Abstract
Background and Purpose
The impact of cancer and its treatments on balance and functional mobility in older adults remains unknown but is increasingly important, given the evolution of cancer treatments. Subacute and more persistent side effects such as chemotherapy-induced peripheral neuropathy are on the rise, and the effects on mobility and balance, as well as the prognosis for resolution of any functional deficits, must be established before interventions can be trialed. The purpose of this case report is to describe the severity and long-term persistence of mobility decline in an older adult who received neurotoxic chemotherapy. To our knowledge, this is the first case report to describe an older adult with chemotherapy-induced peripheral neuropathy using results of standardized balance and mobility tests and to focus on prognosis by repeating these measures more than 2 years after chemotherapy.
Case Description
An 81-year-old woman received a neurotoxic agent (paclitaxel) after curative mastectomy for breast cancer. Baseline testing prior to taxane therapy revealed a socially active woman with no reported functional deficits or neuropathic symptoms, 1.2-m/s gait speed, and performance at the ceiling on balance and gait portions of a standardized mobility measure.
Outcomes
After 3 cycles, paclitaxel therapy was stopped by the oncologist because of neurotoxicity. Declines as large as 50% were seen in performance-based measures at 12 weeks and persisted at 2.5 years, and the patient reported recurrent falls, cane use, and mobility-related disability.
Discussion
This case highlights the extent to which function can decline in an older individual receiving neurotoxic chemotherapy, the potential for these deficits to persist years after treatment is stopped, and the need for physical therapy intervention and further research in this population.
Little is known about the impact of cancer and its treatments on balance and functional mobility in older adults for at least 2 reasons. First, although more than 65% of survivors of cancer are over the age of 65 years, older adults often are excluded from cancer research. Exclusions can be based on age alone or on the comorbid conditions that often accompany increasing age.1 Second, few studies to date have included mobility and balance as primary outcomes.2
Studying balance and mobility in older adults with cancer is important, given the evolution of cancer treatments in recent years. Success of supplemental agents has reduced the incidence of dose-limiting hematologic toxicities such as infection and bleeding, allowing higher chemotherapy doses to be administered.3 As a result, subacute and more persistent side effects such as chemotherapy-induced peripheral neuropathy (CIPN) are on the rise.4–7
Much is known about the risks for and mechanisms behind CIPN. Like diabetic polyneuropathy, CIPN tends to produce a distal, symmetrical axonopathy or neuronopathy.5–7 The result is most often a stocking and glove sensory loss that affects the lower extremities before the upper extremities. Although primarily sensory in nature, mixed sensorimotor (usually distal myopathy) and even autonomic neuropathies (including orthostatic intolerance) are possible,5,6 and any of these neuropathies could increase fall risk. Chemotherapy-induced peripheral neuropathy is dose dependent and worse when neurotoxic drugs are administered in combination or in succession.4–8 Symptoms can progress, or even begin, after treatment with the causative agent has ended, in a phenomenon termed “coasting.”6,9,10 The chemotherapies most likely to result in neuropathy include taxane derivatives (paclitaxel, docetaxel), vinca alkaloids (vincristine), platinum complexes (cisplatin, oxaliplatin), and newer agents (thalidomide, suramin).4–7,9,10 These drugs are widely used to treat the most common cancers, including breast and lung, which predominate in older adults.
Chemotherapy-induced peripheral neuropathy is more likely to occur in individuals with baseline peripheral neuropathy,11 and the prevalence of peripheral sensory neuropathy was found to increase with age in a cohort of nearly 800 community-dwelling adults aged 65 years and older.12 Mold et al12 identified neuropathy in 26% to 54% of these older adults, many of whom were unaware of these deficits and reported not having neurodegenerative pathologies such as diabetes. Unfortunately, most studies of CIPN exclude individuals with findings of baseline peripheral neuropathy or potentially neurotoxic systemic diseases.4,13–15 As a result, these studies likely underestimate the impact of neurotoxic chemotherapies on the larger population of older adults seen clinically. Still, the risk for and severity of CIPN appear to increase with advancing age,4 and neuroprotective pharmacologic agents have had limited success.16
Although CIPN is common, little is known about its effects on mobility or the time course for resolution of any functional deficits, particularly in older adults. Neuropathy reportedly occurs in as many as 70% to 95% of individuals receiving taxane chemotherapy,4,7,17 and there is evidence that it persists after treatment, particularly after paclitaxel therapy.8 Signs and symptoms of chronic neurotoxicity after oxaliplatin therapy have been reported to partially resolve in most patients within 4 to 6 months and to completely resolve in about 40% of patients within 6 to 8 months.18 Recovery from vinca alkaloid neurotoxicity can take up to 2 years,6 and persistent symptoms have been reported in one third of patients.9 In general, it appears that the majority of patients continue to experience neurotoxic symptoms months after treatment, and these symptoms could be accompanied by long-term effects on mobility and balance. Weakness and disturbance of gait and balance have been described as motor characteristics of CIPN; however, few studies have quantified these deficits using standardized measures.2,16
We suspect that older adults may experience greater functional decline than younger people who receive the same antineoplastic therapy and that they may have greater difficulty recovering from these deficits. Studies have shown that older adults are more likely to experience chemotherapy side effects, and chemotherapy doses often are reduced clinically, based on concern for comorbidities and declining organ function.1,19 A side effect of the same severity could translate into a more pronounced functional decline in an older adult. Age-associated conditions such as sarcopenia and neuropathy may leave older adults fewer resources with which to compensate for a new impairment. In addition, the rapid onset of CIPN (within weeks or even days after treatment) may make compensation more challenging than in slowly progressive neurotoxic conditions such as diabetes. Older adults, often with baseline visual and vestibular deficits, may benefit from additional time to develop compensatory postural control strategies. Finally, any recovery of nerve and mobility function after chemotherapy ends may be slower in the older adult, as age-related declines in postinjury nerve regeneration have been reported.20 A summary of selected publications related to differential effects of chemotherapy based on age is presented in eAppendix 1.
As health care providers, we have not adapted to the shift in chemotherapy side effects. Unlike blood counts, nerve function and mobility are not routinely monitored over the course of cancer treatment, and referrals to prevent or rehabilitate new deficits are not a universal standard of care. As in cancer clinics, performance-based measures of mobility and balance are seldom used in cancer research. Most studies to date have focused on outcomes at the impairment level, but physical performance measures could be useful in determining functional limitations and restricted participation in response to side effects such as neuropathy and fatigue.2 A summary of current literature related to CIPN's impact on balance and mobility is presented in eAppendix 2.
The purpose of this case report is to describe the rapid onset and long-term persistence of mobility decline in an older adult receiving neurotoxic chemotherapy after curative tumor resection. This case report adds to the sparse literature on prognosis for mobility deficits that arise during chemotherapy. To our knowledge, this is the first case report to describe an older adult with CIPN using results of standardized balance and mobility tests and to include measurements both before and years after treatment.
Patient History and Review of Systems
An 81-year-old retired nurse had undergone a right mastectomy for stage II breast cancer at the end of 2004, 2 months prior to enrollment in a 12-week observational pilot study designed to explore the feasibility of measuring effects of cancer treatments (radiation and systemic chemotherapy) on function and daily activity in adults aged 65 years and older and the willingness of this population to participate in research. The only specific recruitment criteria were: age, active cancer diagnosis, and comprehension of study procedures sufficient to provide informed consent. On the day of study enrollment, the patient received the third of 4 planned cycles of biweekly chemotherapy with Adriamycin* and Cytoxan† (A/C), drugs that can cause side effects such myelosuppression and cardiotoxicity but that are not recognized as neurotoxic.5,6,21 After completing chemotherapy with A/C, she was to receive 4 cycles of Taxol† or paclitaxel (ACT regimen) on a biweekly dosing schedule, followed by localized radiation and aromatase inhibitor therapy with Arimidex.‡
The patient appeared much younger than her stated age. She reported a past medical history of controlled hypertension, atrial fibrillation, and treated hypothyroidism, but she reported no symptoms of peripheral neuropathy or morbidities such as diabetes that are more commonly associated with neuropathy. Her gait was steady and coordinated with good speed and without gross deviation. She reported no prior assistive device use or falls. She described herself as active, volunteering as a teacher at week-long youth camps, and hooking rugs as a hobby.
Clinical Impression
The patient was a high-functioning, socially active, 81-year-old woman with minimal comorbidity and no reported mobility limitation. Unlike older adults who already have frank mobility deficits and restricted social participation, any sequelae experienced as she shifts to neurotoxic chemotherapy should present as a decline from baseline levels and interfere with her hobbies. Along with more detailed self-reports of function, we recognized the importance of obtaining timed measurements of balance and mobility to quantify her baseline status using measures that should respond to even subtle changes as she receives neurotoxic chemotherapy.
Examination
Outcome measures were the Short Physical Performance Battery (SPPB)22 and a self-report measure of basic and instrumental activities of daily living (ADL/IADL) taken from the National Health Interview Survey (NHIS).23 All data were collected by a single research physical therapist as part of pilot work with older adults receiving cancer treatment.
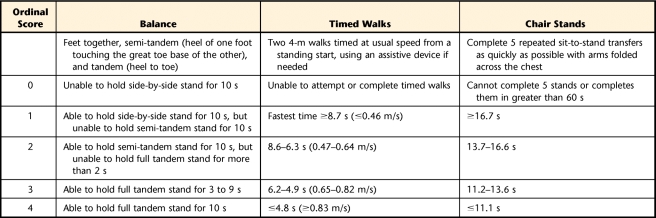
The SPPB quantifies functional mobility using 3 categories: standing balance, walking, and repeated chair stands. Performance in each category is timed, and the time is converted to an ordinal score ranging from 0 (unable to perform) to 4 (best performance), which is used to calculate a summary score (maximum=12). Specifics of each category are shown in Table 1. The full protocol and training instructions can be downloaded from the National Institutes of Health Web site (http://www.nih.gov/). Test-retest and interrater reliability of each of the 3 categories range from .73 to .97,24–26 and the reliability of the summary scale was established using internal consistency (Cronbach alpha=.76).22 Originally validated in more than 5,000 adults aged 71 years and older, summary scores correlated strongly with self-reported ADL disability for tasks such as walking across a room and transferring from bed to chair and with self-reported ability to walk up and down steps or walk 0.8 km (½ mile) without help.27 Summary scores also were found to strongly predict mortality and nursing home admission.22 Although currently more popular in research than in clinics, the SPPB is feasible for clinic use, requiring about 10 minutes to complete and a stopwatch, a chair, and a 6-m walkway.
Table 1.
Short Physical Performance Battery22
For individuals with cancer, we recommend monitoring subscores in addition to the summary score in an attempt to discriminate the contribution of change in each SPPB domain to any change in overall score. Different antineoplastic agents have different side effects (eg, peripheral sensory neuropathy, proximal myopathy, fatigue) that vary with the individual,4,5,11 and it is possible that each agent may affect the 3 SPPB domains differentially.
The ADL/IADL scale measures self-reported ability to perform 16 tasks, including the use of assistance for each task, whether from another person or from specialized equipment. Among the tasks are getting up from a bed or a chair, walking, stair climbing, getting outside the home, and shopping. For any tasks reported as difficult, self-perceived reasons were recorded. Intraclass correlation coefficients above .90 for interrater and test-retest reliability have been reported in community-dwelling older adults.28
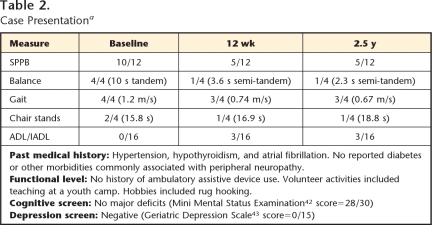
Table 2 summarizes the patient's baseline performance, recorded on the day she received her third of 4 cycles of A/C chemotherapy. Physical performance tests indicated that her functional level was high for her age, as only 4.5% of women over 80 years of age in one multi-center epidemiologic study walked at least 1.0 m/s.29 Of those who walked at that speed, 94.6% had no mobility or ADL disability. Guralnik et al29 provided equations to estimate the probability of developing mobility disability (defined as the inability to walk 0.8 km or climb stairs without assistance) over the next year based on the patient's gait speed, age, and gender. Using those equations, the patient's probability of developing mobility disability was only .059. Her baseline SPPB summary score of 10 fell in the highest quartile of function established by Guralnik et al, and she achieved the highest scores possible on balance and gait subscales by maintaining full tandem stance for 10 seconds and walking at a self-selected speed of 1.2 m/s. She scored in only the second quartile of performance for repeated chair stands, but she reported having no difficulty with any of the 16 ADL/IADL tasks.
Table 2.
Case Presentationa
SPPB=Short Physical Performance Battery, ADL/IADL=basic and instrumental activities of daily living.
Clinical Impression
Examination results confirmed that the patient was high functioning and without mobility disability. Her low probability for developing such disability in the next year under normal conditions should strengthen the connection between any observed decline and the chemotherapy agent received. At this point, we planned to repeat baseline measures in 12 weeks in accordance with the research study protocol. This visit would fall about 2 weeks after anticipated completion of taxane chemotherapy.
Intervention
Over the next 12 weeks, the patient received the final cycle of A/C chemotherapy, followed by 3 cycles of paclitaxel on a 2-week dosing schedule. Symptoms of sensory neuropathy were recognized as early as the second dose, and the fourth dose of biweekly paclitaxel had been withheld by the oncologist due to neurotoxicity. After the third dose, her symptoms had reached grade 3 of 4 on the National Cancer Institute's Common Toxicity Criteria (NCI-CTC 2.0) (available at: http://ctep.cancer.gov), indicating severe sensory loss or paresthesia that interferes with self-care ADL tasks. The other NCI-CTC grades for peripheral sensory neuropathy range from 1 (“mild, loss of deep tendon reflexes or paresthesia not interfering with function”) and 2 (“moderate, objective sensory loss or paresthesia interfering with function [IADL] but not with ADL”) to 4 (“permanent sensory loss interfering with function”), but grades of 3 are generally dose limiting.30 The patient subsequently refused the planned radiation therapy out of frustration with the adverse effects of her chemotherapy, but she began aromatase inhibitor therapy shortly after the 12-week intervention.
Outcome
At 12 weeks, exactly 4 weeks after the third and final dose of paclitaxel and before starting Arimidex, the patient's gait had deteriorated. It was now wide-based and unsteady, and she fell into the clinic wall when turning her head to talk. She was not using an assistive device, and noted that no intervention had been suggested for her new walking difficulty. Although the patient's participation in the parent study ended at 12 weeks, we were granted permission from the local institutional review board to conduct a long-term follow-up visit with this participant. At 2.5 years, her gait remained unsteady, and she lost her balance twice during an hour-long visit in her home. She had fallen without injury in the week prior to the visit, attributing the fall to a quick turn, and she estimated falling at least 12 times in the first year after starting taxane therapy.
She now used a cane when walking on uneven surfaces or in unfamiliar environments, for longer distances, or in the community alone. She reported walking with caution and preferred to be accompanied by her husband when leaving her home. Her social participation had declined from baseline levels, and she reported no volunteer activity with the youth group or any other organization. Overall, she endorsed dropping many of her previous activities and interests.
Consistent with observed and reported mobility deficits at 12 weeks, considerable decline was noted in her SPPB score, and this decline persisted at 2.5 years. Table 2 lists SPPB scores at all time points. According to Perera et al,31 a 1-point change in SPPB summary score is clinically significant. At 12 weeks, the patient's summary score had decreased by 5 points (50%). Her balance score was now 1 out of 4, as she maintained semi-tandem stance for only 3.6 seconds (2.3 seconds at 2.5 years), and she could not assume or maintain full tandem stance. Although her gait score dropped only 1 point from her baseline score, her speed slowed by 0.46 m/s at 12 weeks and by an additional 0.07 m/s at 2.5 years. Change of only 0.05 m/s is considered significant, and a change of 0.10 m/s is substantial and clinically meaningful.31 Time to complete 5 chair stands had increased to 16.9 seconds at 12 weeks, lowering her SPPB score by 1 point. At 2.5 years, her time had further worsened by almost 2 seconds, and she reported using caution when moving to stand.
Mobility-related disability was found at both 12 weeks and 2.5 years, with difficulty reported in 3 ADL/IADL tasks at both time points. At 12 weeks, the tasks were walking, climbing stairs, and shopping for personal items, but at 2.5 years, heavy housework replaced shopping. She attributed the difficulty with all tasks to “balance problems.” She specifically denied having weakness, nausea, and diarrhea at 2.5 years and reported feeling tired only “a little of the time,” as opposed to “most of the time” at 12 weeks.
Symptoms consistent with neuropathy were reported as interfering with both upper- and lower-limb function. At 12 weeks, the patient could not button the cuffs of her blouse, and she described this as a new problem that appeared around the same time as her imbalance. At 2.5 years, she reported difficulty with tasks such as writing and picking up small objects and noted that she had only recently resumed rug hooking. She reported residual numbness, more severe in her feet than her hands; estimated that partial recovery of balance deficits did not begin until about 1 year after onset; and noted that her deficits had not yet fully resolved. As a retired nurse, she frequently used the term “neuropathy” and openly attributed her persistent functional deficits to this condition. She stated that she did not have neuropathy before chemotherapy but that she “sure (does) now.”
Discussion
This case highlights the rapid onset and long-term persistence of mobility disability in an 81-year-old woman who received chemotherapy, the last dose withheld due to sensory neurotoxicity. Because this case report describes functional outcomes for a single patient and no measurements of nerve function were collected, neurotoxicity cannot be established as the cause of her new limitations. However, at least 2 aspects of this case support CIPN as a factor in her mobility decline. First, the oncologist recognized CIPN and discontinued chemotherapy based on sensory deficits interfering with the patient's self-care ADL tasks after her third cycle of paclitaxel, a drug that has been shown to preferentially affect A-beta myelinated fibers in the periphery.32 Second, the patient's self-report of peripheral nerve symptoms (numbness and tingling) coincided with her loss of function for mobility and dexterity tasks, and the persistence of these same symptoms paralleled the persistence of her functional limitations 2.5 years later.
Although she had begun chemotherapy before baseline measurements were taken, the baseline time point does represent her status before neurotoxic drug therapy. This finding may actually strengthen the case for paclitaxel as the offending agent, as her function remained high after 3 cycles of the first combination, declining only after paclitaxel was introduced and stabilizing after it was discontinued and as Arimidex therapy was initiated. Overall, we believe this case meets most of the 6 criteria proposed by Hill33 for establishing causation rather than simple association between medication and peripheral neuropathy: strong dose-response relationship, consistent manifestations, close proximity of symptoms to exposure, stabilization or improvement after drug cessation, and reproduction in animal models or characteristic neuropathology. Unfortunately, the “exclusion of other causes” criterion cannot be verified and is lacking if we attempt to apply these same criteria to the relationship between her peripheral somatosensory symptoms and functional decline.
More important than the specific cause are the rapidity, severity, and persistence of her functional decline. This patient became less active and independent and experienced falls 2.5 years later. Although the descriptions of some of her falls are consistent with underlying vestibular pathology, paclitaxel and the other ACT regimen drugs are not recognized as particularly ototoxic or toxic to the central nervous system.34 Whether the outcome was due to CIPN, another aspect of chemotherapy intervention, or even simply having cancer, this case highlights the extent to which function can decline in an older individual receiving medical treatment for cancer and the need for physical therapy intervention and further research for these individuals.
Previous studies of functional deficits in patients receiving taxane therapy have been limited to upper-extremity function using a peg-board test32 and gait and balance function using semi-quantitative scales.13 Only recently has performance on timed measures such as the Timed “Up & Go” Test been reported.35,36 Studies of the impact of cancer and its treatments on physical function in older adults tend to utilize self-report outcome measures not supplemented with performance-based measures.37–39 In searching the literature for use of timed measures of function in this age group, we have located only case reports without a pre-chemotherapy baseline.40
The case of this 81-year-old woman with decline after chemotherapy makes a novel contribution to the sparse literature on the functional impact of cancer and its treatments from at least 3 perspectives. First, this patient falls into the older adult age group commonly excluded from cancer studies.1,19 We believe that this is the group for which research utilizing functional measures is needed most, as it is the age group in which cancer is most prevalent and there is evidence for greater chemotoxicity.4,19 Second, we demonstrate the successful application of a timed physical performance measure that has been validated in older adults without cancer. To our knowledge, this is the earliest evidence that the SPPB is feasible for administration to older adults receiving chemotherapy and may be responsive to change in this population. Third, and most importantly, the 2.5-year follow-up data presented in this case report provide early evidence for prognosis after functional decline. To date, most sources report that about half of patients with CIPN improve over a period of months, but this improvement is rarely measured using functional outcomes and even more rarely using timed performance tests.8,9,18
The decision to follow up on the patient's status at 2.5 years was made primarily out of convenience. Although finding continued symptoms and disability so long after treatment is significant from a prognostic standpoint, we recognize that conducting interim visits between 12 weeks and 2.5 years would have better established her functional course over this period and may have better elucidated any role played by CIPN in her persistent limitations. Even though we cannot rule out the impact of health-related factors on her mobility status at 2.5 years, the patient reported having no new or worsening medical conditions or change in environment, other than the mobility-related restrictions in social participation described previously. Certainly, restricted activity and participation could result in new or worsened impairments over time; however, the ultimate cause would still be the cancer treatments that led to the initial restrictions. Arimidex is not known to be neurotoxic, so is unlikely to have contributed to her continued neuropathic symptoms, but it is associated with side effects (fatigue, joint pain, increased fracture risk)41 that could impede return of mobility function. Although at 2.5 years this patient reported lessening of fatigue and no injuries following the 12-week time point, she did report “mild” pain not reported at either baseline or 12 weeks. She attributed this pain to arthritis.
We are concerned that the degree of functional return experienced after any chemotherapy ends may decrease with age. The potential for residual deficits, subsequent injury, and loss of independence may be greatest in the oldest individuals. At least one group of researchers found no difference in CIPN incidence and severity between older and younger adults, but outcomes were measured primarily at the impairment level, and mobility was assessed using a 6-point scale of broad categories.14,15 Timed measures of mobility may have detected more subtle differences between age groups in this cohort, and age effects may have been diluted by the exclusion of patients with baseline peripheral neuropathy or potentially neurotoxic comorbidities. Additionally, the age difference between the 2 groups may have been too narrow, as a cohort of participants age 65 to 77 years was compared with one in which all participants were at least 50 years of age. Although we suspect that the advanced age of the patient in our case report contributed to her limited recovery of balance and mobility function, our project design did not allow such conclusions to be made, and we cannot confirm that her status at 2.5 years reflects CIPN rather than age-related decline of other systems or even side effects of Arimidex therapy. Instead, we recognize the potential interplay of all factors.
In a recent literature review, insufficient evidence was found to recommend any nonpharmacologic or pharmacologic intervention for CIPN.16 Evidence is mounting for the merits of physical activity in preventing and managing side effects of cancer treatment such as nausea, pancytopenia, fatigue, and depression, but its role in the maintenance or recovery of balance and mobility function has not been demonstrated. Although physical therapy has been shown to improve balance and mobility in older adults without cancer, evidence that it can be tolerated by those who are experiencing other chemotherapy-related side effects is needed, as are evidence-based guidelines for adjusting rehabilitative interventions on a session-by-session basis in response to such effects.
This patient received chemotherapy in a community hospital that also housed an outpatient physical therapy department, but she had received no rehabilitative intervention by 2.5 years after the initial onset of her deficits, even though it had been recommended by the research team in the pilot study from which this case was derived to both the patient and the oncologist. In our experience studying the feasibility of cancer and aging research, older individuals with cancer, particularly those receiving active treatment, appear to be underrepresented in physical therapy clinics. This underrepresentation probably is due, at least in part, to lack of awareness of available services. Many members of the medical oncology staff are concerned about the functional decline they observe, and it is understandable that this concern is second in the fight to eradicate cancer. However, in some cases, providers have not been educated about the role of physical therapy or may feel that older adults with cancer will either not tolerate or not respond to rehabilitative services. As physical therapists, we can educate both providers and patients so that older adults with cancer are provided the option of receiving potentially beneficial intervention. The Figure lists considerations for clinicians who work with clients who will or have received neurotoxic chemotherapy.
Figure.
Considerations for the clinician.
Suggestions for future research are focused on larger cohort studies of mobility and fall risk in older adults receiving chemotherapy or other medical interventions for cancer, with long-term follow-up to establish prognosis for resolution of any deficits. Inclusion of both self-report and performance-based outcome measures is recommended to capture the functional consequences of chemotherapy-induced impairment, and baseline nerve function should be recorded. To reflect the population of older adults who typically receive cancer treatments, exclusion criterion should be loosened to allow enrollment of individuals with comorbid conditions and baseline frailty.
Randomized controlled trials of physical therapy interventions individualized to mobility and balance deficits that arise while receiving chemotherapy could provide the strongest evidence for rehabilitative intervention in this arena. Ultimately, studies should focus on preventative measures, such as pretreatment balance and mobility training programs designed to optimize baseline functional reserve. Based on our own experience and experiences detailed by other authors,1 these studies may be difficult to conduct due to recruitment and retention challenges unique to the older adult population and especially individuals with active cancer.
To our knowledge, the long-term outcome for older individuals with CIPN, or for those whose function has otherwise declined after receiving chemotherapy, has not been established. We have demonstrated that the decline in balance and mobility experienced by one older adult while receiving chemotherapy persisted at least 2.5 years after treatment, although we cannot conclude that CIPN was responsible for her persistent mobility disability. Regardless of the specific cause, we suspect that functional decline experienced during a course of antineoplastic therapy may persist for other older adults with cancer. For some of these older individuals, function may never return to baseline levels. Even if complete resolution of balance and mobility deficits is realized, there is still a period of increased risk for falls and loss of independence, and it is during this time that rehabilitative interventions should be offered.
Supplementary Material
Footnotes
Dr Hile and Dr Studenski provided concept/idea/project design, data collection and analysis, and project management. All authors provided writing. Dr Studenski provided fund procurement and institutional liaisons. Dr Fitzgerald and Dr Studenski provided consultation (including review of manuscript before submission).
This case report and the larger pilot study from which the case was derived meet the HIPAA requirements for disclosure of protected health information as outlined by the University of Pittsburgh Institutional Review Board. All data were collected with approval from the University of Pittsburgh Institutional Review Board.
The baseline and 12-week data presented in this case report were presented at the Combined Sections Meeting of the American Physical Therapy Association; February 14–18, 2007; Boston, Massachusetts.
This project was funded by the Cancer and Aging Program at the University of Pittsburgh (NIH grant P20 CA103730) and the Pittsburgh Claude D. Pepper Older Americans Independence Center (NIH/NIA grant P30 AG024827).
Bedford Laboratories, 300 Northfield Rd, Bedford, OH44146.
Bristol-Myers Squibb Co, PO Box 4500, Princeton, NJ 08543-4500.
AstraZeneca Pharmaceuticals LP, 1800 Concord Pike, PO Box 15437, Wilmington, DE 19850.
References
- 1.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124 [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist LS, Galantino ML, Wampler M, et al. A framework for assessment in oncology rehabilitation. Phys Ther. 2009;89:286–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller JH, Storer B, Tutsch K, et al. Phase I trial of 3-hour infusion of paclitaxel with or without granulocyte colony-stimulating factor in patients with advanced cancer. J Clin Oncol. 1994;12:241–248 [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Schlaich M, Luck HJ, et al. Evaluation of neurotoxicity induced by paclitaxel second-line chemotherapy. Support Care Cancer. 1999;7:354–361 [DOI] [PubMed] [Google Scholar]
- 5.Park SB, Krishnan AV, Lin CS, et al. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15:3081–3094 [DOI] [PubMed] [Google Scholar]
- 6.Malik B, Stillman M. Chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2008;8:56–65 [DOI] [PubMed] [Google Scholar]
- 7.Pace A, Bove L, Aloe A, et al. Paclitaxel neurotoxicity: clinical and neurophysiological study of 23 patients. Ital J Neurol Sci. 1997;18:73–79 [DOI] [PubMed] [Google Scholar]
- 8.Pignata S, De Placido S, Biamonte R, et al. Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study. BMC Cancer. 2006;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verstappen CC, Koeppen S, Heimans JJ, et al. Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology. 2005;64:1076–1077 [DOI] [PubMed] [Google Scholar]
- 10.LoMonaco M, Milone M, Batocchi AP, et al. Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol. 1992;239:199–204 [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry V, Chaudhry M, Crawford TO, et al. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60:337–340 [DOI] [PubMed] [Google Scholar]
- 12.Mold JW, Vesely SK, Keyl BA, et al. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–318 [DOI] [PubMed] [Google Scholar]
- 13.Visovsky C, Daly BJ. Clinical evaluation and patterns of chemotherapy-induced peripheral neuropathy. J Am Acad Nurse Pract. 2004;16:353–359 [DOI] [PubMed] [Google Scholar]
- 14.Argyriou AA, Polychronopoulos P, Koutras A, et al. Peripheral neuropathy induced by administration of cisplatin- and paclitaxel-based chemotherapy: could it be predicted? Support Care Cancer. 2005;13:647–651 [DOI] [PubMed] [Google Scholar]
- 15.Argyriou AA, Polychronopoulos P, Koutras A, et al. Is advanced age associated with increased incidence and severity of chemotherapy-induced peripheral neuropathy? Support Care Cancer. 2006;14:223–229 [DOI] [PubMed] [Google Scholar]
- 16.Visovsky C, Collins M, Abbott L, et al. Putting evidence into practice: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2007;11:901–913 [DOI] [PubMed] [Google Scholar]
- 17.Forsyth PA, Balmaceda C, Peterson K, et al. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol. 1997;35:47–53 [DOI] [PubMed] [Google Scholar]
- 18.Brienza SVJ, Itzahki M, Krikorian A. Oxaliplatin (L-OHP): Global safety in 682 patients (abstract 513). Proc Am Soc Clin Oncol. 1995;14:209 [Google Scholar]
- 19.Giovanazzi-Bannon S, Rademaker A, Lai G, Benson AB., III Treatment tolerance of elderly cancer patients entered onto phase II clinical trials: an Illinois Cancer Center study. J Clin Oncol. 1994;12:2447–2452 [DOI] [PubMed] [Google Scholar]
- 20.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208 [DOI] [PubMed] [Google Scholar]
- 21.Pratt RW, Weimer LH. Medication and toxin-induced peripheral neuropathy. Semin Neurol. 2005;25:204–216 [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 23.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987:21–115 [PubMed] [Google Scholar]
- 24.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls: a prospective study. JAMA. 1989;261:2663–2668 [PubMed] [Google Scholar]
- 25.Seeman TE, Charpentier PA, Berkman LF, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol. 1994;49:M97–M108 [DOI] [PubMed] [Google Scholar]
- 26.Winograd CH, Lemsky CM, Nevitt MC, et al. Development of a physical performance and mobility examination. J Am Geriatr Soc. 1994;42:743–749 [DOI] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322 [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postma TJ, Heimans JJ, Muller MJ, et al. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9:739–744 [DOI] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 32.Dougherty PM, Cata JP, Cordella JV, et al. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142 [DOI] [PubMed] [Google Scholar]
- 33.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarafraz M, Ahmadi K. Paraclinical evaluation of side-effects of Taxanes on auditory system. Acta Otorhinolaryngol Ital. 2008;28:239–242 [PMC free article] [PubMed] [Google Scholar]
- 35.Wampler MA, Topp KS, Miaskowski C, et al. Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Arch Phys Med Rehabil. 2007;88:1002–1008 [DOI] [PubMed] [Google Scholar]
- 36.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148 [DOI] [PubMed] [Google Scholar]
- 37.Stafford RS, Cyr PL. The impact of cancer on the physical function of the elderly and their utilization of health care. Cancer. 1997;80:1973–1980 [DOI] [PubMed] [Google Scholar]
- 38.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006;24:3465–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003;58:M1119–M1124 [DOI] [PubMed] [Google Scholar]
- 40.Wampler MA, Hamolsky D, Hamel K, et al. Case report: painful peripheral neuropathy following treatment with docetaxel for breast cancer. Clin J Oncol Nurs. 2005;9:189–193 [DOI] [PubMed] [Google Scholar]
- 41.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62 [DOI] [PubMed] [Google Scholar]
- 42.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 43.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version in Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986:165–173 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.