Abstract
Background
Muscle architecture is known to be predictive of muscle function. However, it is unknown whether this relationship is similar in children and adolescents with and without cerebral palsy (CP).
Objective
The objective of this study was to determine whether the architecture of the rectus femoris (RF) and vastus lateralis (VL) muscles was predictive of maximum voluntary knee extensor torque in children and adolescents with and without CP and whether these measures were related to activity and participation levels.
Design
A case-control design was used.
Methods
Eighteen participants with CP (mean age=12.0 years, SD=3.2) at Gross Motor Function Classification System (GMFCS) levels I through IV and 12 age-matched peers with typical development (mean age=12.3 years, SD=3.9) were evaluated. Muscle thickness, fascicle length, and fascicle angle of the RF and VL muscles were measured with 2-dimensional, B-mode ultrasound imaging. The activity and participation measures used for participants with CP were the Pediatric Outcomes Data Collection Instrument (PODCI) and the Activities Scale for Kids, Performance Version (ASKp).
Results
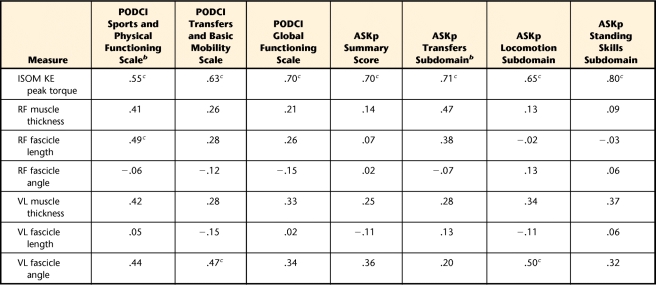
When age and GMFCS level were controlled for, VL muscle thickness was the best predictor of knee extensor isometric torque in the group with CP (R2=.85). This prediction was similar to the prediction from VL muscle thickness and age in participants with typical development (R2=.91). Rectus femoris muscle fascicle length was significantly correlated with the Sports and Physical Functioning Scale of the PODCI (ρ=.49), and VL muscle fascicle angle was correlated with the Transfers and Basic Mobility Scale of the PODCI (r=.47) and with ASKp Locomotion subdomain (r=.50).
Limitations
A limitation of this study was the small sample size.
Conclusions
Ultrasound measures of VL muscle thickness, adjusted for age and GMFCS level, were highly predictive of maximum torque and have the potential to serve as surrogate measures of voluntary strength (force-generating capacity) in children and adolescents with and without CP.
Cerebral palsy (CP) is a group of disorders of movement and posture, often characterized by impairments such as muscle weakness, spasticity (hypertonicity), stiffness, and excessive co-contraction.1 In children and adolescents with CP, exercise and resistance training are important components of rehabilitation programs for counteracting these impairments at the muscle level, thus improving muscle force output and efficiency.2–4 However, little is known about the mechanisms underlying these improvements. Muscle architecture is defined as the internal arrangement of muscle fibers within a muscle and has been described as the primary determinant of muscle function.5 Because the architecture of a muscle determines the force-velocity properties of the muscle, including outcome measures of muscle architecture is of clinical importance. Such measures include physiological and anatomical cross-sectional areas, muscle thickness, and length and angle of fascicles (bundles of muscle fibers). However, researchers are only beginning to understand the differences in muscle architecture in children and adolescents with CP and those with typical development (TD). Furthermore, information in the literature about how muscle architecture predicts muscle function in CP is scarce. Even more scarce are studies reporting on how muscle architecture predicts other impairments or activity levels in CP, which are important for informing clinical practice.
It is well documented in the literature that children and adolescents with CP have lower activity levels than their peers with TD.6,7 Whether primary weakness is the cause of decreased activity levels or whether decreased activity levels result in secondary weakness is unknown. Over time, the interaction of these 2 properties, in conjunction with abnormal movement patterns, may perpetuate a continuous cycle of inactivity and weakness, accompanied by disuse atrophy and alterations in muscle architecture.8 However, existing studies of muscle morphology in CP are cross-sectional and describe morphological characteristics in school-aged children with CP.9–11 Therefore, the possible role of muscle dysgenesis, or abnormal development of muscle architecture, in weakness, spasticity, and disuse is unknown. It has been shown that weight-bearing muscles, particularly the quadriceps and gastrocnemius muscles, are most affected by disuse and inactivity in humans because of their antigravity function.12,13 Therefore, it is possible that muscle architecture is altered in these same muscles in people with CP because of muscle disuse and low activity levels.
Most knowledge about muscle architecture in CP has been obtained from studies of the gastrocnemius muscles. Elder et al14 and Mohagheghi et al11 reported decreased anatomical cross-sectional area and thickness of the gastrocnemius muscles, respectively, on the paretic and nonparetic sides in children with hemiplegic CP. Shortland et al9 were the first investigators to use ultrasound imaging to evaluate muscle architecture in a small group of children with CP and a control group with TD. They observed no differences in fascicle angles and normalized fascicle lengths between the groups. However, other authors have reported differences in the fascicle angle15,16 and fascicle length11,15,16 of the gastrocnemius muscles in children with CP.
The quadriceps muscles are particularly important for transitioning from sitting to standing, ascending and descending stairs, and antigravity control during the stance phase of gait, among other functional activities.17,18 In people with CP, the strength (force-generating capacity) of the knee extensor muscles alone is significantly correlated with the standing, walking, running, and jumping components of the Gross Motor Function Measure and with the total Gross Motor Function Measure score.18,19 Primary weakness and secondary disuse of this muscle group, coupled with abnormal movement patterns, may lead to muscle atrophy and rearrangement of the internal muscle architecture, thus adversely affecting function. We previously reported measures of muscle architecture and the sizes of the vastus lateralis (VL) and rectus femoris (RF) muscles in a group of children and adolescents with CP and their peers with TD. We observed decreased cross-sectional areas and thicknesses of both muscles, as well as shorter fascicle lengths of the RF muscle and smaller fascicle angles of the VL muscle.20 Ohata et al10 reported decreased combined thickness of the RF and vastus intermedius muscles in adults with CP and severe motor involvement relative to those with moderate motor involvement. They suggested that the combined muscle thickness measure could be used as a surrogate measure of strength but did not provide any evidence to support this claim.
A surrogate measure of strength in CP would be highly beneficial because of the difficulty in obtaining quantitative measures of voluntary maximum force generation in children with selective motor control deficits resulting in a decreased or an absent ability to isolate movements, as well as in those with cognitive, hearing, visual, behavioral, or other impairments related to the ability to receive and communicate information.1 In healthy muscle, muscle architecture determines the force-velocity properties of that muscle. Three major principles should be considered. First, the number of sarcomeres in parallel, reflected by the size of the muscle, is directly proportional to the amount of force that the muscle can produce. Second, the number of sarcomeres in series, reflected by the length of the muscle fibers, is directly related to the maximum shortening velocity and the excursion of the muscle. Third, the angle of pennation of the muscle fibers is inversely related to force and shortening velocity, so that the greater the pennation angle, the lesser the force that is transmitted to the tendon and the lesser the shortening velocity.21 However, the effect on force production usually is offset by the fact that a larger angle of pennation allows more contractile material to be attached within a given volume.22 Because intact fibers are difficult to isolate, fascicles are typically measured in studies of human muscle.5
Therefore, the overall purpose of this study was to investigate the critical relationships between muscle architecture and muscle function (body structures and function) and the relationships between muscle architecture and activity and participation, as outlined in the World Health Organization's International Classification of Functioning, Disability and Health (ICF) model.23 More specifically, the first aim was to develop a predictive regression model of maximum voluntary knee extensor torque in children and adolescents with and without CP on the basis of measures of the thicknesses of the RF and VL muscles obtained from 2-dimensional (2D) ultrasound imaging. We hypothesized that the physiological relationships of muscle thickness and maximum voluntary knee extensor torque would be similar in participants with and without CP and across muscle groups. Although physiological and anatomical cross-sectional areas are more commonly used in predictive models for maximum force-generating capacity, measures of muscle thickness also have been used in predictive models for maximum force and have been shown to be significantly correlated with anatomical cross-sectional area.24,25 In addition, muscle thickness can be measured easily and relatively quickly with 2D B-mode ultrasound; in contrast, magnetic resonance imaging and computerized tomography are more costly and time-consuming. Furthermore, the added value of including other muscle architectural variables known to influence force production, such as fascicle angle and fascicle length, was investigated.
The second aim was to quantify the relationships between muscle architectural parameters and measures of activity and participation in participants with CP. We hypothesized that fascicle length measurements would show the strongest relationships with higher-level activity scales that would be more likely to involve speed and power, whereas muscle thickness and fascicle angle measurements would be more closely related to overall global function, activity, and participation.
The clinical importance of this study is threefold: (1) to provide insight into the relationship between muscle architecture and weakness in CP, (2) to propose a surrogate measure of strength (based on ultrasound imaging) that could be used for better characterization of patients and as an outcome measure in intervention studies, and (3) to elucidate the relationships between muscle architecture and activity and participation to better interpret how a change in muscle architecture in response to an intervention (therapeutic, surgical, pharmacological, or other) would affect activity and participation in daily life, as emphasized by the World Health Organization's ICF model.23 A clinical example would be the use of VL muscle thickness to estimate strength in a child with CP who has cognitive impairments and is undergoing orthopedic surgery for crouch gait. This measure could be used for preoperative surgical planning, to develop a postoperative physical therapy plan of care, as an outcome measure to assess changes in strength in response to the surgical and therapeutic interventions, and to predict ambulatory outcome.
Method
Participants
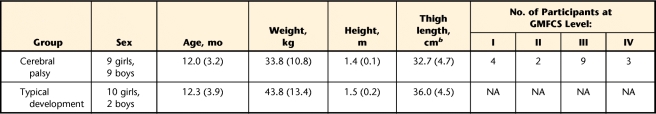
Eighteen young people with spastic CP (mean age=12.0 years, SD=3.2, range=7–19) and 12 young people with TD (mean age=12.3 years, SD=3.9, range=7–20) were recruited for this prospective study. Participants with CP were recruited from a local hospital and an outpatient rehabilitation center. Table 1 shows the characteristics of the participants. Participants with CP were at Gross Motor Function Classification System (GMFCS) levels I through IV and had bilateral lower-extremity involvement. Exclusion criteria were orthopedic or neurological surgery within 6 months before testing or botulinum toxin injections to the quadriceps muscle within 3 months before testing. In addition, participants were excluded if they had cognitive or other behavioral impairments that interfered with the ability to understand and follow directions. Written informed consent was obtained from each participant over 18 years of age, and parental consent was obtained for those under 18 years of age.
Table 1.
Participant Characteristicsa
Reported as mean (SD). GMFCS=Gross Motor Function Classification System, NA=not applicable.
b Calculated as distance from greater trochanter to lateral femoral condyle.
Procedure
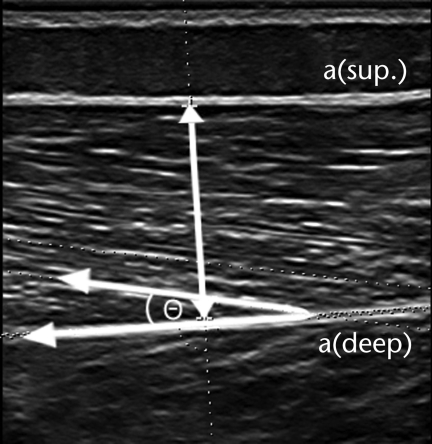
Ultrasound (Voluson 730 Expert*) images of the RF and VL muscles were recorded in real time with a 6- to 12-MHz linear-array transducer in 2D B-mode. Imaging was performed prior to strength assessment, and the right lower extremity was chosen for measurement. Participants rested comfortably in the supine position with the knee joint near the natural resting position of 10 degrees; a towel roll was placed under the knee as needed for positioning or to aid comfort and muscle relaxation. Participants were supine for approximately 10 to 15 minutes prior to imaging. The resting angle of the knee was measured and recorded with a goniometer. Participants were instructed to relax their muscles during scanning. Images were recorded only when the muscle was fully relaxed, as evidenced in real time. Images of the RF muscle were taken at 50% of the distance between the anterior superior iliac spine and the superior border of the patella. Images of the VL muscle were taken at the midpoint between the most prominent portion of the greater trochanter and the lateral femoral epicondyle. To ensure proper placement of the probe, each midpoint was clearly marked on the skin with a surgical pen. To eliminate compression of the muscle, a generous amount of gel was applied to the skin, and the examiner held the probe suspended on top of the gel with the forearm supported. Images were taken with the probe oriented in the sagittal plane and perpendicular to the skin, and the following measurements were calculated with the ultrasound system software (Fig. 1): muscle thickness was calculated as the distance between the superficial and the deep aponeuroses in the middle of the ultrasound image at a 90-degree angle from the deep aponeurosis, fascicle angle was measured as the positive angle in degrees between the deep aponeurosis and the line of the fascicle, and fascicle length was estimated as muscle thickness/sin(fascicle angle).9 Three images were taken per muscle, and the average value from the 3 images for each measurement was used in the analyses. Intrasession reliability was previously reported to be high for all measurements obtained with this protocol (for the CP group, the intraclass correlation coefficient was ≥.93; for the TD group, the intraclass correlation coefficient was ≥.88), with particular emphasis on RF and VL muscle thickness (intraclass correlation coefficient of ≥.98) for both groups.20
Figure 1.
Muscle thickness was calculated as the distance between the superficial aponeurosis [a(sup.)] and the deep aponeurosis [a(deep)] in the middle of the ultrasound image at a 90-degree angle from the deep aponeurosis, as indicated by the double-headed arrow. The fascicle angle was the positive angle between the deep aponeurosis and the line of the fascicle, as indicated by theta (θ) and corresponding arrows.
Maximum voluntary knee extensor torque was tested isometrically at a knee angle of 60 degrees of flexion with an isokinetic dynamometer (System 3 Pro†). The 60-degree position was chosen because it approximates the optimal point on the length-tension curve for generating force.26 The peak isometric torque of the highest of 3 repetitions was used as the measure of strength, or maximum force-generating capacity.
The Pediatric Outcomes Data Collection Instrument (PODCI) parent report and the Activities Scale for Kids (ASK), Performance Version (ASKp), were administered as measures of activity and participation. The PODCI was designed to assess self-reported physical function and psychosocial aspects of health status in children with mild to moderate musculoskeletal disability.27 The following scales, with scores ranging from 0 (worst) to 100 (best), were used for analysis: Transfers and Basic Mobility Scale, Sports and Physical Functioning Scale, and Global Functioning Scale, which includes the preceding 2 scales in addition to an Upper-Extremity Scale and a Pain and Comfort Scale. The PODCI has been widely administered in children with CP; it has high internal consistency,27,28 moderate to good test-retest reliability,27 moderate to excellent concurrent validity with the Gross Motor Function Measure,28 and the ability to discriminate across motor groups for certain domains28 and is responsive to change after orthopedic surgery.29 The ASK is a child self-report questionnaire that assesses physical functioning in the community for children with musculoskeletal disorders, including CP.30 The ASK (ASKp and ASK capacity version) has excellent test-retest and interrater reliability and good validity (content, concurrent, and construct) in children who are 5 to 15 years of age and have musculoskeletal disorders.30,31 The ASKp measures what the child “did do” during the previous week and serves as a measure of activity and participation under the performance qualifier of the ICF model. In addition, the ASKp has been shown to discriminate among GMFCS levels in adolescents with CP across all subdomains.32 In addition to the overall summary score, the following subdomains of the ASKp were used for analysis because they are related to lower-extremity functioning: Locomotion, Standing Skills, and Transfers.
Data Analysis
For the first aim, which was to develop a predictive model for knee extensor force-generating capacity, hand-fitted univariate multiple regression with a conceptual theoretical model was used to determine the predictive power of RF and VL muscle thickness for the response variable (knee extensor torque) in the CP and TD groups separately. Control variables (age, height, thigh length, and weight for both groups and GMFCS level for the CP group) and muscle architectural variables (fascicle length and fascicle angle) were entered individually into the model to determine which variables improved the predictive value of the model. This strategy was based on the theoretical perspective that these variables could have an additional or competing influence on torque values. If the addition of a control variable resulted in a P value of less than .15 and changed the beta coefficient of the variable of interest significantly, the variable was kept in the model and the next variable was entered. This process resulted in an increase in the R2 value, with the R2 and adjusted R2 values moving closer together. The significance level for the overall regression model was set at .05. This process was continued until the final, best model was determined for the CP and TD groups separately. There were no missing data in the multiple regression analyses; missing data can significantly alter the results.
For the first aim, to achieve a power of .80 and a medium to large effect size (Cohen f2=.15 and .35), a sample size of between 25 and 55 was predicted to produce a significant model. We performed post hoc power analyses33 for the predictive models for the TD and CP groups (Tab. 2). We obtained greater than 99% power for the predictive model for the TD group with 2 variables (and n=12) and greater than 99% power for the predictive model for the CP group with 3 variables (and n=18). Thus, our sample size appeared to be adequate for the model.
Table 2.
Multiple Linear Regression Analyses of the Prediction of Knee Extensor Muscle Strength From Vastus Lateralis (VL) Muscle Thickness for Children With Cerebral Palsy (CP) and Children With Typical Development (TD)
a GMFCS=Gross Motor Function Classification System.
For the second aim, Pearson correlation coefficients were calculated to determine the relationships between muscle architectural variables and functional measures of activity and participation (ASKp and PODCI). Spearman rho correlation coefficients (ρ) were calculated for the scales that were not normally distributed. Missing data were removed from the correlation analyses; the numbers of observations are reported in the appropriate tables. The significance level was set at .05. All data were analyzed with SAS (version 9.2).‡
Role of the Funding Source
Dr Moreau was a postdoctoral fellow in the Movement Science Program at Washington University, St Louis, Missouri, when this study was conducted and was supported by a National Center for Medical Rehabilitation Research/National Institutes of Health grant (T32HD007434-16). This project also was supported by a clinical research grant from the Section on Pediatrics of the American Physical Therapy Association. This research was supported, in part, by the Intramural Research Program of the NIH Clinical Center.
Results
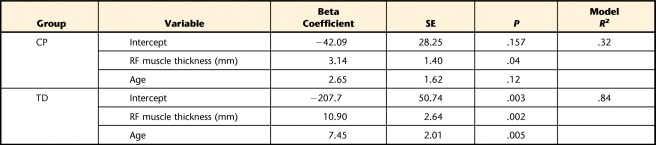
Height, thigh length, fascicle angle, and fascicle length for both the RF and the VL muscles were not significant contributors to the knee extensor torque predictive model for either group. As expected from the literature, weight competed for variance with muscle thickness and reduced the predictive power of the model. Combining VL muscle thickness and RF muscle thickness into the same regression model also diminished the predictive power of either one alone because of the high correlation between the 2 variables. However, age was a significant control variable for both the TD and the CP groups, significantly improving the predictive power of the model over that obtained with either RF muscle thickness or VL muscle thickness.
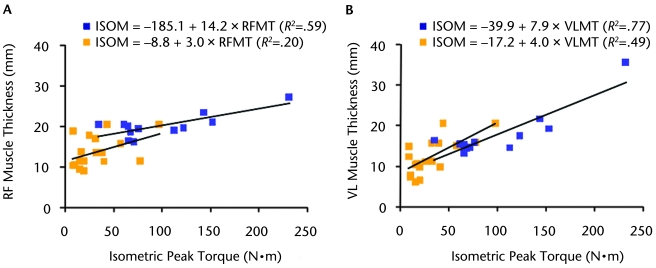
As shown in Figure 2, RF muscle thickness and VL muscle thickness alone explained 59% and 77% of the variance in knee extensor torque for the TD group and 20% and 49% of the variance for the CP group, respectively. When age was controlled for, VL muscle thickness had the highest explanatory power for both the TD and the CP groups, explaining 91% and 62% of the variance in knee extensor torque, respectively (Tab. 2). When age was controlled for, RF muscle thickness explained 84% and 32% of the variance for the TD and CP groups, respectively (Tab. 3).
Figure 2.
Linear regression of isometric knee extensor peak torque and rectus femoris (RF) muscle thickness (A) and vastus lateralis (VL) muscle thickness (B) in both participants with cerebral palsy (CP) (gold squares) and participants with typical development (blue squares). All relationships were statistically significant, except for the relationship between RF muscle thickness and knee extensor torque in the CP group. In the regression equations, ISOM=isometric knee extensor peak torque (N·m), RFMT=RF muscle thickness (millimeters), and VLMT=VL muscle thickness (millimeters).
Table 3.
Multiple Linear Regression Analyses of the Prediction of Knee Extensor Muscle Strength From Rectus Femoris (RF) Muscle Thickness for Children With Cerebral Palsy (CP) and Children With Typical Development (TD)
We added GMFCS levels to the regression model for the CP group to determine the effects of different GMFCS levels (Tab. 2). When both age and GMFCS levels were controlled for, VL muscle thickness predicted 85% of the variance in knee extensor torque.
Table 4 shows the correlation coefficients for the relationships between muscle architecture and functional measures of activity and participation. The fascicle angle of the VL muscle was significantly correlated with the Transfers and Basic Mobility Scale of the PODCI (r=.47, P=.05) and with the ASKp Locomotion subdomain (r=.50, P=.03). As hypothesized, the fascicle length of the RF muscle was significantly correlated with the Sports and Physical Functioning Scale of the PODCI (ρ=.49, P=.04). The correlation of RF muscle thickness with the ASKp Transfers subdomain approached significance (ρ=.47, P=.06). Knee extensor torque was directly correlated with all of the measures of activity and participation (r=.55–.80).
Table 4.
Correlation Coefficients for Measures of Muscle Architecture and Size and Measures of Activity and Participationa
PODCI=Pediatric Outcomes Data Collection Instrument; ASKp=Activities Scale for Kids, Performance Version; ISOM KE torque=isometric knee extensor peak torque (N·m); RF=rectus femoris; VL=vastus lateralis. n=18 for all variables except ASKp Transfers subdomain (n=16) and ASKp Standing Skills subdomain (n=17) because of missing data. Coefficients are Pearson correlation coefficients (r), unless otherwise indicated.
b Spearman rho correlation coefficient (ρ) because of a nonnormal distribution.
c P<.05.
Discussion
The main finding of the present study was that VL muscle thickness was the best predictor of maximum voluntary knee extensor torque for both the TD and the CP groups when age was controlled for. The regression model for the TD group had higher explanatory power than that for the CP group, explaining 91% and 62% of the variance, respectively. However, when GMFCS levels were added to the CP model, the explanatory power of the model increased to 85%.
The clinical implications of the utility of this model are noteworthy. It is not always possible to obtain accurate, quantitative measures of voluntary strength in people with CP because motor impairments often are accompanied by cognitive, hearing, visual, communicative, or behavioral impairments.1 These impairments can occur across GMFCS levels and can result in a diminished ability to accurately or successfully administer strength assessments. In addition, poor selective motor control often results in the inability to isolate the muscle group of interest for an isometric contraction or to isolate the joint movement of interest for a dynamic contraction, resulting in mass patterned movements. The method used to measure muscle architecture also has considerable relevance for physical therapist practice. Ultrasound is less expensive, more portable, and more accessible than magnetic resonance imaging and computerized tomography and could easily be implemented into a rehabilitation setting with proper training. The results of the present study indicate that a simple, 2D ultrasound measure of VL muscle thickness taken at 50% of thigh length, along with the age of the child and the GMFCS level, is highly predictive of knee extensor torque and may have value as a substitute for a voluntary strength assessment. Furthermore, the significant relationship between VL muscle thickness and knee extensor torque supports the use of this measure as an outcome tool for the assessment of muscle size changes in response to an intervention.
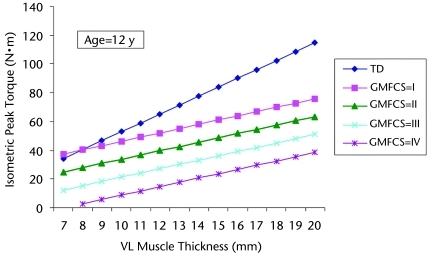
Figure 3 shows the prediction of maximum voluntary knee extensor torque from various VL muscle thickness values for a representative 12-year-old child with CP at GMFCS levels I through IV and for a representative 12-year-old child with TD by use of the regression equations from Table 2. The slope was greater for the child with TD, so that at higher muscle thickness values, the child with TD produced higher levels of extensor torque than the child with CP at all GMFCS levels. Although the slopes were the same at all GMFCS levels for the 12-year-old child with CP, the torque values decreased as impairment levels increased (ie, higher GMFCS levels). The difference in the predictive power of the muscle thickness model for the CP and TD groups could be attributed to several potential factors. Voluntary activation of the quadriceps muscle group was previously reported to be 33% lower in children with CP than in children with TD.34 In addition, co-contraction of the hamstring muscles during isometric knee extension was observed to decrease net torque by 12% in the CP group but only 5% in the TD group, with the effect diminishing with increasing knee flexion angles.35 Furthermore, Elder et al14 measured specific tension, cross-sectional area, and muscle volume with magnetic resonance imaging for the gastrocnemius-soleus muscle complex in children with CP and children with TD (controls). They concluded that the ability to produce levels of torque consistent with cross-sectional area was impaired in the children with CP, a finding that could partially explain our results. Kanehisa and colleagues36,37 reported that children generally produced less force per unit of cross-sectional area than adults and that this ability increased throughout adolescence.
Figure 3.
Prediction of isometric knee extensor peak torque from various vastus lateralis (VL) muscle thickness values for a representative 12-year-old child with cerebral palsy (CP) at Gross Motor Function Classification System (GMFCS) levels I through IV and for a representative 12-year-old child with typical development (TD). The equations are based on the VL muscle thickness predictive equations in Table 2 for the CP and TD groups; ISOM=isometric knee extensor peak torque (N·m), VLMT=VL muscle thickness (millimeters). For the CP group: ISOM=−7.6 + (3.0 × VLMT) + (3.0 × age) − (12.5 × GMFCS); for the TD group: ISOM=−79.7 + (6.2 × VLMT) + (5.9 × age).
Although the force-generating capacity of a muscle should be related to its cross-sectional area, literature reports of predictive relationships between muscle size and strength in children and adolescents are limited. Kanehisa et al36 reported that cross-sectional area multiplied by thigh length was a significant predictor of isokinetic knee extensor muscle strength in children between 6 and 9 years of age (r=.71–.83). Our results were similar in participants with TD, with correlation coefficients of .77 and .88 for the RF and VL muscles, respectively, and for the VL muscle in participants with CP (r=.70). Predictive models based on anatomical cross-sectional area and muscle strength are more prevalent for adults.24,25,38 Muscle thickness was observed to be highly correlated with cross-sectional area in adults,24,25 was reported to be a better predictor of isometric elbow flexor muscle force than cross-sectional area,25 and was used as an accurate predictor of muscle volume.39 Our unpublished data showed similar correlations between VL muscle thickness and extensor torque (r=.70) and between RF muscle cross-sectional area and strength (r=.65) in children and adolescents with CP. Collectively, these data support the use of muscle thickness measures as potential predictors of knee extensor torque in children and adolescents with CP and without CP.
A clinically meaningful observation was the significant relationship between RF fascicle length and the Sports and Physical Functioning Scale of the PODCI. This relationship was hypothesized because of the direct physiological relationship of fiber length with shortening velocity and excursion. Interestingly, we previously reported shorter RF fascicle lengths in participants with CP than in participants with TD.20 Therefore, decreased fascicle lengths may have direct functional implications in that they are related to lower activity levels and reduced participation in higher-level sports and activities requiring speed and power.
Also of clinical relevance was the finding that the VL fascicle angle was significantly correlated with the Transfers and Basic Mobility Scale of the PODCI and with the ASKp Locomotion subdomain. We also previously compared VL fascicle angles in children and adolescents with CP and TD.20 Our findings had functional implications in that the decreased fascicle angles reported for participants with CP could have been a cause or a consequence of lower levels of mobility and locomotion. Our observations were not surprising because larger fascicle angles were reported to be associated with stronger muscles and muscles undergoing hypertrophy.22 The underlying rationale is that a larger angle of pennation allows more muscle fibers to be packed within a given space.
The VL fascicle angle was not correlated with the ASKp Transfers subdomain. There are 2 possible explanations for this finding. First, the ASKp Transfers subdomain was not normally distributed and was analyzed with the Spearman rho correlation coefficient (Tab. 4). Second, the ASKp Transfers subdomain included only 5 items related specifically to transfers (ie, getting in and out of an automobile, a chair, and a bed; getting down to the ground and back up; and sitting on the floor), whereas the PODCI included 11 items related to both transfers and basic mobility. In addition to items related to transferring in and out of bed or on and off the toilet, it also posed questions associated with mobility and even gait. Some of these questions were related to the scoring of the difficulty level for certain tasks, such as putting on a coat, walking 1 block, climbing a flight of stairs, and sitting without holding on. Thus, the PODCI is more encompassing of overall mobility than transfers only.
Despite the fact that knee extensor muscle strength was correlated with all of the measures of activity and participation, muscle thickness was not significantly associated with any of the measures. However, some of the measures approached significance, with a correlation coefficient of .42 (P=.08) for the relationship between the PODCI Sports and Physical Functioning Scale and VL muscle thickness and a correlation coefficient of .41 (P=.09) for the relationship between this scale and RF muscle thickness. Furthermore, the relationship between RF muscle thickness and the ASKp Transfers subdomain approached statistical significance (r=.47, P=.06). Ohata et al40 observed a statistically significant relationship between quadriceps femoris muscle thickness and scores on the 66-item Gross Motor Function Measure and the Pediatric Evaluation of Disability Inventory. It is possible that the combined measurement of the RF and vastus intermedius muscles, as performed in their study, is more predictive of activity and participation than measurement of the RF muscle or the VL muscle alone. Our data suggest that the direct measurement of knee extensor muscle strength produces the strongest associations with activity and participation.
Although the present study provides useful, clinically relevant information that can be directly applied in a clinical setting, it does have some limitations. There are limitations in the use of VL muscle thickness as a surrogate measure of strength. For example, if VL muscle thickness were used as an outcome measure for an intervention, only muscle adaptations would be accounted for. Therefore, strength gains due solely to neural adaptations would not be reflected in the muscle architecture (for a review on neural adaptations, see Sale41). In addition, although the PODCI and the ASKp are both reliable and valid self-report measures of activity and participation, a limitation is that we did not use direct measures of activity and participation. Another limitation is that our sample size was not adequate to statistically compare slopes between the CP group and the TD group. However, the primary aim of the present study was to determine a predictive model for torque generation from muscle thickness in children and adolescents with CP rather than to compare the groups. Finally, a causal relationship cannot be determined from the data; that is, which came first: muscle weakness or muscle architecture adaptations?
In summary, VL muscle thickness was the best predictor of maximum voluntary knee extensor torque in our participants with CP as well as those with TD. When age and GMFCS level were controlled for, VL muscle thickness explained 85% of the variance in extensor torque in participants with CP, similar to the prediction from VL muscle thickness and age in participants with TD (R2=.91). This predictive equation has clinical applicability because of the ease of 2D real-time ultrasound measurement, especially when strength testing is not feasible for a population. Thus, our results show promise for the use of VL muscle thickness as a surrogate measure of strength in children and adolescents with CP and as an evaluative tool for quantifying changes in muscle size in response to an intervention, such as strength training, serial casting, and even surgery. Furthermore, the relationships between RF fascicle length and sports and physical functioning and between VL fascicle angle and mobility were consistent with muscle architecture principles. An understanding of these relationships is important for determining the most effective type of exercise or resistance training for targeting specific muscle architecture parameters with the aims of influencing muscle force production and increasing activity and participation.
The Bottom Line
What do we already know about this topic?
In individuals without impairments, the force-velocity properties of a muscle are determined by the architecture of that muscle. However, it is unknown whether these relationships are similar in people with cerebral palsy.
What new information does this study offer?
Vastus lateralis muscle thickness was highly predictive of knee extensor strength in people with cerebral palsy, particularly when age and Gross Motor Function Classification System level were considered. Rectus femoris fascicle length was related to sports and physical functioning, and vastus lateralis fascicle angle was related to locomotion, transfers, and basic mobility.
If you're a patient, what might these findings mean for you?
The study proposes an alternative measure of strength using 2-dimensional ultrasound imaging that can be used when strength testing is not feasible in people with cerebral palsy. The study also provides a better understanding of how a change in muscle architecture in response to an intervention (such as a therapy, surgery, or medication) would affect muscle strength, activity, and participation in daily life.
Footnotes
Dr Moreau, Dr Teefey, and Dr Damiano provided concept/idea/research design. Dr Moreau and Dr Simpson provided writing. Dr Moreau provided data collection. Dr Moreau, Dr Simpson, and Dr Damiano provided data analysis. Dr Moreau provided project management and fund procurement. Dr Teefey and Dr Damiano provided facilities/equipment. Dr Teefey provided institutional liaisons. Dr Simpson and Dr Damiano provided consultation (including review of manuscript before submission).
The authors thank Chris Stanley for assistance with data collection and processing and Dr Janice Brunstrom-Hernandez and Jennifer Miros, PT, MPT, for their support and assistance with recruitment. They thank GE Healthcare and Tania Gordley, Applications Specialist for GE Healthcare Ultrasound, for their support and technical assistance with this project.
The study was approved by the Institutional Review Board at Washington University in St Louis, Missouri.
Portions of this article were presented at the 62nd Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine; September 17–20, 2008; Atlanta, Georgia; and the 2009 Combined Sections Meeting of the American Physical Therapy Association; February 9–12, 2009; Las Vegas, Nevada; and published in the proceedings in abstract format.
Dr Moreau was a postdoctoral fellow in the Movement Science Program at Washington University, St Louis, Missouri, when this study was conducted and was supported by a National Center for Medical Rehabilitation Research/National Institutes of Health grant (T32HD007434-16). This project also was supported by a clinical research grant from the Section on Pediatrics of the American Physical Therapy Association. This research was supported, in part, by the Intramural Research Program of the NIH Clinical Center.
GE Healthcare, Kretztechnik, Zipf, Austria.
Biodex Medical Systems, 20 Ramsay Rd, Shirley, NY 11967-4704.
SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513-2414.
References
- 1.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–576 [DOI] [PubMed] [Google Scholar]
- 2.Damiano DL, Abel MF. Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–125 [DOI] [PubMed] [Google Scholar]
- 3.Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol. 2003;45:652–657 [DOI] [PubMed] [Google Scholar]
- 4.Verschuren O, Ketelaar M, Gorter JW, et al. Exercise training program in children and adolescents with cerebral palsy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2007;161:1075–1081 [DOI] [PubMed] [Google Scholar]
- 5.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg-Emons RJ, van Baak MA, de Barbanson DC, et al. Reliability of tests to determine peak aerobic power, anaerobic power and isokinetic muscle strength in children with spastic cerebral palsy. Dev Med Child Neurol. 1996;38:1117–1125 [DOI] [PubMed] [Google Scholar]
- 7.Bjornson KF, Belza B, Kartin D, et al. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–1540 [DOI] [PubMed] [Google Scholar]
- 9.Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol. 2002;44:158–163 [DOI] [PubMed] [Google Scholar]
- 10.Ohata K, Tsuboyama T, Ichihashi N, Minami S. Measurement of muscle thickness as quantitative muscle evaluation for adults with severe cerebral palsy. Phys Ther. 2006;86:1231–1239 [DOI] [PubMed] [Google Scholar]
- 11.Mohagheghi AA, Khan T, Meadows TH, et al. Differences in gastrocnemius muscle architecture between the paretic and non-paretic legs in children with hemiplegic cerebral palsy. Clin Biomech (Bristol, Avon). 2007;22:718–724 [DOI] [PubMed] [Google Scholar]
- 12.de Boer MD, Maganaris CN, Seynnes OR, et al. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer MD, Seynnes OR, di Prampero PE, et al. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol. 2008;104:401–407 [DOI] [PubMed] [Google Scholar]
- 14.Elder GC, Kirk J, Stewart G, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–550 [DOI] [PubMed] [Google Scholar]
- 15.Mohagheghi AA, Khan T, Meadows TH, et al. In vivo gastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev Med Child Neurol. 2008;50:44–50 [DOI] [PubMed] [Google Scholar]
- 16.Cheatwood AP. Changes in Medial Gastrocnemius Architecture With Spasticity and Contracture [doctoral dissertation] Los Angeles, CA: University of Southern California; 1995 [Google Scholar]
- 17.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23:1083–1090 [DOI] [PubMed] [Google Scholar]
- 18.Damiano DL, Quinlivan J, Owen BF, et al. Spasticity versus strength in cerebral palsy: relationships among involuntary resistance, voluntary torque, and motor function. Eur J Neurol. 2001;8(suppl 5):40–49 [DOI] [PubMed] [Google Scholar]
- 19.Goh HT, Thompson M, Huang WB, Schafer S. Relationships among measures of knee musculoskeletal impairments, gross motor function, and walking efficiency in children with cerebral palsy. Pediatr Phys Ther. 2006;18:253–261 [DOI] [PubMed] [Google Scholar]
- 20.Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2009;51:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;October:275–283 [PubMed] [Google Scholar]
- 22.Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–2744 [DOI] [PubMed] [Google Scholar]
- 23.International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 24.Sipila S, Suominen H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve. 1991;14:527–533 [DOI] [PubMed] [Google Scholar]
- 25.Akagi R, Kanehisa H, Kawakami Y, Fukunaga T. Establishing a new index of muscle cross-sectional area and its relationship with isometric muscle strength. J Strength Cond Res. 2008;22:82–87 [DOI] [PubMed] [Google Scholar]
- 26.Kues JM, Rothstein JM, Lamb RL. The relationships among knee extensor torques produced during maximal voluntary contractions under various test conditions. Phys Ther. 1994;74:674–683 [DOI] [PubMed] [Google Scholar]
- 27.Daltroy LH, Liang MH, Fossel AH, Goldberg MJ; Pediatric Outcomes Instrument Development Group, Pediatric Orthopaedic Society of North America The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. J Pediatr Orthop. 1998;18:561–571 [DOI] [PubMed] [Google Scholar]
- 28.McCarthy ML, Silberstein CE, Atkins EA, et al. Comparing reliability and validity of pediatric instruments for measuring health and well-being of children with spastic cerebral palsy. Dev Med Child Neurol. 2002;44:468–476 [DOI] [PubMed] [Google Scholar]
- 29.Damiano DL, Gilgannon MD, Abel MF. Responsiveness and uniqueness of the Pediatric Outcomes Data Collection Instrument compared to the Gross Motor Function Measure for measuring orthopaedic and neurosurgical outcomes in cerebral palsy. J Pediatr Orthop. 2005;25:641–645 [DOI] [PubMed] [Google Scholar]
- 30.Young NL, Yoshida KK, Williams JI, et al. The role of children in reporting their physical disability. Arch Phys Med Rehabil. 1995;76:913–918 [DOI] [PubMed] [Google Scholar]
- 31.Young NL, Williams JI, Yoshida KK, Wright JG. Measurement properties of the Activities Scale for Kids. J Clin Epidemiol. 2000;53:125–137 [DOI] [PubMed] [Google Scholar]
- 32.Palisano RJ, Copeland WP, Galuppi BE. Performance of physical activities by adolescents with cerebral palsy. Phys Ther. 2007;87:77–87 [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed.Hillsdale, NJ: Lawrence Erlbaum Associates; 1988 [Google Scholar]
- 34.Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31:594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda AJ, Abel MF, Granata KP, Damiano DL. Quantification of co-contraction in spastic cerebral palsy. Electromyogr Clin Neurophysiol. 1998;38:497–504 [PubMed] [Google Scholar]
- 36.Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional area of knee extensor muscles in children. Eur J Appl Physiol Occup Physiol. 1994;68:402–405 [DOI] [PubMed] [Google Scholar]
- 37.Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional areas of reciprocal muscle groups in the upper arm and thigh during adolescence. Int J Sports Med. 1995;16:54–60 [DOI] [PubMed] [Google Scholar]
- 38.Kanehisa H, Ikegawa S, Fukunaga T. Comparison of muscle cross-sectional area and strength between untrained women and men. Eur J Appl Physiol Occup Physiol. 1994;68:148–154 [DOI] [PubMed] [Google Scholar]
- 39.Miyatani M, Kanehisa H, Kuno S, et al. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur J Appl Physiol. 2002;86:203–208 [DOI] [PubMed] [Google Scholar]
- 40.Ohata K, Tsuboyama T, Haruta T, et al. Relation between muscle thickness, spasticity, and activity limitations in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2008;50:152–156 [DOI] [PubMed] [Google Scholar]
- 41.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20:S135–S145 [DOI] [PubMed] [Google Scholar]