Abstract
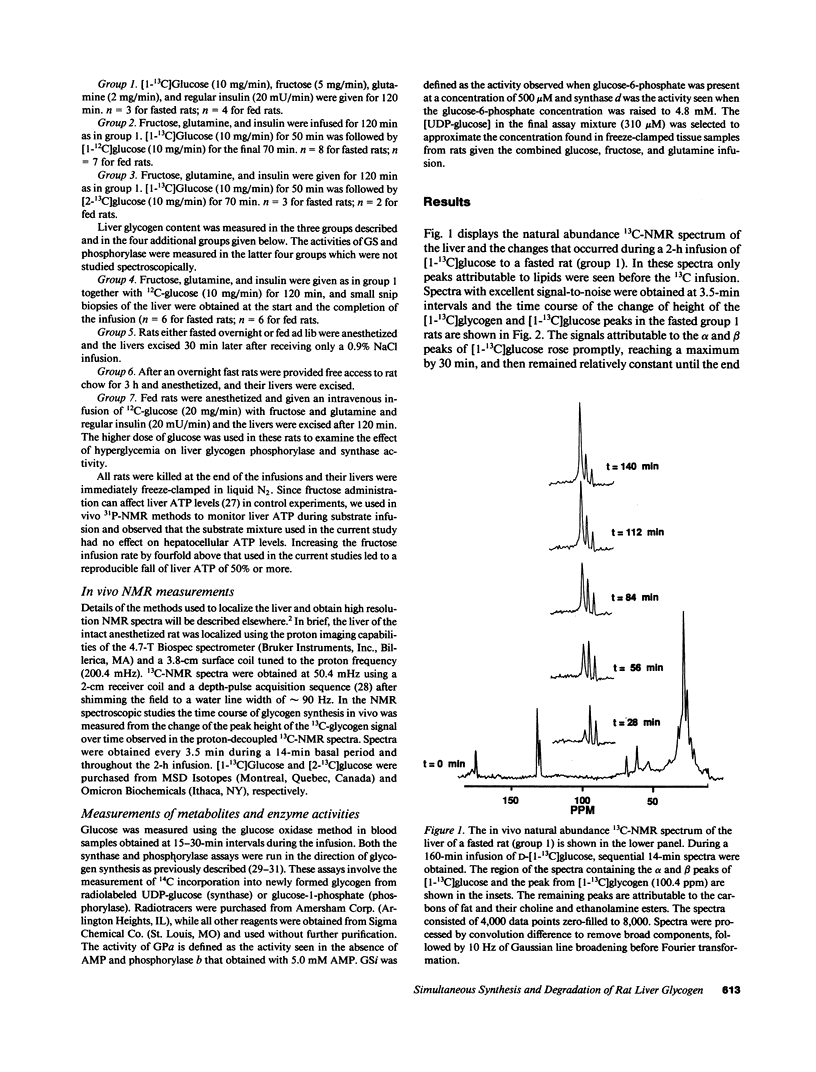
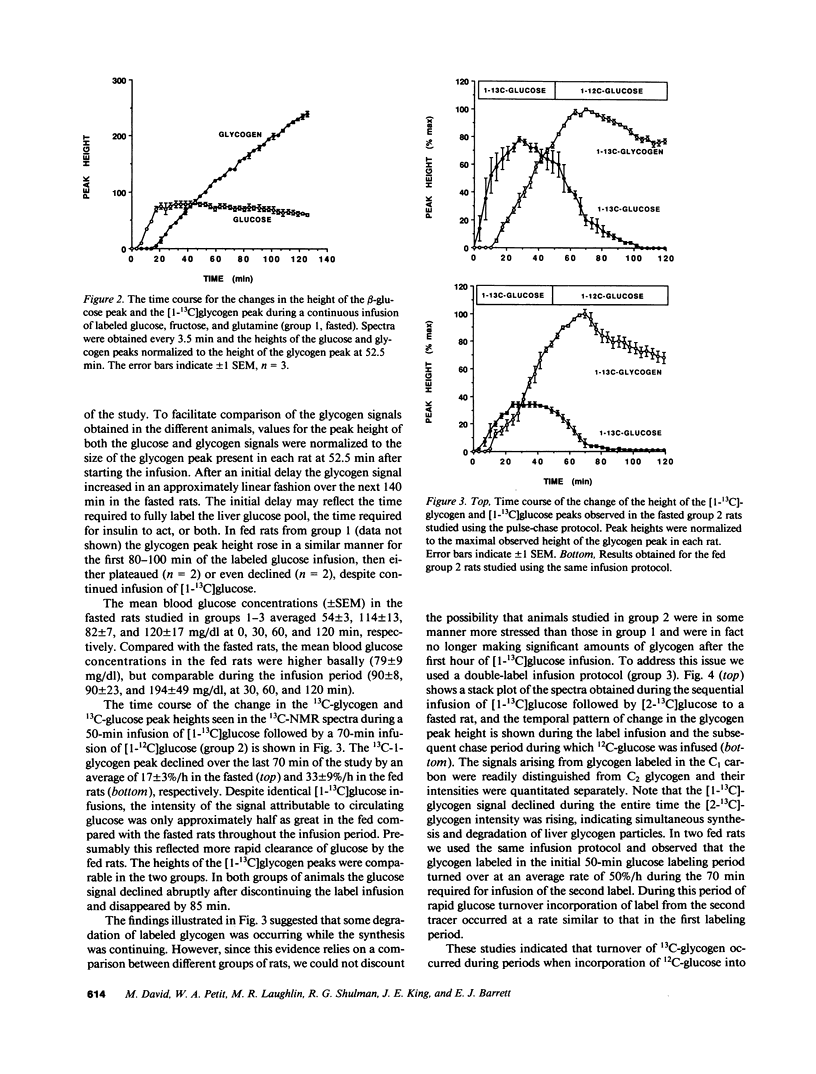
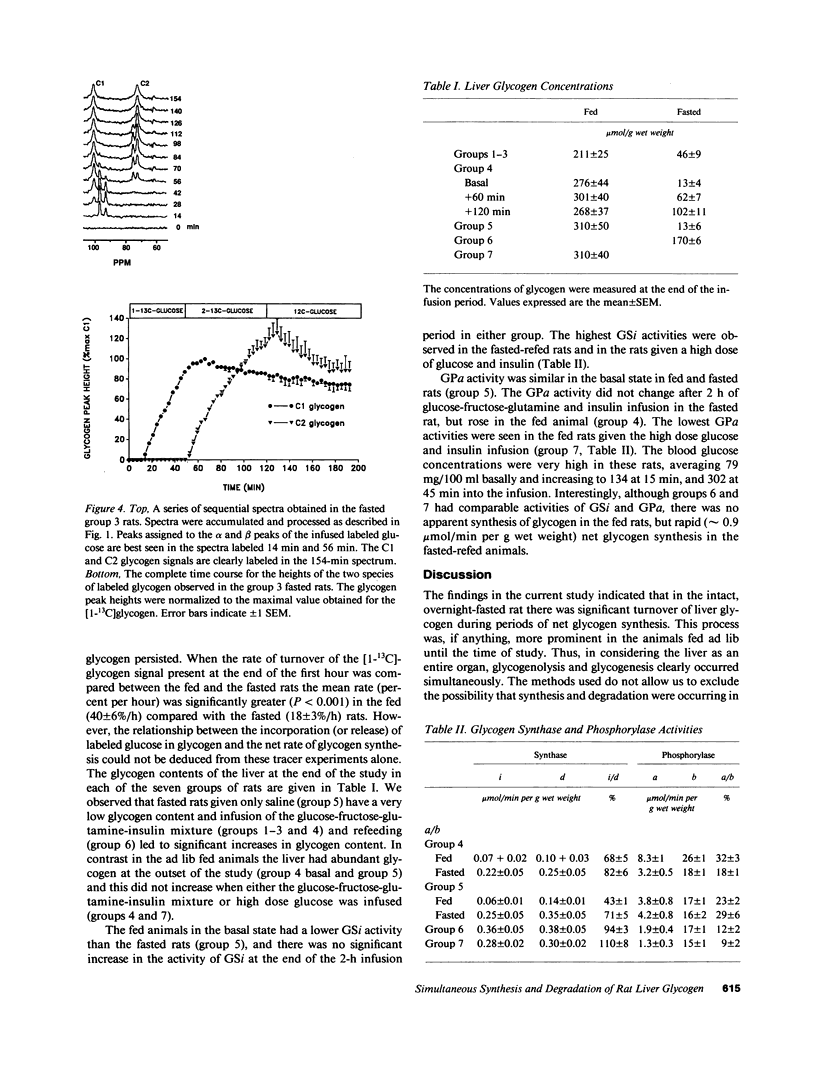
Using 13C nuclear magnetic resonance spectroscopic methods we examined in vivo the synthesis of liver glycogen during the infusion of D-[1-13C]glucose and the turnover of labeled glycogen during subsequent infusion of D-[1-13C]glucose. In fasted rats the processes of glycogen synthesis and degradation were observed to occur simultaneously with the rate of synthesis much greater than degradation leading to net glycogen synthesis. In fed rats, incorporation of infused D-[1-13C]glucose occurred briskly; however, over 2 h there was no net glycogen accumulated. Degradation of labeled glycogen was greater in the fed versus the fasted rats (P less than 0.001), and the lack of net glycogen synthesis in fed rats was due to degradation and synthesis occurring at similar rates throughout the infusion period. There was no indication that suppression of phosphorylase a or subsequent activation of glycogen synthase was involved in modulation of the flux of tracer into liver glycogen. We conclude that in both fed and fasted rats, glycogen synthase and phosphorylase are active simultaneously and the levels of liver glycogen reached during refeeding are determined by the balance between ongoing synthetic and degradative processes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Crabtree B., Newsholme E. A. Hormonal regulation of the rate of the glycogen/glucose-1-phosphate cycle in skeletal muscle. Eur J Biochem. 1987 Feb 16;163(1):205–210. doi: 10.1111/j.1432-1033.1987.tb10756.x. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Stevenson R. W., Steiner K. E., Davis M. A., Myers S. R., Adkins B. A., Abumrad N. N., Williams P. E. Insulin, glucagon, and glucose as regulators of hepatic glucose uptake and production in vivo. Diabetes Metab Rev. 1987 Jan;3(1):307–332. doi: 10.1002/dmr.5610030114. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Abou-Mourad N., Lacy W. W., Steiner K. E., Liljenquist J. E. Insulin as a mediator of hepatic glucose uptake in the conscious dog. Am J Physiol. 1982 Feb;242(2):E97–101. doi: 10.1152/ajpendo.1982.242.2.E97. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K. Metabolic zonation of liver parenchyma: significance for the regulation of glycogen metabolism, gluconeogenesis, and glycolysis. Diabetes Metab Rev. 1987 Jan;3(1):269–293. doi: 10.1002/dmr.5610030112. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann U., Froesch E. R. Inhibition of phosphorylase-a by fructose-1-phosphate, alpha-glycerophosphate and fructose-1,6-diphosphate: explanation for fructose-induced hypoglycaemia in hereditary fructose intolerance and fructose-1,6-diphosphatase deficiency. Eur J Clin Invest. 1973 Sep;3(5):407–413. doi: 10.1111/j.1365-2362.1973.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Laughlin M. R., Petit W. A., Jr, Dizon J. M., Shulman R. G., Barrett E. J. NMR measurements of in vivo myocardial glycogen metabolism. J Biol Chem. 1988 Feb 15;263(5):2285–2291. [PubMed] [Google Scholar]
- Mulmed L. N., Gannon M. C., Gilboe D. P., Tan A. W., Nuttall F. Q. Glycogen synthase, synthase phosphatase, and phosphorylase response to glucose in somatostatin-pretreated intact rats. Diabetes. 1979 Mar;28(3):231–236. doi: 10.2337/diab.28.3.231. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Niewoehner C. B., Gilboe D. P., Nuttall F. Q. Metabolic effects of oral glucose in the liver of fasted rats. Am J Physiol. 1984 Jan;246(1 Pt 1):E89–E94. doi: 10.1152/ajpendo.1984.246.1.E89. [DOI] [PubMed] [Google Scholar]
- Niewoehner C. B., Gilboe D. P., Nuttall G. A., Nuttall F. Q. Metabolic effects of oral fructose in the liver of fasted rats. Am J Physiol. 1984 Oct;247(4 Pt 1):E505–E512. doi: 10.1152/ajpendo.1984.247.4.E505. [DOI] [PubMed] [Google Scholar]
- Niewoehner C. B., Nuttall F. Q. Disposition of a glucose load in fed rats and rats adapted to a high-carbohydrate diet. Am J Physiol. 1989 Jun;256(6 Pt 1):E811–E817. doi: 10.1152/ajpendo.1989.256.6.E811. [DOI] [PubMed] [Google Scholar]
- Nuttall F. Q., Gilboe D. P., Gannon M. C., Niewoehner C. B., Tan A. W. Regulation of glycogen synthesis in the liver. Am J Med. 1988 Nov 28;85(5A):77–85. doi: 10.1016/0002-9343(88)90400-7. [DOI] [PubMed] [Google Scholar]
- Oberhaensli R. D., Galloway G. J., Taylor D. J., Bore P. J., Radda G. K. Assessment of human liver metabolism by phosphorus-31 magnetic resonance spectroscopy. Br J Radiol. 1986 Jul;59(703):695–699. doi: 10.1259/0007-1285-59-703-695. [DOI] [PubMed] [Google Scholar]
- Olavarria J. M., Gödeken O. G., Sandruss R., Flawia M. Recovery of the liver glycogen in fasted rats. Biochim Biophys Acta. 1968 Aug 6;165(1):183–188. [PubMed] [Google Scholar]
- Regan J. J., Jr, Doorneweerd D. D., Gilboe D. P., Nuttall F. Q. Influence of fructose on the glycogen synthase and phosphorylase systems in rat liver. Metabolism. 1980 Oct;29(10):965–969. doi: 10.1016/0026-0495(80)90040-2. [DOI] [PubMed] [Google Scholar]
- Shalwitz R. A., Reo N. V., Becker N. N., Ackerman J. J. Visibility of mammalian hepatic glycogen to the NMR experiment, in vivo. Magn Reson Med. 1987 Nov;5(5):462–465. doi: 10.1002/mrm.1910050508. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rossetti L., Rothman D. L., Blair J. B., Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987 Aug;80(2):387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Chung Y., Rossetti L., Petit W. A., Jr, Barrett E. J., Shulman R. G. 13C NMR studies of glycogen turnover in the perfused rat liver. J Biol Chem. 1988 Apr 15;263(11):5027–5029. [PubMed] [Google Scholar]
- Sillerud L. O., Shulman R. G. Structure and metabolism of mammalian liver glycogen monitored by carbon-13 nuclear magnetic resonance. Biochemistry. 1983 Mar 1;22(5):1087–1094. doi: 10.1021/bi00274a015. [DOI] [PubMed] [Google Scholar]
- Stalmans W., Bollen M., Mvumbi L. Control of glycogen synthesis in health and disease. Diabetes Metab Rev. 1987 Jan;3(1):127–161. doi: 10.1002/dmr.5610030107. [DOI] [PubMed] [Google Scholar]
- Stalmans W., De Wulf H., Hue L., Hers H. G. The sequential inactivation of glycogen phosphorylase and activation of glycogen synthetase in liver after the administration of glucose to mice and rats. The mechanism of the hepatic threshold to glucose. Eur J Biochem. 1974 Jan 3;41(1):127–134. doi: 10.1111/j.1432-1033.1974.tb03252.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., Laloux M., Hers H. G. The interaction of liver phosphorylase a with glucose and AMP. Eur J Biochem. 1974 Nov 15;49(2):415–427. doi: 10.1111/j.1432-1033.1974.tb03847.x. [DOI] [PubMed] [Google Scholar]
- Tan A. W., Nuttall F. Q. Characteristics of the dephosphorylated form of phosphorylase purified from rat liver and measurement of its activity in crude liver preparations. Biochim Biophys Acta. 1975 Nov 20;410(1):45–60. doi: 10.1016/0005-2744(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Van de Werve G., Jeanrenaud B. Synthase activation is not a prerequisite for glycogen synthesis in the starved liver. Am J Physiol. 1984 Aug;247(2 Pt 1):E271–E275. doi: 10.1152/ajpendo.1984.247.2.E271. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Werve G., Jeanrenaud B. The onset of liver glycogen synthesis in fasted-refed lean and genetically obese (fa/fa) rats. Diabetologia. 1987 Mar;30(3):169–174. doi: 10.1007/BF00274223. [DOI] [PubMed] [Google Scholar]



