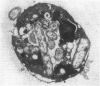
Abstract
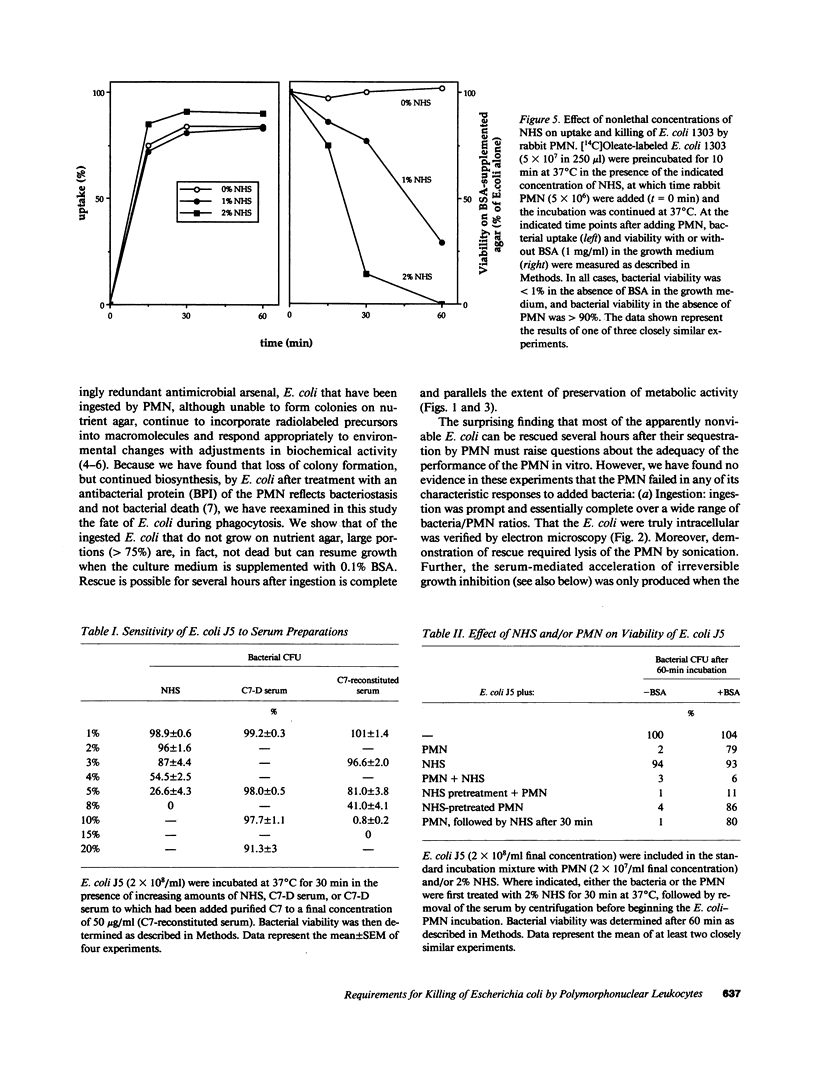
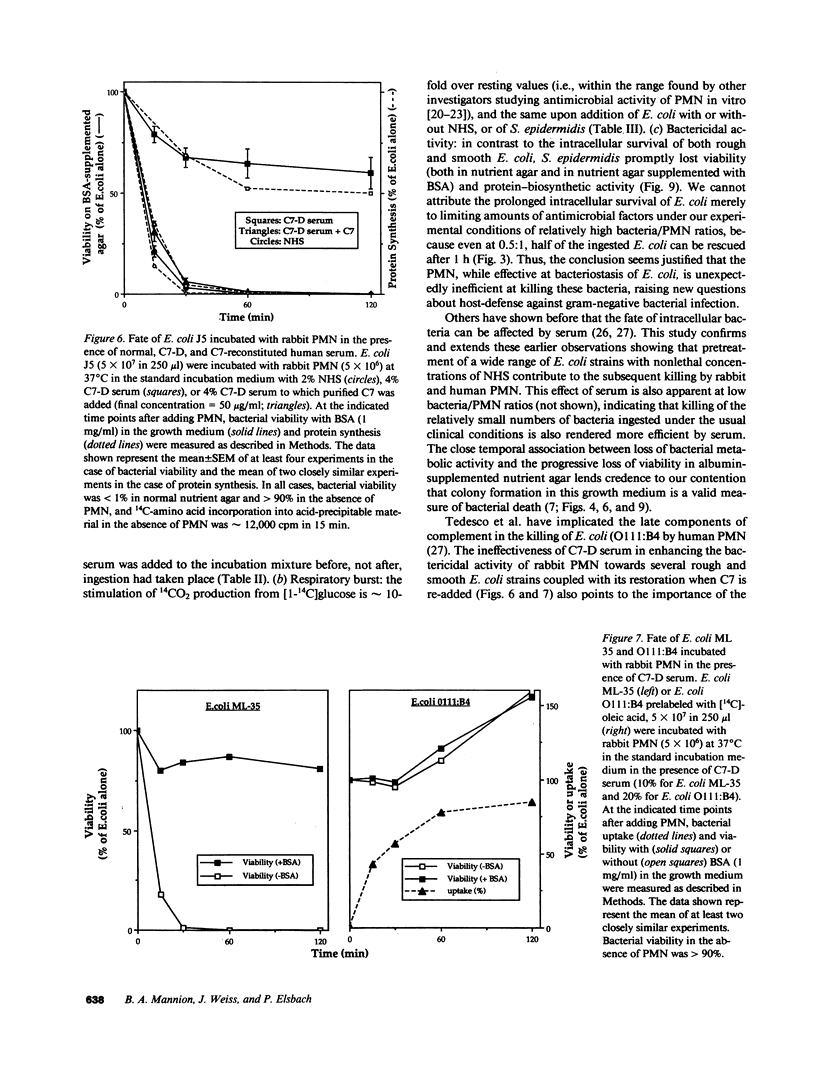
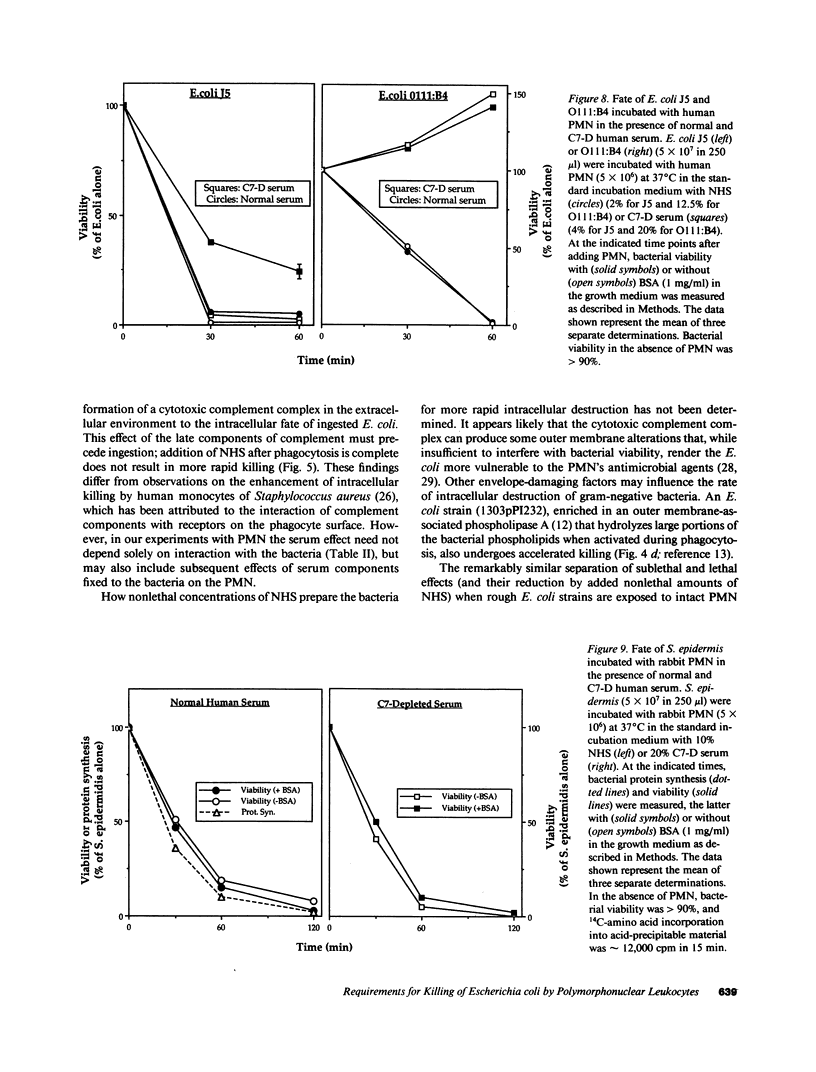
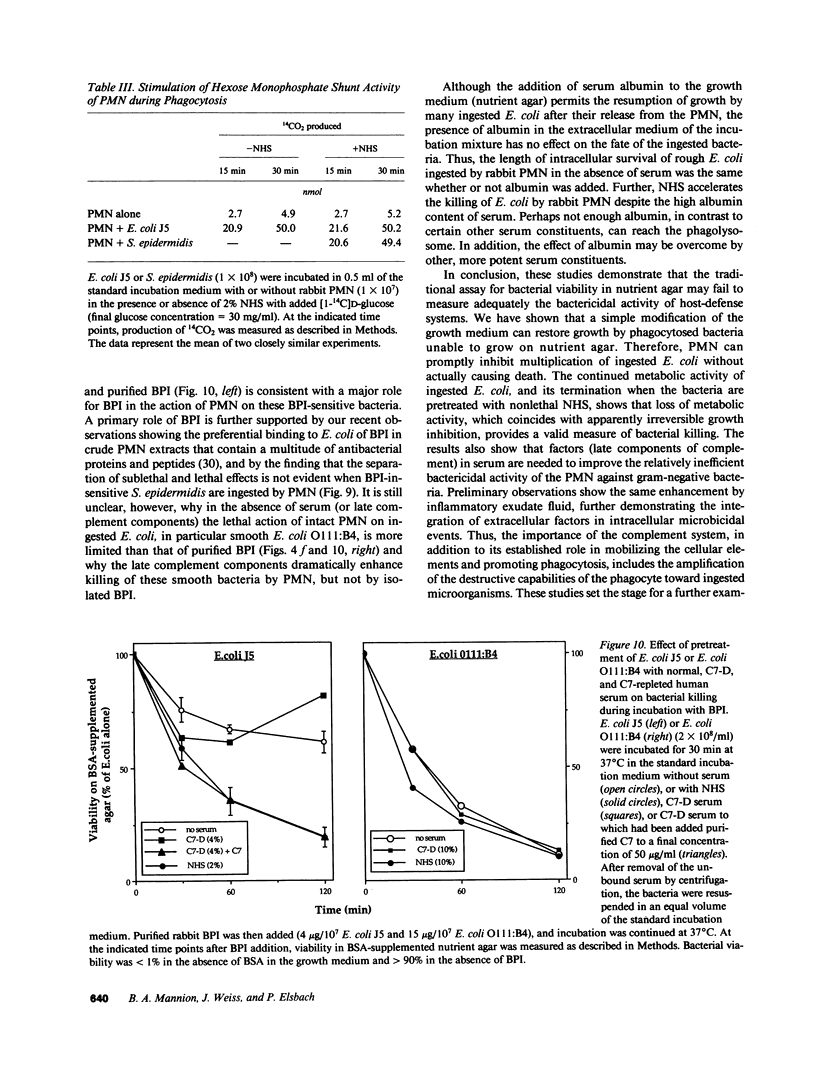
Escherichia coli ingested by PMN promptly stop growing and form no colonies in nutrient agar, but metabolize near normally for up to several hours. The bactericidal/permeability increasing protein (BPI) of PMN also inhibits E. coli growth without initial metabolic impairment. We recently showed that BPI-treated E. coli, although unable to grow in normal nutrient agar, can form colonies in this medium plus 0.1% BSA, as long as their metabolism is maintained, indicating that biochemical impairment is a better indicator of death than growth arrest (1990. J. Clin. Invest. 85:853-860). We have now reexamined the fate of ingested E. coli. Rabbit PMN ingest greater than 85% of several rough E. coli strains in 15 min, but greater than 80% of these bacteria, while unable to form colonies in conventional agar, grow normally on agar plus 0.1% BSA. Thus, the PMN under these conditions promptly stop growth of ingested E. coli without killing. Adding nonlethal concentrations of normal human serum (NHS) before, but not after ingestion, accelerates killing and, in parallel, loss of bacterial metabolism (t1/2 less than 0.5 h vs. greater than 3 h, respectively, with and without NHS). The rapid killing of both rough and smooth E. coli pretreated with NHS is lost after C7 depletion (C7-D) and restored when C7 is replenished. Similar results are obtained with human PMN. In contrast, ingested Staphylococcus epidermidis, opsonized with either NHS or C7-D serum rapidly stop metabolizing and do not form colonies in nutrient agar with or without BSA. Respiratory burst activity is the same during ingestion of E. coli (with or without NHS) and S. epidermidis. Killing of E. coli J5 (however, not of O111-B4) by BPI is also accelerated by pretreatment with NHS but not C7-D human serum. These findings indicate that late complement components are needed for efficient killing of both rough and smooth E. coli by PMN, and that BPI is the principal intracellular agent acting on ingested rough E. coli.
Full text
PDF

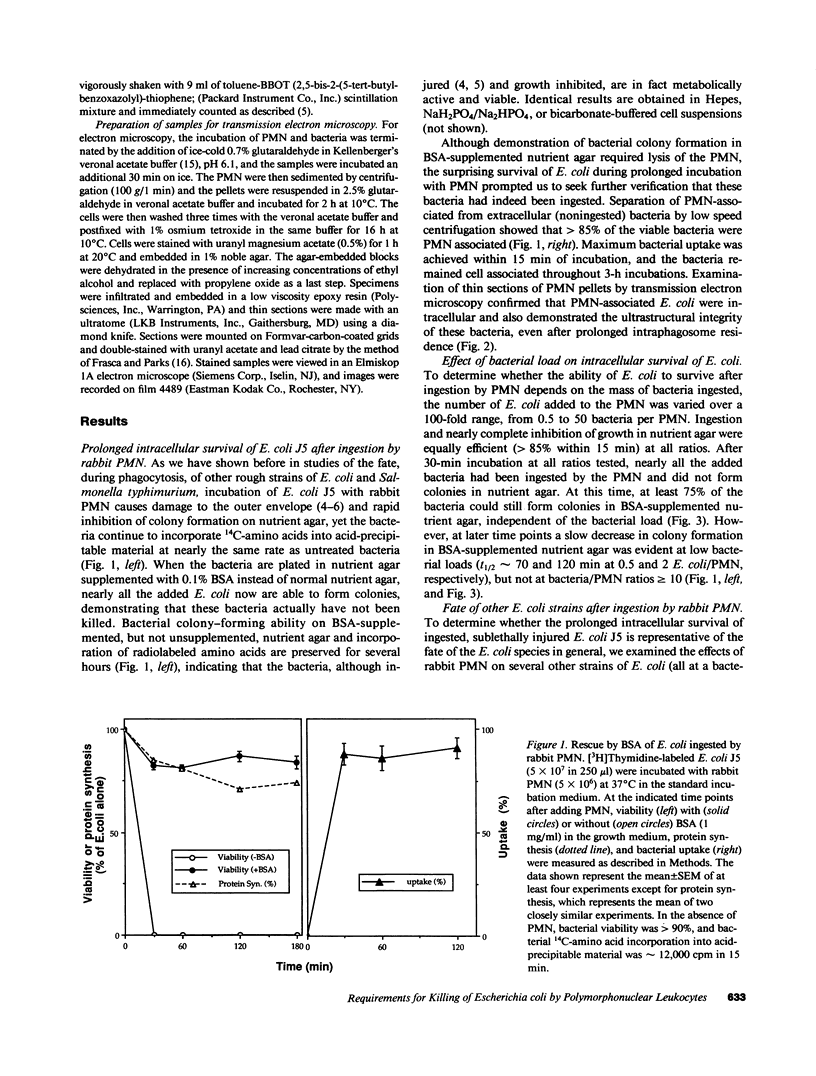

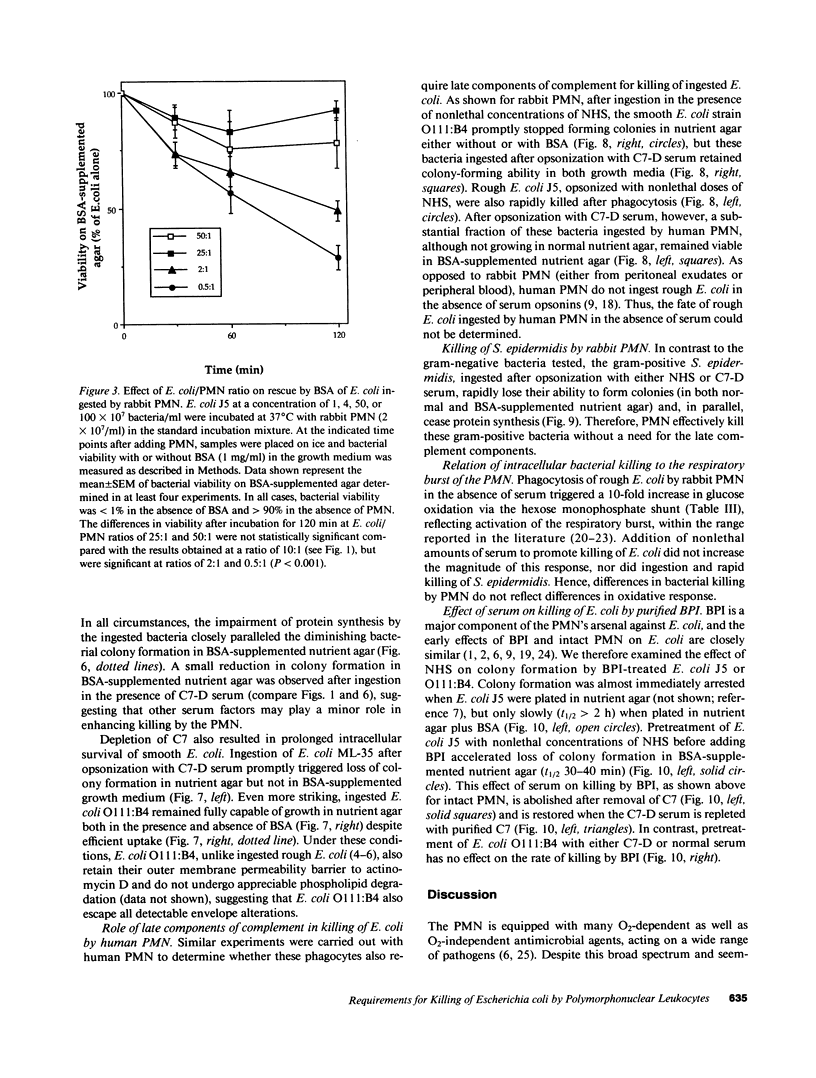
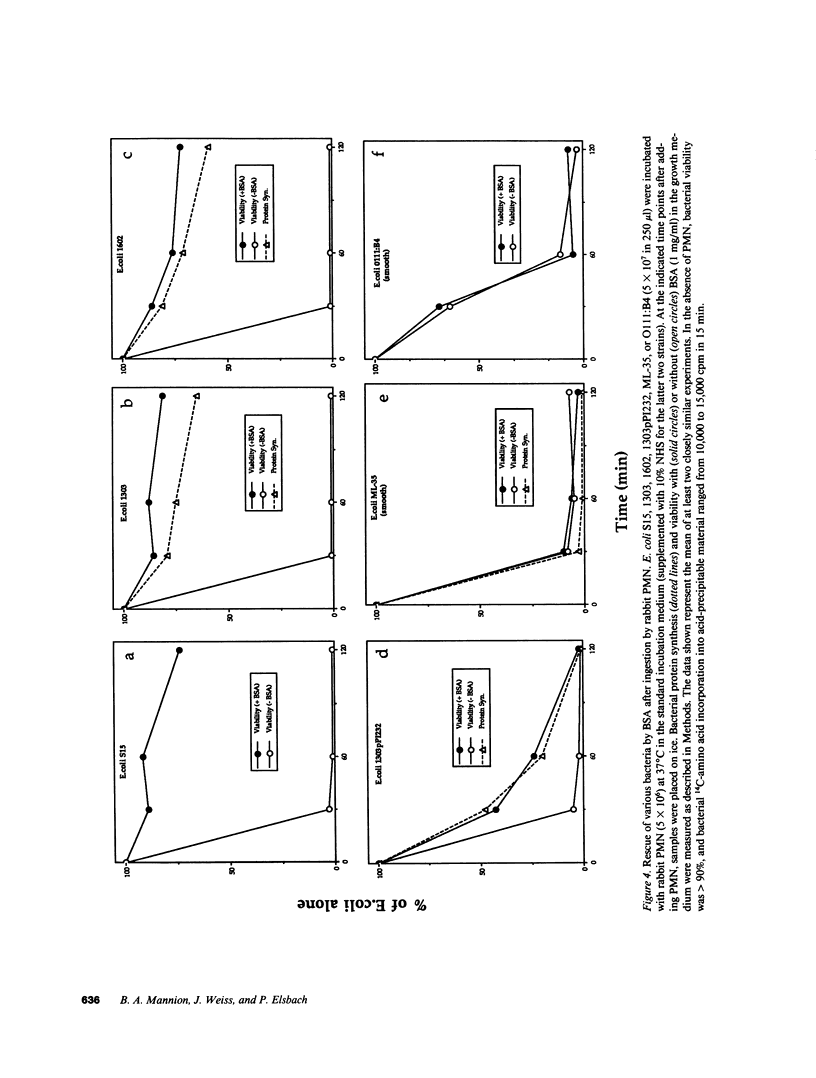

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Kuller G., Muhly M., Fromm S., Seibert G., Parrisius J. Formation of transmural complement pores in serum-sensitive Escherichia coli. Infect Immun. 1987 Jan;55(1):206–210. doi: 10.1128/iai.55.1.206-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSBACH P., SCHWARTZ I. L. Studies on the sodium and potassium transport in rabbit polymorphonuclear leukocytes. J Gen Physiol. 1959 May 20;42(5):883–898. doi: 10.1085/jgp.42.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSBACH P. UPTAKE OF FAT BY PHAGOCYTIC CELLS. AN EXAMINATION OF THE ROLE OF PHAGOCYTOSIS. I. RABBIT POLYMORPHONUCLEAR LEUKOCYTES. Biochim Biophys Acta. 1965 Apr 5;98:402–419. doi: 10.1016/0005-2760(65)90133-5. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion B. A., Kalatzis E. S., Weiss J., Elsbach P. Preferential binding of the neutrophil cytoplasmic granule-derived bactericidal/permeability increasing protein to target bacteria. Implications and use as a means of purification. J Immunol. 1989 Apr 15;142(8):2807–2812. [PubMed] [Google Scholar]
- Mannion B. A., Weiss J., Elsbach P. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. J Clin Invest. 1990 Mar;85(3):853–860. doi: 10.1172/JCI114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SIMON E. J., VANPRAAG D. INHIBITION OF RNA SYNTHESIS IN ESCHERICHIA COLI BY LEVORPHANOL. Proc Natl Acad Sci U S A. 1964 May;51:877–883. doi: 10.1073/pnas.51.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHELIN H., KARNOVSKY M. L., FARNHAM A. E., SUTER E. Studies on the interaction between phagocytes and tubercle bacilli. III. Some metabolic effects in guinea pigs associated with infection with tubercle bacilli. J Exp Med. 1957 Mar 1;105(3):265–277. doi: 10.1084/jem.105.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Tedesco F., Rottini G., Patriarca P. Moculating effect of the late-acting components of the complement system on the bactericidal activity of human polymorphonuclear leukocytes on E. coli 0111:B4. J Immunol. 1981 Nov;127(5):1910–1915. [PubMed] [Google Scholar]
- Tesh V. L., Duncan R. L., Jr, Morrison D. C. The interaction of Escherichia coli with normal human serum: the kinetics of serum-mediated lipopolysaccharide release and its dissociation from bacterial killing. J Immunol. 1986 Aug 15;137(4):1329–1335. [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Weiss J., Kao L., Victor M., Elsbach P. Oxygen-independent intracellular and oxygen-dependent extracellular killing of Escherichia coli S15 by human polymorphonuclear leukocytes. J Clin Invest. 1985 Jul;76(1):206–212. doi: 10.1172/JCI111947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Olsson I. Cellular and subcellular localization of the bactericidal/permeability-increasing protein of neutrophils. Blood. 1987 Feb;69(2):652–659. [PubMed] [Google Scholar]
- Weiss J., Victor M., Stendhal O., Elsbach P. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J Clin Invest. 1982 Apr;69(4):959–970. doi: 10.1172/JCI110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. C., Weiss J., Kim K. S., Verheij H., Elsbach P. Bacterial phospholipid hydrolysis enhances the destruction of Escherichia coli ingested by rabbit neutrophils. Role of cellular and extracellular phospholipases. J Clin Invest. 1990 Jun;85(6):1925–1935. doi: 10.1172/JCI114655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster N., Elsbach P., Simon E. J., Pettis P., Lebow S. The effects of the morphine analogue levorphanol on leukocytes. Metabolic effects at rest and during phagocytosis. J Clin Invest. 1971 May;50(5):1091–1099. doi: 10.1172/JCI106580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus P., van Die I., Bergmans H., Tommassen J., de Haas G. Molecular cloning of pldA, the structural gene for outer membrane phospholipase of E. coli K12. Mol Gen Genet. 1983;190(1):150–155. doi: 10.1007/BF00330338. [DOI] [PubMed] [Google Scholar]