Abstract
Effort thrombosis, or Paget-Schroetter Syndrome, refers to axillary-subclavian vein thrombosis associated with strenuous and repetitive activity of the upper extremities. Anatomical abnormalities at the thoracic outlet and repetitive trauma to the endothelium of the subclavian vein are key factors in its initiation and progression. The role of hereditary and acquired thrombophilias is unclear. The pathogenesis of effort thrombosis is thus distinct from other venous thromboembolic disorders. Doppler ultrasonography is the preferred initial test, while contrast venography remains the gold standard for diagnosis. Computed tomographic venography and magnetic resonance venography are comparable to conventional venography and are being increasingly used. Conservative management with anticoagulation alone is inadequate and leads to significant residual disability. An aggressive multimodal treatment strategy consisting of catheter-directed thrombolysis, with or without early thoracic outlet decompression, is essential for optimizing outcomes. Despite excellent insights into its pathogenesis and advances in treatment, a significant number of patients with effort thrombosis continue to be treated suboptimally. Hence, there is an urgent need for increasing physician awareness about risk factors, etiology and the management of this unique and relatively infrequent disorder.
INTRODUCTION
Effort thrombosis refers to axillary-subclavian vein thrombosis (ASVT) associated with strenuous and repetitive activity of the upper extremities. The earliest description of spontaneous ASVT was by Cruveilhier in 1816, and the first elaborate account was provided by James Paget in 1875.1,2 In 1894, von Schroetter was the first to identify vascular trauma from muscle strain as a potential etiologic factor.3 In 1948, Hughes coined the term Paget-Schroetter Syndrome (PSS) and published the first review.4, 5 PSS accounts for 30– 40 % of spontaneous ASVT and for 10–20 % of all upper extremity deep venous thrombosis (UEDVT). 6 Other important predisposing factors for UEDVT include indwelling hardware (central venous catheter, ports, and pacemakers), occult or overt malignancy and other thrombophilic states. 6–10
PATHOGENESIS
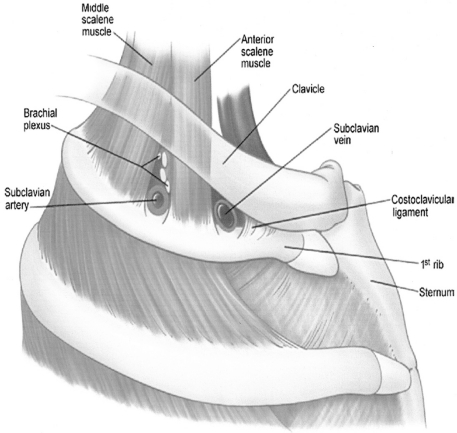
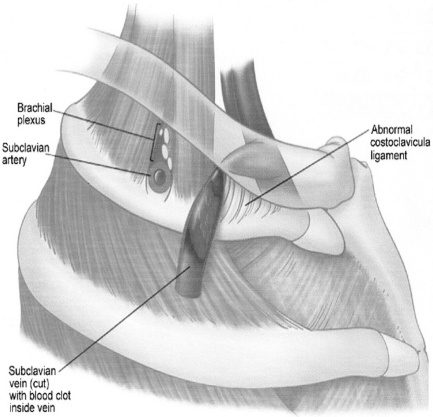
Effort thrombosis usually follows sporting activities, such as wrestling, playing ball, gymnastics and swimming, which involve vigorous and sustained upper extremity movements.11 It is believed that retroversion, hyperabduction and extension of the arm involved with these activities impose undue strain on the subclavian vein leading to microtrauma of the endothelium and activation of the coagulation cascade. Substantial evidence now supports the role of anatomical abnormalities involving the thoracic outlet (cervical rib, congenital bands, hypertrophy of scalenus tendons and abnormal insertion of the costoclavicular ligament) in the pathogenesis of effort thrombosis. 11, 12 (Figures 1 and 2 depict the normal/abnormal anatomy of the thoracic outlet.) The narrow costoclavicular space leads to compression of the vein and to stasis in the flow. More importantly, it restricts the mobility of the subclavian vein, making it more susceptible to trauma from arm use. These lead to a self-perpetuating cycle of endothelial trauma, thrombosis and recanalization. The repetitive endothelial trauma leads to intimal hyperplasia, inflammation and fibrosis, resulting in venous webs, extensive collateral formation and perivenular fibrosis. This in turn worsens the stasis and costoclavicular crowding. Effort thrombosis has therefore been rightfully categorized as a venous variant of thoracic outlet syndrome. 12, 13
Figure 1.
Normal anatomy of the thoracic outlet. Foot note: Reproduced with permission from Urschel et al, Ann Thorac Surg 2008;86:254–60 12 Copyright Elsevier Inc, 2008.
Figure 2.
Abnormal lateral insertion of the costoclavicular ligament in Paget-Schroetter syndrome. Foot note: Reproduced with permission from Urschel et al, Ann Thorac Surg 2008;86:254–60 12 Copyright Elsevier Inc, 2008.
Some investigators have reported a higher frequency of factor V Leidin, Prothrombin gene mutation and other inherited thrombophilic states in patients with idiopathic UEDVT.14 –16 In fact, in one recent study, Cassada et al. demonstrated that approximately two-thirds of patients with PSS had concurrent thrombophilia. 17 In addition, they showed that concurrent thrombophilia led to increased postoperative complications following corrective surgery. However, others have refuted this association by demonstrating that the frequency of inherited thrombophilias in patients with effort thromboses was comparable to that of the general population. They found that the increased frequency of concurrent thrombophilic disorders was limited to patients with idiopathic UEDVT, which was not effort related.18 Thus, unlike venous thromboses elsewhere (lower extremities and visceral), the role of inherited and acquired thrombophilic disorders in the development and progression of effort thrombosis is unclear. In summary, costoclavicular crowding due to anatomical abnormalities and repetitive endothelial trauma from muscular strain are the key pathogenic factors in the initiation and progression of effort thrombosis. Inflammation, by leading to perivenular fibrosis and adhesions, seems to play a contributory role in the perpetuation of obstruction. The role of inherited and acquired thrombophilic disorders remains unresolved and needs further investigation.
CLINICAL FEATURES
Not surprisingly, effort thrombosis is more common in young and otherwise healthy men. It preferentially involves the dominant arm. Unlike those with UEDVT secondary to central venous catheters, patients with effort thrombosis are usually symptomatic. Swelling and arm discomfort are the most frequent presenting problems.12, 13, 19 Other symptoms include heaviness, redness of arm, cyanosis and dilated, visible veins across the shoulder and upper arm (Urschel’s sign).12 Symptom onset is usually acute or sub-acute but an occasional patient can present with chronic symptoms. Not infrequently, symptoms can be nonspecific and can even mimic a muscular strain.20 A majority of patients report a discrete precipitating event, usually sports-related arm exertion. Occasionally, minor and relatively innocuous day-to-day activities can precipitate effort thrombosis.11, 12 Complications include pulmonary embolism, post-thrombotic syndrome and recurrent thrombosis.12, 13 While some investigators report a lower incidence of pulmonary embolism compared to patients with lower extremity DVT and catheter related UEDVT, others have refuted this.19, 21, 22 Irrespective of the relative risk, it is important to bear in mind that the risk of pulmonary embolism with effort thrombosis is real and significant. Post-thrombotic syndrome (characterized by pain, heaviness, and swelling), on the other hand, is more frequent in effort thrombosis, compared to secondary UEDVT, and is the major contributor to the morbidity associated with disease.12, 13, 23, 24 In one recent review, it was shown that up to 45% (15% on average) with UEDVT develop post-thrombotic syndrome. 24 The fact that effort thrombosis preferentially affects young and active individuals makes even minor degrees of residual disability very relevant.
DIAGNOSIS
Notably, the symptoms and signs of UEDVT have poor specificity, and less than 50% of those with suggestive symptoms actually have deep venous thrombosis (DVT). 12, 13, 25 Confirmatory tests are therefore crucial following a presumptive clinical diagnosis.23, 25 Compression ultrasonography with color Doppler by virtue of its ease, availability, portability and low cost is currently the preferred initial test in the evaluation of suspected UEDVT.26 Contrast venography has traditionally been the gold standard for the diagnosis of UEDVT. However, invasive nature, high cost, and the accuracy of non-invasive tests have relegated venography to the background.12, 13, 27, 28 Radionuclide, magnetic resonance and computed tomographic venography are superior to ultrasonography. Magnetic resonance venography has the highest sensitivity (100%) and specificity (97%) among all the non-invasive diagnostic modalities and has the potential to replace digital subtraction angiography as the gold standard.27 However, the higher cost and limited availability limits the applicability of magnetic resonance venography.12, 13, 22, 26–28 Conversely, computed tomographic venography is widely available but is associated with risks of radio contrast administration. These tests are therefore second line and reserved for patients with high clinical probability of effort thrombosis and negative ultrasound. Though venography is not necessary for diagnosis, it is almost always done as a part of multimodal treatment strategy to deliver catheter-directed thrombolysis and plan thoracic outlet decompression surgery. 12
MANAGEMENT
Contemporary management of effort thrombosis varies widely, and there is no broad consensus as to what constitutes the best approach. The relative rarity of the disease, paucity of awareness and lack of large randomized trails are factors contributing to this confusion. For many years patients with effort thrombosis were managed conservatively with limb elevation and anticoagulation alone. However, subsequent long-term data demonstrated an unacceptably high incidence of residual symptoms, disability and recurrent thrombosis with this conservative strategy.12, 29–31 This has prompted clinicians to devise and evaluate aggressive treatment strategies involving thrombolysis, thrombectomy, percutaneous and surgical venoplasty, venous bypass and stents.
Systemic fibrinolysis is superior to anticoagulation in achieving vein patency but is associated with higher rates of complications such as intracranial hemorrhage.32 Local catheter-directed thrombolysis has the therapeutic value of systemic thrombolysis without significant systemic side effects and is currently recommended in all patients presenting early.33 The precise time interval for defining early presentation is unclear. While some authors recommend using fibrinolytics only in patients presenting within two weeks of symptom onset, others have reported fair outcomes even with a delay of about four to six weeks.12, 34–36 Of note, the success of thrombolysis diminishes as the time from symptom onset to treatment increases, underscoring the need for prompt recognition and treatment.12 Newer fibrinolytics like alteplase and reteplase have replaced urokinase and streptokinase due to their better safety profile. The duration of treatment and dose of these agents is not standardized and therefore varies among various institutions. Most patients require prolonged infusion of the fibrinolytic agent for catheter directed thrombolysis; average durations varying between 24–48 hours.33
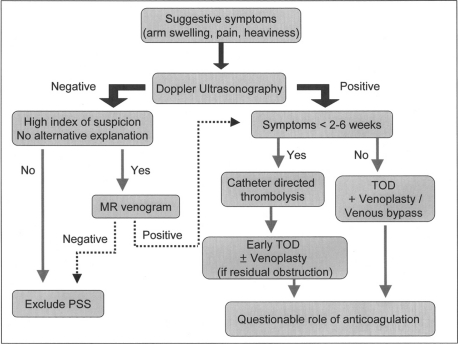
Therapy directed at thoracic outlet decompression (TOD) has become an integral part in the management of effort thrombosis with the recognition of the central role of thoracic outlet obstruction. TOD involves resection of the first rib, division of the scalenus muscles and the costoclavicular ligament using either a transaxillary or infraclavicular approach. Some investigators reserve TOD only for patients with persistent or recurrent symptoms following catheter-directed thrombolysis. Lee et al. used this restrictive strategy and reported that less than 25% of patients needed surgery after a mean follow up of 13 months. 37 However, others recommend routine and early TOD in all patients.36, 38 Suboptimal results with delayed surgery vis-à-vis early surgery; in the form of higher incidence of residual symptoms, disability from post-thrombotic syndrome and recurrent thrombosis, argue in favor of early TOD.34–36 Controversy remains regarding the best surgical approach to achieve TOD. Some suggest that the anterior, or sub-clavicular, approach is superior because it allows better exposure of the proximal subclavian and innominate veins, thereby ensuring more optimal vein repair.36 However, others have reported excellent results with the relatively easier transaxillary approach.12 Either approach can be associated with complications like pneumothorax, bleeding, nerve or arterial injury, underscoring the need for careful patient selection. Moreover, thoracic outlet decompression is a complicated surgery and should be undertaken only by experienced surgeons at centers with expertise in managing patients of effort thrombosis. The need for a detailed discussion with patients regarding the risks and benefits of surgery cannot be overstated. So far, definite markers predicting failure of thrombolysis and need for surgery have not been identified. Lee et al. reported that age < 28 years was a predictor of recurrent thrombosis following thrombolysis.37 However, further validation is awaited. Surgical thrombectomy, balloon venoplasty and stenting have practically been abandoned due to the limited success, high procedural morbidity, and high rates of stent fracture.12, 36–38 Patch venoplasty and venous bypass have been successfully used in some patients with residual stenosis after TOD.35, 36 Based on our own experience and review of the literature, we suggest the algorithm in Figure 3 as a guide to the diagnosis and management of patients with suspected effort thrombosis. It is reasonable to seek early surgical (vascular and thoracic) consultation in all young patients with UEDVT especially, in the absence of obvious precipitating factors, such as a central venous line. Finally, the need for and the duration of anticoagulation in patients treated with a combination of catheter-directed thrombolyis and TOD remains unclear. While some authors recommend no anticoagulation when good surgical results are obtained, others prefer anticoagulation for at least two to three months12, 36 As already mentioned, the role of inherited and acquired thrombophilic states in effort thrombosis is unclear. Nevertheless, it is reasonable to test at least selected patients for these abnormalities as they might help predict postoperative complications, recurrence rates and determine the need for long-term anticoagulation. It appears reasonable to recommend long-term anticoagulation in patients with coexistent thrombophilias and for those presenting late and having suboptimal surgical results. However, the efficacy of this strategy in preventing recurrent thrombosis and alleviating symptoms is unclear and needs further study.
Figure 3.
Diagnostic and management algorithm for patients with suspected effort thrombosis.
TOD, thoracic outlet decompression
CONCLUSION
In summary, effort thrombosis is a complex and relatively infrequent disorder with a distinct pathogenesis. Most physicians unfamiliar with effort thrombosis manage it similarly to classic lower extremity DVT. Instead, effort thrombosis is ideally managed using a multimodal approach consisting of routine catheter-directed thrombolysis, early TOD in appropriate patients and physical and occupational therapy. Long-term anticoagulation may be reasonable in patients with coexistent thrombophilia and suboptimal surgical results. Increasing awareness among primary care and emergency physicians will ensure early recognition, timely thrombolysis, and prompt referral to a thoracic or vascular surgeon. Future research should focus on defining the benefit of thrombolytic therapy in patients presenting late, identifying factors that predict failure of thrombolysis and need for surgery. Other avenues for research include assessment of the need for and duration of anticoagulation following TOD, and cost benefit analysis of the various treatment strategies.
Footnotes
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
- 1.Cruveilhier LJB. Essai sur l’anatomie pathologique en général et sur les transformations et productions organiques en particulier. 1816. Doctoral thesis. Paris,
- 2.Paget J. Clinical Lectures and Essays. London: Longman Green; 1875. p. 292. [Google Scholar]
- 3.Von Schrötter L. Nathnagel’s Handbuch der speciellen Pathologie und Therapie. Vienna, Austria: Holder; 1884. Erkrankungen der Gefässe. [Google Scholar]
- 4.Hughes ESR. Venous obstruction in the upper extremity (Paget-Schroetter’s syndrome) Br J Surg. 1948;36:155–63. doi: 10.1002/bjs.18003614206. [DOI] [PubMed] [Google Scholar]
- 5.Hughes ES. Venous obstruction in the upper extremity; Paget-Schroetter’s syndrome; A review of 320 cases. Surg Gynecol Obstet. 1949;88:89–127. [PubMed] [Google Scholar]
- 6.Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32:729–36. doi: 10.1055/s-2006-951458. [DOI] [PubMed] [Google Scholar]
- 7.Spencer FA, Emery C, Lessard D, Goldberg RJ. Upper extremity deep vein thrombosis: a community-based perspective. Am J Med. 2007;120:678–84. doi: 10.1016/j.amjmed.2006.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joffe HV, Kucher N, Tapson VF, et al. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605–11. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- 9.De Cicco M, Matovic M, Balestreri L, et al. Central venous thrombosis: an early and frequent complication in cancer patients bearing long-term silastic catheters. A prospective study. Thromb Res. 1997;86:101–13. doi: 10.1016/s0049-3848(97)00054-6. [DOI] [PubMed] [Google Scholar]
- 10.Marinella MA, Kathula SK, Markert RJ. Spectrum of upper extremity deep venous thrombosis in a community teaching hospital. Heart Lung. 2000;29:113–7. [PubMed] [Google Scholar]
- 11.Zell L, Kindermann W, Marschall F, et al. Paget-Schroetter syndrome in sports activities--case study and literature review. Angiology. 2001;52:337–42. doi: 10.1177/000331970105200507. [DOI] [PubMed] [Google Scholar]
- 12.Urschel HC, Jr, Patel AN. Surgery remains the most effective treatment for Paget-Schroetter syndrome: 50 years‘ experience. Ann Thorac Surg. 2008;86:254–60. doi: 10.1016/j.athoracsur.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Kommareddy A, Zaroukian MH, Hassouna HI. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2002;28:89–99. doi: 10.1055/s-2002-20567. [DOI] [PubMed] [Google Scholar]
- 14.Hendler MF, Meschengieser SS, Blanco AN, et al. Primary upper-extremity deep vein thrombosis: high prevalence of thrombophilic defects. Am J Hematol. 2004;76:330–7. doi: 10.1002/ajh.20131. [DOI] [PubMed] [Google Scholar]
- 15.Martinelli I, Battaglioli T, Bucciarelli P, Passamonti SM, Mannucci PM. Risk factors and recurrence rate of primary deep vein thrombosis of the upper extremities. Circulation. 2004;110:566–70. doi: 10.1161/01.CIR.0000137123.55051.9B. [DOI] [PubMed] [Google Scholar]
- 16.Vaya A, Mira Y, Mateo J, et al. Prothrombin G20210A mutation and oral contraceptive use increase upper-extremity deep vein thrombotic risk. Thromb Haemost. 2003;89:452–7. [PubMed] [Google Scholar]
- 17.Cassada DC, Lipscomb AL, Stevens SL, Freeman MB, Grandas OH, Goldman MH. The importance of thrombophilia in the treatment of Paget-Schroetter syndrome. Ann Vasc Surg. 2006;20:596–601. doi: 10.1007/s10016-006-9106-z. [DOI] [PubMed] [Google Scholar]
- 18.Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Fiessinger JN. Hypercoagulable states in primary upper extremity deep vein thrombosis. Arch Intern Med. 2000;160:382–6. doi: 10.1001/archinte.160.3.382. [DOI] [PubMed] [Google Scholar]
- 19.Joffe HV, Kucher N, Tapson VF, et al. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605–11. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- 20.Louis J. Axillary vein thrombosis mimicking muscular strain. J Accid Emerg Med. 1999;16:233–4. doi: 10.1136/emj.16.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooij JD, van der Zant FM, van Beek EJ, et al. Pulmonary embolism in deep venous thrombosis of upper extremity: more often in catheter-related thrombosis. Neth J Med. 1997;50:238–42. doi: 10.1016/s0300-2977(97)00020-x. [DOI] [PubMed] [Google Scholar]
- 22.Monreal M, Lafoz E, Ruiz J, et al. Upper-extremity deep venous thrombosis and pulmonary embolism. A prospective study. Chest. 1991;99:280–3. doi: 10.1378/chest.99.2.280. [DOI] [PubMed] [Google Scholar]
- 23.Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;13:57–62. 157: [PubMed] [Google Scholar]
- 24.Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res. 2006;117:609–14. doi: 10.1016/j.thromres.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Leebeek FW, Kappers-Klunne MC, Gomez-Garcia EB. Deep venous thrombosis of the arm: etiology, diagnosis and treatment. Ned Tijdschr Geneeskd. 2000;144:361–4. [PubMed] [Google Scholar]
- 26.Mustafa BO, Rathbun SW, Whitsett TL, et al. Sensitivity and specificity of ultrasonography in the diagnosis of upper extremity deep vein thrombosis: A systematic review. Arch Intern Med. 2002;162:401–4. doi: 10.1001/archinte.162.4.401. [DOI] [PubMed] [Google Scholar]
- 27.Chang YC, Su CT, Yang PC, et al. Magnetic resonance angiography in the diagnosis of thoracic venous obstruction. J Formos Med Assoc. 1998;97:38–43. [PubMed] [Google Scholar]
- 28.Fraser JD, Anderson DR. Venous protocols, techniques, and interpretations of the upper and lower extremities. Radiol Clin North Am. 2004;42:279–96. doi: 10.1016/j.rcl.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Donayre CE, White GH, Mehringer SM, et al. Pathogenesis determines late morbidity of axillosubclavian vein thrombosis. Am J Surg. 1986;152:179–84. doi: 10.1016/0002-9610(86)90238-2. [DOI] [PubMed] [Google Scholar]
- 30.AbuRahma AF, Robinson PA. Effort subclavian vein thrombosis: evolution of management. J Endovasc Ther. 2000;7:302–8. doi: 10.1177/152660280000700408. [DOI] [PubMed] [Google Scholar]
- 31.Urschel HC, Razzuk MA. Paget - Schroetter syndrome: What is the best management? Ann Thorac Surg. 2000;69:1663–9. doi: 10.1016/s0003-4975(00)01151-6. [DOI] [PubMed] [Google Scholar]
- 32.Sabeti S, Schillinger M, Mlekusch W, et al. Treatment of subclavian-axillary vein thrombosis: long-term outcome of anticoagulation versus systemic thrombolysis. Thromb Res. 2002;108:279–85. doi: 10.1016/s0049-3848(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 33.Grunwald MR, Hofmann LV. Comparison of urokinase, alteplase, and reteplase for catheter-directed thrombolysis of deep venous thrombosis. J Vasc Interv Radiol. 2004;15:347–52. doi: 10.1097/01.rvi.0000121407.46920.15. [DOI] [PubMed] [Google Scholar]
- 34.Melby SJ, Vedantham S, Narra VR, et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget-Schroetter syndrome) J Vasc Surg. 2008;47:809–20. doi: 10.1016/j.jvs.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 35.Doyle A, Wolford HY, Davies MG, et al. Management of effort thrombosis of the subclavian vein: today’s treatment. Ann Vasc Surg. 2007;21:723–9. doi: 10.1016/j.avsg.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Molina JE, Hunter DW, Dietz CA. Protocols for Paget-Schroetter syndrome and late treatment of chronic subclavian vein obstruction. Ann Thorac Surg. 2009;87:416–22. doi: 10.1016/j.athoracsur.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 37.Lee JT, Karwowski JK, Harris EJ, et al. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43:1236–43. doi: 10.1016/j.jvs.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Urschel HC, Jr, Patel AN. Paget Schroetter syndrome therapy: failure of intravenous stents. Ann Thorac Surg. 2003;75:1693–6. doi: 10.1016/s0003-4975(03)00116-4. [DOI] [PubMed] [Google Scholar]