs, as their name suggest, comprise of hydrophobic moieties linked to hydrophilic, functional peptide sequences [25–28]. Due to their amphiphilic nature, they are capable of self-assembly into nanofibers. These PAs can carry enzyme mediated degradable sites and cell adhesive ligands, and can therefore, mimic the biochemical aspect of ECM. This coating of PAs onto ePCL nanofibers constitutes the hybrid biomimetic nanomatrix, as shown in Figure 1. The PAs used in the study consist of matrix metalloprotease-2 (MMP-2) degradable site Gly-Thr-Ala-Gly-Leu-Ile-Gly-Gln (GTAGLIGQ). MMPs are constitutively produced by healthy cells, and therefore, the presence of this site promotes the remodeling of the nanomatrix by the cells[27, 28]. The PAs also contain laminin derived Tyr-Ile-Gly-Ser-Arg (YIGSR) sequence, which is known to promote endothelial cell adhesion and spreading[27, 29], and a polylysine (KKKKK) sequence that acts as an NO donor[23, 24, 30]. The incorporation of NO donating residues into the PA is expected to provide a controlled release of NO from the hybrid nanomatrix into the local blood stream where it will limit smooth muscle cell proliferation and platelet adhesion while enhancing re-endothelialization. This novel, multifunctional approach may be able to provide current electrospun scaffolds with an attractive solution to tackle restenosis, thrombosis, and re-endothelialization which are critical issues for cardiovascular implant applications. Therefore, the goal of this study was to develop a hybrid nanomatrix by combining electrospun polycaprolactone (ePCL) nanofibers with self-assembling NO releasing PAs. These PAs confer bioactivity on ePCL nanofibers and enable them to tackle the limitations faced by current cardiovascular implant devices.
Figure 1.
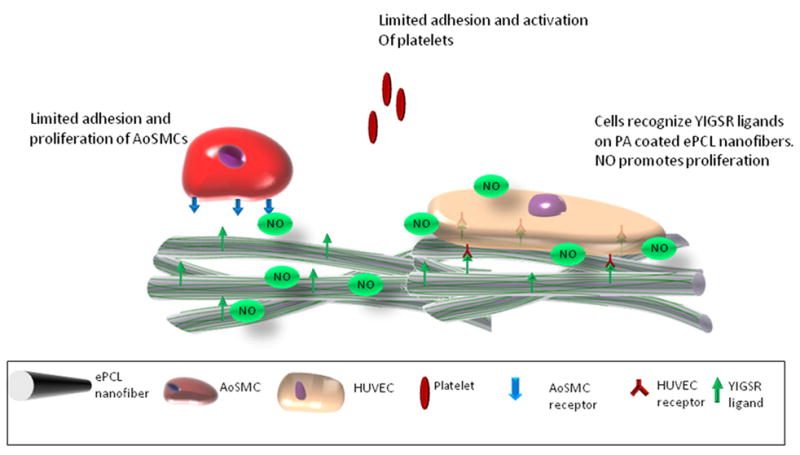
ePCL nanofibers are coated with NO releasing PAs to help them tackle the issues faced by current cardiovascular implants. The PAs possess cell adhesive ligands and NO donor residues, and are self-assembled onto the surface of ePCL nanofibers by solvent evaporation technique. Presence of YIGSR ligands and release of NO promote the adhesion and proliferation of endothelial cells, while simultaneously limiting the adhesion and proliferation of smooth muscle cells, and the adhesion and activation of platelets.
2.0 Materials and methods
2.1 Fabrication of electrospun polycaprolactone nanofibers
A 1:1 (v/v) solvent system of chloroform: methanol was used to dissolve polycaprolactone (PCL) pellets (Sigma Aldrich, St. Louis, MO; Mn = 80,000). The 22.5 wt% viscous polymer solution thus obtained was transferred to a 25G blunt tip needle capped syringe. The syringe was placed in a syringe pump (KD Scientific, Holliston, MA) that was set at a flow rate of 1 ml/hr. The needle tip was connected to a high voltage source (Gamma High-Voltage Research, Ormond Beach, FL) through which a voltage of +21 kV was applied to the needle tip. The resulting electrospun PCL (ePCL) nanofibers were deposited onto a stationary grounded aluminum collector that was placed 28 cm from the needle tip. The network of nanofibers deposited onto the collector has no preferred directional orientation. The collectors with ePCL sheets on them were then stored in a vacuum dessicator for 2–3 days to remove any residual solvents.
2.2 Synthesis of peptide amphiphiles
The peptides were synthesized using Fmoc chemistry in an Aapptech Apex 396 Peptide synthesizer, as described before[26–28, 30]. Two different peptides, each thirteen amino acids long, consisting of MMP-2 sensitive sequences (GTAGLIGQ) with cell-adhesive sequence YIGSR or NO donating residue KKKKK were synthesized. These peptides were alkylated to be linked to a 16 carbon palmityl chain, thus forming amphiphiles. Therefore, two different PAs, C16-GTAGLIGQ-YIGSR (PA-YIGSR) and C16-GTAGLIGQ-KKKKK (PA-KKKKK) were synthesized.
2.3 Self-assembly of PAs onto ePCL nanofibers
The PAs were dissolved in deionized (DI) water to make a 1 wt % stock solution. The pH of this solution was adjusted to 7 using 1 M Sodium hydroxide. For all studies, a 0.1 wt% solution was obtained by diluting the 1 wt% solution. A Humboldt boring machine (Fisher Scientific) was used to cut 16 mm diameter discs out of ePCL sheets. These discs were then sterilized with serially decreasing concentrations of ethanol. Then, these discs were coated with 200 μl of 0.1 wt. % PAs. These scaffolds were then placed on a shaker for 24 hours and then the PAs were allowed to self-assemble onto ePCL nanofibers via solvent evaporation by drying in a chemical hood for 48 hours.
2.4 Scanning electron microscopy (SEM)
SEM was used to characterize the morphology of PA coated ePCL nanofibers. The nanofibers were sputter coated with gold/palladium and their morphology was observed under a Philips SEM 510 at an accelerating voltage of 20 kV. As a control, uncoated ePCL was characterized by SEM under similar conditions.
2.5 Transmission electron microscopy (TEM)
TEM was used to characterize and confirm the coating of PAs onto the surface of ePCL nanofibers. PCL was electrospun onto 600 mesh hexagonal TEM grids and dessicated in a vacuum dessicator for 48 hours. They were then coated with 5 μl 0.1 wt % PAs. The grids were then allowed to dry over a 24 hour period in a chemical hood. These grids were then stained with 2% PTA for 30 seconds. The grids were then imaged using a FEI Technai T12 TE microscope at 60 kV accelerating voltage. As a control, uncoated ePCL nanofibers were imaged under the same conditions.
2.6 Cell maintenance
All cells and media were purchased from Lonza Inc. (Walkersville, MD). Human umbilical vein endothelial cells (HUVECs) were cultured in endothelium basal medium (EBM) that was supplemented with endothelial growth media (EGM) SingleQuot® Kit (2% FBS (Fetal Bovine Serum), 0.1% hEGF (human Epidermal Growth Factor), 0.1% hydrocortisone, 0.1% gentamycin A, 0.4% bovine brain extract). This cell culture medium was used in all HUVEC experiments. 0.05% trypsin/EDTA was used for passaging cells, which were then subcultured at a density of approximately 5000 cells/cm2. Human aortic smooth muscle cells (AoSMCs) were grown in smooth muscle cell basal medium (SmBM) that was supplemented with Smooth muscle cell growth media (SmGM-2) SingleQuot® Kit (5% FBS, 0.1% Insulin, 0.2% hFGF-B (human Fibroblast Growth Factor-B), 0.1% gentamycin A, 0.1% hEGF). This cell culture medium was used in all AoSMC experiments. Cells were passaged by trypsinizing (0.05% trypsin/EDTA) and subculturing at a density of 3500 cells/cm2. All cell cultures were maintained under normal culture conditions (37°C, 95% relative humidity, and 5% CO2). For all cell studies, passage numbers 3–5 were used.
2.7 Optimization of ratio of PA-YIGSR and PA-KKKKK
HUVECs were seeded at density of 30,000 cells/cm2 on different molar ratios of PA-YIGSR and PA-KKKKK coated onto ePCL. These samples were named, based on the molar ratios, as ePCL-YK 90 (90% PA-YIGSR, 10% PA-KKKKK), ePCL-YK 75 (75% PA-YIGSR, 25% PA-KKKKK), ePCL-YK 50 (50% PA-YIGSR, 50% PA-KKKKK), and ePCL-YK 25 (25% PA-YIGSR, 75% PA-KKKKK). Uncoated ePCL was used as a control. After a 2 hour incubation period, the media was aspirated; cells were trypsinized using 400 μl of 0.25% clear trypsin for 30 minutes. The trypsinized cells were collected at 1:1 Dilutions with PBS into 1.5 ml eppendorf tubes and store in −80°C. The samples thus collected were subjected to a Picogreen DNA assay to evaluate the DNA content which was proportional to the cell number[27].
Briefly, the cells were permeabilized by repeated freeze-thaw cycles. Then, PicoGreen dye was added to the cell sample. The PicoGreen dye binds to double stranded DNA in cells and allows its quantification. Known volumes of calf thymus dsDNA were used as standards. The fluorescence was measured in a microplate fluorescence reader (Synergy HT, Bio-Tek Instruments, VT). The amount of DNA was correlated to cell number by an empirical value of 8 pg DNA per cell[27].
2.8 Preparation and characterization of the NO releasing hybrid nanomatrix
NO releasing PAs were synthesized by reacting PA solutions overnight with pure NO under high pressure in a 100 ml round bottom flask. Pure NO was obtained by the process of scrubbing. Commercially available NO gas is passed through 5 M potassium hydroxide to remove impurities such as higher oxide species. Prior to NO scrubbing, the apparatus is degassed by passing argon through the system.
Based on the results of the optimization study previously described, the optimum ratio of PA-YIGSR and PA-KKKKK was chosen. This mixture, henceforth referred to as PA-YK, was reacted with NO to obtain NO releasing PA, which was called PA-YK-NO. PA-YK-NO was coated onto 16 mm diameter ePCL discs, as described earlier, to obtain the NO releasing hybrid nanomatrix, called ePCL-PA-YK-NO. As a control for all studies, unreacted PA-YK, which is essentially PA-YK 90, was coated onto ePCL nanofibers to form ePCL-PA-YK. ePCl-PA-YK and ePCL-PA-YK-NO was characterized by SEM and TEM.
For NO release studies, the 16 mm ePCL scaffolds were coated with 200 μl 1 wt% PA-YK-NO. These discs were then placed in a shaker for 24 hours, followed by 48 hours in a chemical hood to allow self-assembly of PAs on to ePCL nanofibers. These scaffolds were then incubated in 400 μl HEPES buffered saline (HBS) in a 48 well tissue culture plate. Samples were collected at 0h, 2h, 4h, 6h, 24h, 48h, 4 day, 6 day, 10 day, 14 day, 21 day and 28 day time points and stored at −80°C. As a control, ePCL scaffolds were coated with PA-YIGSR-NO in similar conditions. PA-YIGSR-NO was obtained by reacting PA-YIGSR with pure NO. This accounts for non-specific binding of NO to PAs. After samples were collected, the NO released was measured by using Greiss Assay (Promega), where sulfanilamide and N-1 napthylethylenediamine dihydrochloride are used to determine the nitrite content. Nitrite is the primary degradation product of NO. 50 μl of sample was treated with 50 μl sulfanilamide and 50 μl of N-1 napthylethylenediamine dihydrochloride. After incubation for 15 minutes, absorbance was read at 540 nm using a microplate reader (Synergy HT, Bio-Tek Instruments, VT). The difference in nitrite content between ePCL-PA-YK-NO and ePCL-PA-YIGSR-NO accounts for the NO released from the lysine residues of PA-KKKKK. This was plotted as a measure of NO released against time.
2.9 Evaluation of cellular behaviors on hybrid nanomatrix
HUVECs and AoSMCs were seeded at densities of 30,000 cells/cm2 and 15,000 cells/cm2, respectively. Initial cell adhesion was analyzed by seeding HUVECs and AoSMCs on 3 different substrates: ePCL, ePCL-YK, and ePCL-YK-NO. After a 2 hour incubation period, the samples were collected as mentioned previously and they were subjected to a Picogreen DNA assay to evaluate the cell number[27].
To evaluate the effect of NO on cell proliferation, 0.1 wt% PA-YK and PA-YK-NO were coated onto 16mm ePCL discs. HUVECs and AoSMCs were seeded at densities of 15,000 cells/cm2. Proliferation of HUVECs and AoSMCs was evaluated by collecting cell samples by trypsinization at day 1 and day 4 time points. The cell numbers in these samples were evaluated by subjecting them to a Picogreen DNA assay. The proliferation of HUVECs and AoSMCs was also analyzed by staining for proliferating cell nuclear antigen (PCNA). HUVECs and AoSMCs were seeded at 15,000 cells/cm2 on ePCL-PA-YK-NO and ePCL-PA-YK. After 48 hrs of incubation, the media was aspirated and the cells were fixed in 10% formalin. After permeabilization by incubation in methanol, the cells were incubated with mouse IgG anti-PCNA primary antibody (Dako Corp., Carpinteria, CA), which was diluted 1:100 in 3% FBS in Phosphate buffered saline (PBS). Then, cells were incubated with secondary anti-mouse IgG HRP (Dako Corp., Carpinteria, CA), which was also diluted 1:100 3% FBS in PBS. The cells were then incubated with aminoethylcarbazole chromogen (Dako Corp., Carpinteria, CA). Mayer’s hematoxylin was used as a counterstain. The cells were then imaged at 20x using phase contrast microscopy. The percentage of proliferating cells per field of view was determined by counting the red proliferating cells and blue non-proliferating cells. Four samples were tested for each condition, and five random fields were imaged for each well. Therefore, the results shown in the graphs represent an average of over 120 images, which were obtained from 3 independent experiments (n=12). To ensure that the reduced proliferation of AoSMCs on NO releasing hybrid nanomatrices was not due to cytotoxicity, cells grown on the nanomatrices were subjected to a Live/Dead assay (Molecular Probes). After 48 hours of incubation, the media was aspirated and replaced with the Live/Dead reagent. Cells were fluorescently imaged using a fluorescence microscope.
2.10 Evaluation of platelet adhesion
Platelet adhesion on hybrid nanomatrices was evaluated by incubating whole blood on ePCL-PA-YK and ePCL-PA-YK-NO. ePCL was used as a control. A collagen film prepared by casting 2.5 mg/ml Collagen I in 3% glacial acetic acid solution was used as a positive control. Whole blood was obtained from a healthy consenting volunteer (IRB approved protocol; dated 07/06/2009) and store temporarily in BD Vacutainer tubes (BD, New Jersey). This blood was then mixed with 10 μM mepacrine to fluorescently label the platelets. This blood was then incubated on the hybrid nanomatrices and the controls for 30 minutes at 37°C. The platelets attached to each surface were then counted per field of view (10x) after obtaining fluorescent microscopic images. Five random fields were imaged and counted per sample.
2.11 Statistical analysis
Each study was performed at least three times, independently. One way ANOVA (SPSS) was used to compare the data for statistical significance. Within ANOVA, Tukey multiple comparisons test was performed to detect significant differences between pairs. A value of p<0.05 was considered to be statistically significant.
3.0 Results and Discussions
Developing the native endothelium mimicking environment is vital to meet the challenges faced by current cardiovascular implants. In spite of their great promise, electrospun nanofibers are limited in their applications towards these implants due to the lack of bioactivity. To meet this challenge, we have developed and characterized a hybrid biomimetic nanomatrix designed to reconstitute the properties of native endothelium onto the electrospun nanofiber surface.
ePCL nanofibers, known to possess the characteristics desired for biomedical applications, were successfully fabricated. As the SEM image in Figure 2a shows, the ePCL nanofibers have uniform morphologies, possess nanoscale diameters between 200 nm – 700 nm, and are free from beads. The random, interwoven nature that is typical of all ePCL nanofibers is also clearly evident from the image. Additionally, the majority of the fibers had diameters measured to be within the range of 300 nm – 400 nm, which is similar to nanofibrillar proteins found in native ECM [31, 32]. This aspect of ECM mimicking nanofibrous topography is favored by cells[33]. ePCL has also shown to provide favourable degradation and healing characteristics for usage as a vascular graft material[34]. However, ePCL lacks bioactivity, and is therefore unable to interact and direct cell behavior. More importantly, due to this lack of bioactivity, ePCL is unable to deal with the challenges faced by current small diameter vascular grafts; lack of re-endothelialization, restenosis, thrombosis and intimal hyperplasia. To limit these conditions and to improve clinical patency of these grafts, it is essential to mimic the native endothelial environment on the surface of these ePCL nanofibers.
Figure 2.
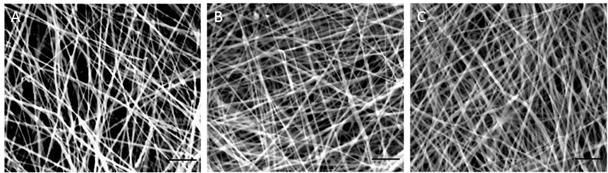
SEM images of hybrid nanomatrices at 5000x. (A) ePCL. (B) ePCL-PA-YK (C) ePCL-PA-YK-NO. In this image, PA-YK refers to PA-YK90. Scale bar = 5μm.
This biomimetic character was introduced to ePCL nanofibers through the use of PAs. PAs are unique in the sense, that they possess cell adhesive ligands to improve cell adhesion and direct their behavior and enzyme mediated degradable sites to promote matrix remodeling[26–28]. Most importantly, these PAs can self-assemble into nanofibers by a simple solvent evaporation technique, and this makes it extremely useful for application on several prosthetic devices with great ease [27, 35]. In this study, two different PAs, PA-YIGSR and PA-KKKKK were successfully synthesized. The PAs contain enzyme-mediated degradable MMP-2 sensitive sequences (GTAGLIGQ), along with YIGSR or a polylysine (KKKKK) group to form NO donating residues. PA-YKs were also designed by mixing different molar ratios of PA-YIGSR and PA-KKKKK. Thus, densities of cell adhesive ligands and NO could be tuned by varying the ratios of PA-YIGSR and PA-KKKKK. To determine the optimal ratio, endothelial cells were seeded on various ratios of ePCL-PA-YKs and cell adhesion was found to be significantly greater with increasing PA-YIGSR concentration (Figure 4). Thus, ePCL-PA-YK90 (9:1 mol/mol), henceforth referred to as ePCL-PA-YK, was used for all further studies.
Figure 4.
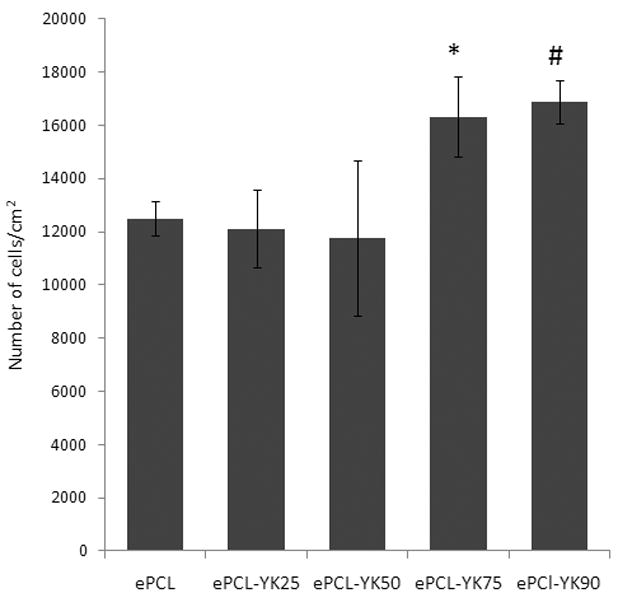
HUVEC adhesion increases with increasing concentrations of PA-YIGSR. ePCL-PA-YK75 (*) and ePCL-PA-YK90 (#) showed significantly increased HUVEC adhesion when compared to ePCL-PA-YK50, ePCL-PA-YK25 and ePCL.
This PA-YK was then coated onto ePCL to create a hybrid nanomatrix (ePCL-PA-YK), which was then characterized by SEM (Figure 2b). It is clear that coating with PA-YK does not affect ePCL nanofiber morphology. ePCL-PA-YK was also characterized by TEM (Figure 3a). From the TEM image, a central ePCL nanofiber (approximately 300 nm in diameter) was observed, and it was coated on either side with PAs (7–8 nm in diameter). Due to a lack of depth in TEM imaging, uniform coating was confirmed by tilting the stage of the electron microscope in the transverse axis to obtain an image (Figure 3b). This image is similar to the untilted image, and therefore it can be reasonably inferred that the coating of PAs on ePCL nanofibers is uniform.
Figure 3.
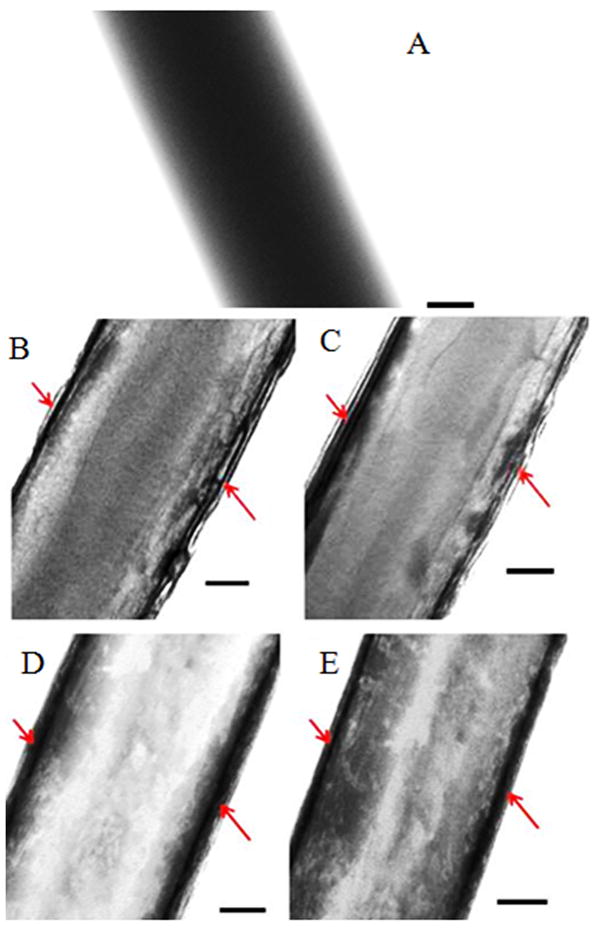
TEM images of hybrid nanomatrices at 67000x showing successful self assembly. (A) ePCL. (B) ePCL-PA-YK. (C) ePCL-PA-YK, tilted to 21°. (D) ePCL-PA-YK-NO. (E) ePCL-PA-YK-NO, tilted to 21°. Scale bar = 50 nm.
PA-YK was reacted with NO to obtain PA-YK-NO. The electron accepting NO reacts with nucleophilic lysines to form diazeniumdiolates or NONOates and these NONOates undergo protonation in buffers to spontaneously release NO [36, 37]. This was then coated onto ePCL to fabricate ePCL-PA-YK-NO, which is the NO releasing, biomimetic hybrid nanomatrix. ePCL-PA-YK-NO was characterized by SEM (Figure 1c), and it was confirmed that PA-YK-NO does not affect the morphology of ePCL nanofibers. To confirm uniform coating, ePCL-PA-YK-NO was then imaged by TEM (Figure 3c). Uniform coating of PA-YK-NO is visible on either side of ePCL nanofibers, and this was confirmed, again, by imaging after tilting the stage in the transverse axis (Figure 3d).
Greiss assay was used to evaluate the NO release profile from the ePCL-PA-YK-NO hybrid nanomatrix which was incubated in HEPES buffered saline. Successful NO release was observed over 28 days. An initial burst release occurred in first 48 hours, followed by a slow sustained release over a period of 4 weeks resulted in a 48% recovery of NO (Figure 5). The percentage recovery was calculated based on the theoretical value obtained by calculating the number of μmoles of lysine in the PA solution that was used to coat ePCL. The initial burst release may be explained as NO release from the surface of the ePCL-PA-YK-NO nanofibrous matrix. This is especially important for limiting the proliferation of smooth muscle cells, which is one of the key events in restenosis, begins as early as one day after injury [38]. Therefore, the 48 hour burst release of NO is critical to arrest neointimal hyperplasia. Subsequent sustained release may be attributed to NO released from the bulk of the hybrid nanofibrous matrix by a combination of diffusion and enzyme degradation. Over time, due to the presence of MMP-2 degradable sites, ePCL-PA-YK-NO degrades slowly, and this is believed to aid in the sustained release of NO from the bulk of the matrix. The presence of enzyme mediated degradable sites is also expected to create a concentration gradient of NO in the hybrid nanomatrix. From the study, it can be observed that 3.8 μmoles of NO was released over the period of 4 weeks from an ePCL-PA-YK-NO disc that was 16 mm in diameter. This amount is comparable to the cumulative NO released by endothelial cells at a rate of 1×10−10mol cm−2 min−1 [39]. The slow sustained release over a longer period is required to maintain the non-proliferative state of smooth muscle cells, anti-thrombogenic nature of the vessel wall, and promote endothelialization during the recovery period, which typically takes several weeks. Another feature of the hybrid nanomatrix is that the amount of NO released can be easily tuned in future applications by changing the number of lysine moieties in PA-KKKKK, by changing the ratio of PA-KKKKK in the mixture or by changing the concentration of the coating.
Figure 5.
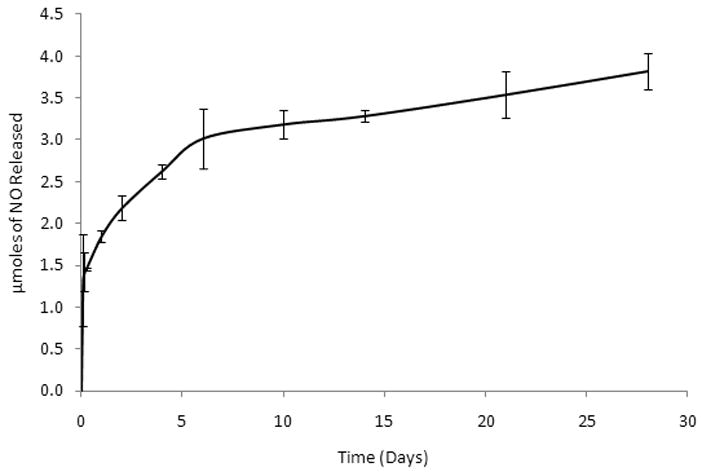
Release profile over 28 days. An initial burst release is observed over the first 48 hours, followed by sustained release through the 28 day time point.
Initial cell adhesion was studied by seeding HUVECs and AoSMCs on ePCL-PA-YK and ePCL-PA-YK-NO. Uncoated ePCL was used as a control. From Figure 6, it can be seen that ePCL-PA-YK (16685±808) and ePCL-PA-YK-NO (15576±2414) show significantly greater HUVEC adhesion when compared to uncoated ePCL (12490±639). It is evident that ePCL-PA-YK (9404±1259) and ePCL-PA-YK-NO (9123±1344) show similar adhesion as ePCL (9310±561), and therefore, do not support AoSMC adhesion. These results clearly show that the hybrid nanomatrices (ePCL-PA-YK and ePCL-PA-YK-NO) promote endothelial cell adhesion while simultaneously not supporting smooth muscle cell adhesion. This is due to PAs endowing the ePCL nanofibers with endothelial cell specific bioactivity in the form laminin derived YIGSR cell adhesive moiety. YIGSR has been known to increase endothelial cell adhesion and spreading [27, 29].
Figure 6.
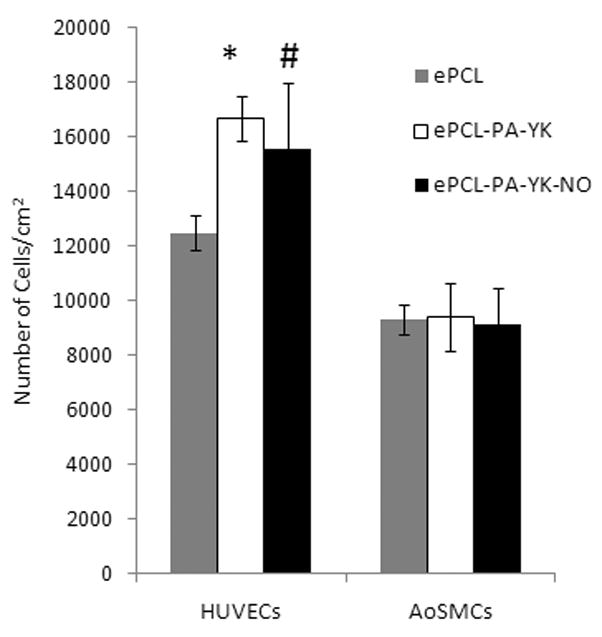
Cell adhesion on hybrid nanomatrix at 2 hrs. HUVECs showed significantly increased adhesion on (*) ePCL-PA-YK and (#) ePCL-PA-YK-NO when compared to ePCL.
The key aspects of improving clinical patency of vascular grafts are re-endothelialization and prevention of restenosis. To achieve this, it is necessary for the hybrid biomimetic nanomatrix to promote the proliferation of endothelial cells, while at the same time, limiting the proliferation of smooth muscle cells. The effect of NO on proliferation of HUVECs and AoSMCs was studied by using the PicoGreen DNA assay over one day and four day time points. As shown in figure 7, HUVECs showed a greater increase in cell number from day one (9391±709) to day four (15196±572) on ePCL-PA-YK-NO when compared to ePCL-PA-YK (10100±1113 on day one and 12742±1141 on day four), while AoSMCs showed no significant increase on ePCL-PA-YK-NO from day one (8876±1169) to day four (10358±631). On ePCL-PA-YK, there was a significant increase in the number of AoSMCs on ePCL-PA-YK from day one (9392±531) to day four (13452±557). Also, on day four, the number of HUVECs on ePCL-PA-YK-NO was significantly greater than the number of HUVECs on ePCL-PA-YK. This shows that ePCL-PA-YK-NO promotes the proliferation of HUVECs while simultaneously limiting the proliferation of AoSMCs. This was corroborated by assaying for PCNA after 48 hours of incubation. PCNA is commonly found in the nucleoli of proliferating cells, and is thus a good marker for proliferation. As shown in Figure 8, the percentage of PCNA positive HUVECs on the ePCL-PA-YK-NO nanomatrix (70±2%) was found to be significantly greater compared to the ePCL-PA-YK nanomatrix (57 ± 3 %). However, the percentage of PCNA positive AoSMCs on ePCL-PA-YK-NO (41±3%) was significantly lower than on ePCL-PA-YK (58±4%). These results indicate that the ePCL-PA-YK-NO nanomatrix enhances endothelial cell growth but limits smooth muscle cell growth, and is therefore consistent with earlier studies on the effect of NO on vascular cell proliferation [23, 24]. Enhancement of endothelialization, while preventing smooth muscle growth, is a crucial step towards improving graft patency by preventing intimal hyperplasia and restenosis. To confirm that the reduced proliferation of AoSMCs was not due to cytotoxicity, these cells were grown on the hybrid nanomatrices for 48 hours. Live/Dead cytotoxicity kit (Molecular Probes) was used to image the cells. From Figure 9, we can see that there were mostly green cells on the hybrid nanomatrices. The images shown in this figure are representative images of over 60 images taken. The live reagent (calcein AM) enters the cells and reacts enzymatically in live nuclei to generate green fluorescence. This is therefore seen only in live cells. The dead reagent (Ethidium homodimer) enters dead cells due to compromised cell membranes and emits red fluorescence. There was no difference between the hybrid nanomatrices and uncoated ePCL. From this, we can reasonably eliminate cytotoxicity as the reason for reduced proliferation.
Figure 7.
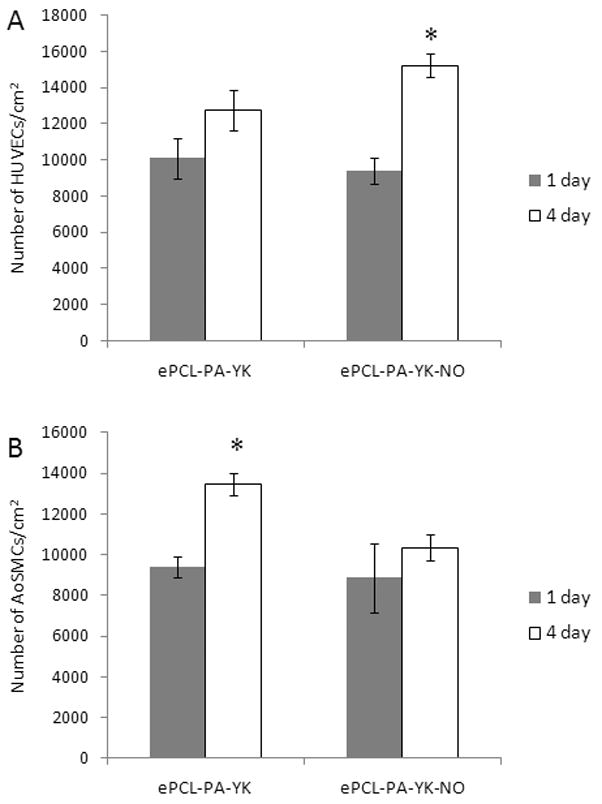
Proliferation of HUVECs and AoSMCs on ePCL-PA-YK-NO. ePCL-PA-YK was used as a control. (A) HUVECs showed significantly increased cell numbers on ePCL-PA-YK-NO (*) over a 4 day period when compared to ePCL-PA-YK. (B) AoSMCs showed a significantly increased cell number on ePCL-PA-YK (*) over 4 days, however, there was no significant increase on ePCL-PA-YK-NO.
Figure 8.
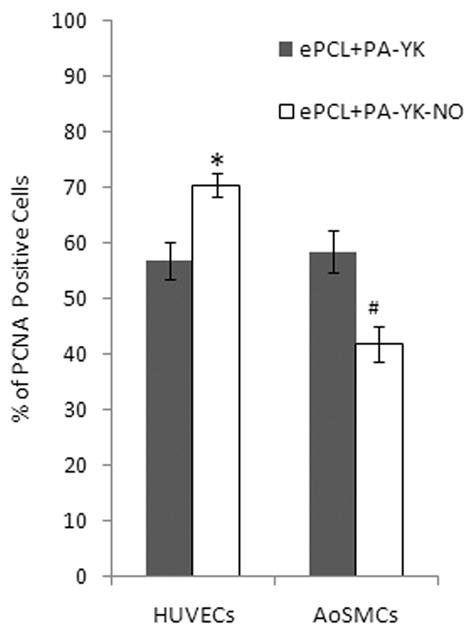
Percentage of proliferating cells on the hybrid nanomatrices. HUVECs showed significantly greater proliferation on ePCL-PA-YK-NO when compared to ePCL-PA-YK(*). AoSMCs showed significantly lower proliferation on ePCL-PA-YK-NO when compared to ePCL-PA-YK(#).
Figure 9.
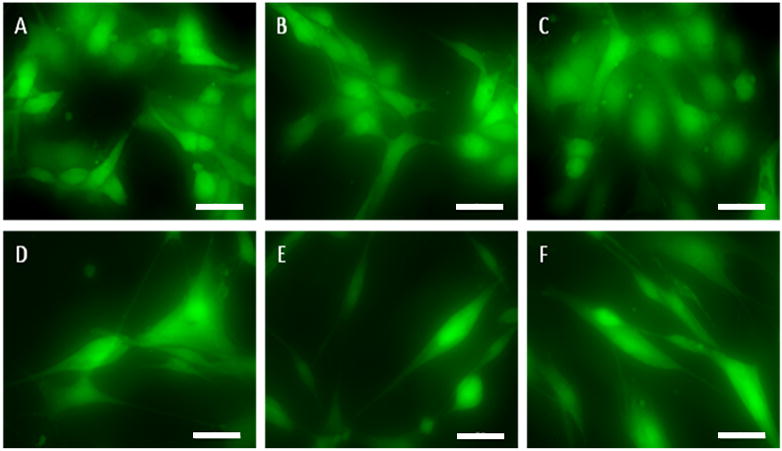
Live/Dead images of HUVECs (A, B and C) and AoSMCs (D, E and F) on hybrid nanomatrices. In both cell types, there is no difference between ePCL-PA-YK-NO (A and D), ePCL-PA-YK (B and E) and the control ePCL (C and F). Scale bar: 10μm
Thrombosis is a major setback suffered by small diameter vascular grafts, and it is absolutely essential to limit the adhesion and activation of platelets to increase the effectiveness of the vascular graft. To evaluate the ability of ePCL-PA-PA-YK-NO on limiting thrombosis, platelet adhesion was studied using mepacrine labeled whole blood from a healthy volunteer. ePCL and ePCL-PA-YK were used as control. Collagen films were used as a positive control. From figure 10, ePCL (388±36) and ePCL-PA-YK (332±42) show significantly reduced platelet adhesion when compared to the collagen film (6272±595). Importantly, ePCL-PA-YK-NO (46±20) showed significantly reduced platelet adhesion when compared to ePCL and ePCL-PA-YK. This shows that NO reduces platelet adhesion, and this biomimetic hybrid nanomatrix is capable of limiting thrombosis, which plagues conventional vascular grafts.
Figure 10.
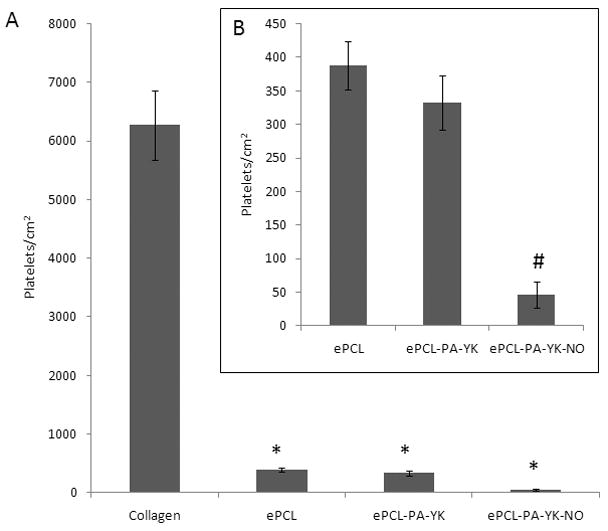
Platelet adhesion on the biomimetic hybrid nanomatrix. (A) Platelets showed significantly reduced adhesion on ePCL, ePCL-PA-YK and ePCL-PA-YK-NO when compared to collagen control (*). (B) ePCL-PA-YK-NO (#) showed significantly reduced platelet adhesion when compared to ePCL and ePCL-PA-YK.
Therefore, this hybrid nanomatrix has great potential for use as biomimetic cardiovascular implants. Conventional cardiovascular implants are limited by a lack of re-endothelialization, restenosis, and thrombosis. NO is known to promote endothelialization, while simultaneously limiting smooth muscle cell proliferation, platelet activation and adhesion [21–24]. Many NO releasing materials have been studied in vitro and in vivo conditions. Consistently, they have been shown to reduce platelet adhesion and intimal hyperplasia [23, 40]. However, one common aspect of these materials is their inability to completely tackle all current clinical challenges that plague conventional implants. They are evidently limited to providing structural support, and are unable to effectively integrate with the host tissue due to their inability to closely mimic the native endothelial environment. This study, therefore, aims to tackle the problems faced by conventional cardiovascular implants using a multipronged approach. While the focus of this study is on the use of biomimetic hybrid nanomatrices as a vascular graft material, another useful application might be in the area of mechanical heart valves. There are several devices available in the market, but they suffer from distinct limitations. Mechanical heart valves are the most commonly used prosthetic cardiac valves, and they are highly thrombogenic. They necessitate the continuous use of anti-coagulation therapy, which reduces, but does not eradicate the risk of thrombosis[41]. The use of this biomimetic nanomatrix, however, could present a solution to this problem, due to its ability to limit platelet adhesion. This could be a significant improvement over the present mechanical valves, and could lead to greatly increased clinical patency.
In summary, a hybrid, NO releasing biomimetic nanomatrix has been successfully developed. This hybrid nanomatrix comprised ePCL nanofibers coated with self-assembling PAs. The ePCL nanofibers provide the mechanical strength and topographical structure, and the PAs comprise enzyme mediated degradable sequence and endothelial cell adhesive ligand or NO donor residue (KKKKK). This hybrid nanomatrix promotes the adhesion and proliferation of endothelial cells, while simultaneously limiting smooth muscle cell proliferation. It also limits the adhesion of platelets. This hybrid nanomatrix, therefore, has great potential for application in cardiovascular implants, including vascular grafts and heart valves.
Acknowledgments
The authors express their gratitude to Melissa Chimento for the use of High Resolution Imaging facility, and Dr. Robin G Foley for the use of the Scanning Electron Microscopic facility. This study was supported by the Wallace H Coulter Foundation and NSF CAREER (CBET-0952974) (HWJ), the Caroline P Ireland Research Scholarship (AA and MK), AHA Greater Southeast Affiliate Predoctoral Fellowship Program (10PRE3500024) for A.A., the NIH T32 predoctoral training grant (NIBIB #EB004312-01), and the Ruth L. Kirschstein National Research Service Award Individual Fellowship (1F31DE021286-01) (JMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Foster ED, Kranc MA. Alternative conduits for aortocoronary bypass grafting. Circulation. 1989 Jun;79(6 Pt 2):I34–39. [PubMed] [Google Scholar]
- 3.Greisler HP. Interactions at the blood/material interface. Annals of vascular surgery. 1990 Jan;4(1):98–103. doi: 10.1007/BF02042699. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. Eur J Vasc Endovasc Surg. 2006 Jun;31(6):627–636. doi: 10.1016/j.ejvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Shuhaiber JH, Evans AN, Massad MG, Geha AS. Mechanisms and future directions for prevention of vein graft failure in coronary bypass surgery. Eur J Cardiothorac Surg. 2002 Sep;22(3):387–396. doi: 10.1016/s1010-7940(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SL, Nicklason LE. Requirements for growing tissue-engineered vascular grafts. Cardiovascular Pathology. 2003;12:59–64. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 7.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. Journal of biomedical materials research. 2003 Dec 15;67(4):1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 8.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008 Jul;29(19):2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005 Sep-Oct;6(5):2583–2589. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 10.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002 Mar–Apr;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 11.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, et al. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008 Jul;29(19):2907–2914. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue engineering. 2005 Jan–Feb;11(1–2):101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 13.Soffer L, Wang X, Zhang X, Kluge J, Dorfmann L, Kaplan DL, et al. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. Journal of biomaterials science. 2008;19(5):653–664. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Liu J, Oh SH, Soker S, Atala A, Yoo JJ. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 2008 Jul;29(19):2891–2898. doi: 10.1016/j.biomaterials.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Yoo JJ, Lim GJ, Atala A, Stitzel J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J Biomed Mater Res A. 2007 Dec 15;83(4):999–1008. doi: 10.1002/jbm.a.31287. [DOI] [PubMed] [Google Scholar]
- 16.Thomas V, Zhang X, Vohra YK. A biomimetic tubular scaffold with spatially designed nanofibers of protein/PDS bio-blends. Biotechnology and bioengineering. 2009 Dec 1;104(5):1025–1033. doi: 10.1002/bit.22467. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Thomas V, Vohra YK. Two ply tubular scaffolds comprised of proteins/poliglecaprone/polycaprolactone fibers. Journal of materials science. 2009 Nov 10; [Google Scholar]
- 18.Inoguchi H, Kwon IK, Inoue E, Takamizawa K, Maehara Y, Matsuda T. Mechanical responses of a compliant electrospun poly(L-lactide-co-epsilon-caprolactone) small-diameter vascular graft. Biomaterials. 2006 Mar;27(8):1470–1478. doi: 10.1016/j.biomaterials.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Williamson MR, Black R, Kielty C. PCL-PU composite vascular scaffold production for vascular tissue engineering: attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials. 2006 Jul;27(19):3608–3616. doi: 10.1016/j.biomaterials.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 20.He W, Ma Z, Teo WE, Dong YX, Robless PA, Lim TC, et al. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A. 2009 Jul;90(1):205–216. doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 21.Kuo PC, Schroeder RA. The emerging multifaceted roles of nitric oxide. Ann Surg. 1995 Mar;221(3):220–235. doi: 10.1097/00000658-199503000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohl KS, West JL. Nitric oxide-generating polymers reduce platelet adhesion and smooth muscle cell proliferation. Biomaterials. 2000 Nov;21(22):2273–2278. doi: 10.1016/s0142-9612(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 24.Lipke EA, West JL. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta biomaterialia. 2005 Nov;1(6):597–606. doi: 10.1016/j.actbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science (New York, NY. 2001 Nov 23;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JM, Andukuri A, Lim DJ, Jun HW. Modulating the gelation properties of self-assembling peptide amphiphiles. ACS nano. 2009 Nov 24;3(11):3447–3454. doi: 10.1021/nn900884n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andukuri A, Minor WP, Kushwaha M, Anderson JM, Jun HW. Effect of endothelium mimicking self-assembled nanomatrices on cell adhesion and spreading of human endothelial cells and smooth muscle cells. Nanomedicine. 2009 Oct 2; doi: 10.1016/j.nano.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun HW, Yuwono V, Paramonov SE, Hartgerink JD. Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv Mater. 2005;17:2612–2617. [Google Scholar]
- 29.Massia SP, Hubbell JA. Human endothelial cell interactions with surface-coupled adhesion peptides on a nonadhesive glass substrate and two polymeric biomaterials. Journal of biomedical materials research. 1991 Feb;25(2):223–242. doi: 10.1002/jbm.820250209. [DOI] [PubMed] [Google Scholar]
- 30.Kushwaha M, Anderson JM, Bosworth CA, Andukuri A, Minor WP, Lancaster JR, Jr, et al. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials. 2010 Nov 11;31:1502–1508. doi: 10.1016/j.biomaterials.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridrikh SV, Yu JH, Brenner MP, Rutledge GC. Controlling the fiber diameter during electrospinning. Physical review letters. 2003 Apr 11;90(14):144502. doi: 10.1103/PhysRevLett.90.144502. [DOI] [PubMed] [Google Scholar]
- 32.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Current opinion in biotechnology. 2003 Oct;14(5):551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids and surfaces. 2004 Dec 10;39(3):125–131. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, et al. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008 Dec 9;118(24):2563–2570. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JM, Kushwaha M, Tambralli A, Bellis SL, Camata RP, Jun HW. Osteogenic differentiation of human mesenchymal stem cells directed by extracellular matrix-mimicking ligands in a biomimetic self-assembled peptide amphiphile nanomatrix. Biomacromolecules. 2009 Oct 12;10(10):2935–2944. doi: 10.1021/bm9007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chemical reviews. 2002 Apr;102(4):1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 37.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods in enzymology. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 38.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007 Sep 3;100(5A):3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998 Jun;274(6 Pt 2):H2163–2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 40.Kaul S, Makkar RR, Nakamura M, Litvack FI, Shah PK, Forrester JS, et al. Inhibition of acute stent thrombosis under high-shear flow conditions by a nitric oxide donor, DMHD/NO. An ex vivo porcine arteriovenous shunt study. Circulation. 1996 Nov 1;94(9):2228–2234. doi: 10.1161/01.cir.94.9.2228. [DOI] [PubMed] [Google Scholar]
- 41.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994 Feb;89(2):635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]


