Abstract
The perpetuation of symbioses through host generations relies on symbiont transmission. Horizontally transmitted symbionts are taken up from the environment anew by each host generation, and vertically transmitted symbionts are most often transferred through the female germ line. Mixed modes also exist. In this Review we describe the journey of symbionts from the initial contact to their final residence. We provide an overview of the molecular mechanisms that mediate symbiont attraction and accumulation, interpartner recognition and selection, as well as symbiont confrontation with the host immune system. We also discuss how the two main transmission modes shape the evolution of the symbiotic partners.
Symbiont transmission maintains symbioses through host generations and has a pivotal role in their evolution1-5. Despite growing evidence that microbial associations are present in diverse animals and plants in virtually every environment, our knowledge of the mechanisms of symbiont transmission is restricted to a few model systems. Most of these involve bacteria and some archaea6,7 (Supplementary information S1 (table)). Two fundamentally different modes of transmission can be distinguished: horizontal (that is, from an environmental, free-living symbiont source) and vertical (that is, inheritance of the symbiont from the mother or, more rarely, from both parents). However, there is great variation, and transmission can also be mixed, involving both vertical and horizontal transfers from the environment and intraspecific or interspecific host switching (FIG. 1).
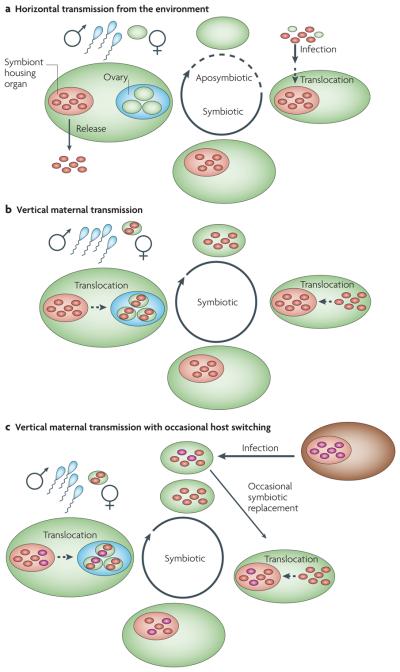
Figure 1. Symbiont transmission pathways.
a | Horizontal transmission from the environment. Host reproduction leads to aposymbiotic descendants, which at a certain life stage are infected with symbionts from the environment; often symbionts are translocated from the initial site of host contact to a putative symbiont housing organ; sometimes the environmental pool is replenished by symbiont release. b | Vertical transmission through the female germ line. Prior to host reproduction, symbionts are typically translocated from the symbiont housing organ to the female gonad, resulting in symbiotic descendants; often symbionts are then translocated from the colonization site to the symbiont housing organ. c | Mixed mode of transmission. Vertical transmission and occasional horizontal transmission can occur through host switching. Besides vertical transmission, occasional horizontal transfer of new symbionts (magenta) from a host population within the same species, but not the parent (intraspecific host switching; not shown), from a different host species (interspecific host switching; shown in brown) or from a free-living population (not shown) occurs.
This Review focuses on those associations that maintain protracted physical contact and involve most of the host population. We use de Bary's definition of symbiosis: living together of differently named organisms8, irrespective of the effects of the interaction on the fitness of the partners9. The key question is how the symbiont is transferred to the host progeny, regardless of the type of symbiosis. Reviews are already available for a range of symbiotic systems: plants10-16, sponges17,18, chemosynthetic bacteria–marine invertebrates19,20, entomopathogenic nematodes21,22, annelids23,24, insects25-30, squid31,32 and the vertebrate gut33-36. Here, we review how the conversation between partners, which ultimately integrates the symbiont into the host's life cycle, is initiated. By identifying the similarities and differences between the two principal types of symbiont transmission, we explore their evolutionary implications.
Horizontal transmission
The cyclic occurrence of aposymbiotic (that is, before the symbiont is acquired) and symbiotic phases in the host's life cycle is intrinsic to horizontally transmitted symbionts (Boxes 1,2; FIG. 2) (Supplementary information S1 (table)). In animal hosts, the aposymbiotic phase includes germ cells (eggs and sperm) and embryonic development. When present, larvae are also aposymbiotic during their pelagic dispersal (in tubeworms, lucinid and bathymodiolin mussels). When development is direct, symbionts can be acquired by juveniles (for example, in squid). Similarly, in plants germ cells and zygotes are aposymbiotic, as are seeds in legumes, and sporophytes and spores in hornworts.
Box 1. Transmission strategies and symbiont genomes.
The sequence of several symbiont genomes or at least their size and GC content is available (Supplementary information S1 (table)). Environmentally acquired symbionts are derived from a large and genetically diverse free-living population. In this case, selection can freely act against mildly deleterious alleles that have arisen by chance in the symbiont genome. The risk that these alleles will accumulate and persist is therefore limited116-118. These versatile symbionts must compete with the rest of the microbial community while free-living and also retain the genetic repertoire that is necessary to associate with their hosts. This reduces selection pressure on genome reduction.
By contrast, during strictly vertical transmission there is a reduction in the purifying activity of natural selection110,117,118. In this case, the symbionts transferred to the next generation are selected out of a restricted genetic population (this is known as a transmission bottleneck). In aphids, for example, the subpopulation of a single mother's bacteriocyte is transferred to the embryo, and later, on cellularization, all symbionts that have penetrated the embryo are subdivided again into different bacteriocytes110. Transmission bottlenecks leave irreparable scars on the genomes of host-relegated symbionts. These genomes, as in asexual populations, irreversibly accumulate harmful mutations (in a process known as Muller's ratchet)117: symbionts that cannot exist in a free-living state cannot purge their genetic repertoire. The accumulation of deleterious mutations results in inactivation and loss of genes, such as DNA repair genes, and, finally, in reduced genomes (reviewed in REFS 27,29,30). Besides high rates of amino acid substitution, the resulting reduced nucleotide base composition does not favour guanidine and cytosine (reviewed in REF. 29). Another possibility to explain the loss of gene function in strictly vertically transmitted symbionts is that many genes may be unnecessary in the nutrient-rich, safe environment provided by the host119,120.
Vertically transmitted symbionts that are either facultative or that only recently became obligate are a special case. Aside from a moderate reduction in size, the genomes of these symbionts (such as those found in mouthless oligochaetes121 and weevils122) have many pseudogenes and insertion sequences123. Increasing disconnection from a free-living pool will eliminate both of these kinds of genetic elements (reviewed in REFS 124,125).
Box 2. Phylogenetic implications of transmission strategies.
Horizontally transmitted symbioses typically show little evidence of phylogenetic congruence or cospeciation. For example, there is no overlap in the tree topologies of mussels and tubeworms and their symbionts20. Several lucinid clam species co-occurring in the same habitat are associated with the same symbiont phylotype126. Burkholderia spp. symbionts of Riptortus clavatus and Leptocorisa chinensis do not cluster according to host, but intermingle together with free-living strains39,127. Congruence between the topology of seven Vibrio spp. strain gapA sequences and 14 squid internal transcribed spacer or cytochrome c oxidase sequences indicates cospeciation128, although the few analysed squid–Vibrio spp. Associations and their large geographical distance raised some disagreement20,129.
The phylogenetic trees of symbionts that are exclusively vertically transmitted are congruent with those of their hosts over long evolutionary time. Several obligate insect symbionts are well-known examples (reviewed in REFS 27-30). Studies on clams showed overlapping symbiont and host genealogies, suggesting cospeciation130,131. However, recent analysis of the northeastern Pacific Vesicomya sp. Mt-II lineage indicates additional horizontal transfer of symbionts in this clade132. A cospeciation pattern was also found between primate lice and Candidatus Riesia spp.133 and between earthworms and Verminephrobacter eiseniae134.
Although many symbionts are transmitted vertically, phylogenetic incongruence points to additional horizontal transfer through host switching or the environment. This was the case for insects harbouring Wolbachia spp.135-137, representatives of four ascidian genera and Prochloron spp.138, gutless oligochaetes139, several bryozoan Bugula spp. and Candidatus Endobugula spp.140, and several solemyid species and their endosymbiotic gamma proteobacteria141. In sponges, recent evidence suggests that horizontal acquisition must also be present to account for the global distribution of closely related microbial lineages in geographically disparate host sponges142.
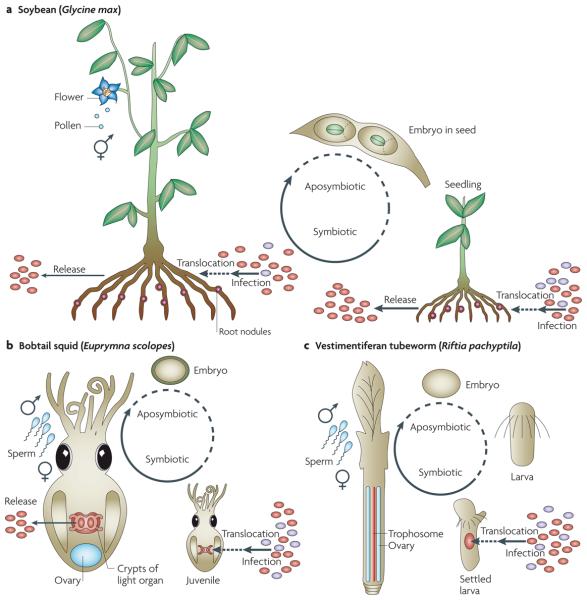
Figure 2. Simplified host life cycles of horizontally transmitted symbionts.
a | Legumes and intracellular rhizobia in root nodules (the example shown is the soybean Glycine max and Bradyrhizobium spp.). Aposymbiotic germ cells are produced by the flower; internal fertilization leads to aposymbiotic seeds in which the embryo develops. After germination, symbiont uptake occurs through infection threads in the roots of seedlings, as long as the plant grows (reviewed in REF. 11). b | The bobtail squid Euprymna scolopes and its extracellular endosymbiont Vibrio fischeri in the light organ crypts. Male and female hosts copulate, egg clutches are laid in the environment and they develop into aposymbiotic juveniles. Free-living V. fischeri is selectively taken up from the environment, and colonization of the light organ is completed within 12 hours after hatching (reviewed in REF. 31). c | The vestimentiferan tubeworm Riftia pachyptila harbours endosymbiotic Candidatus Endoriftia persephone in the trophosome. Sperm are released from males and migrate to females, where eggs are fertilized internally; zygotes are released into the water column, disperse and develop to larvae that settle, and metamorphosis is initiated. Symbionts infect the larval skin, migrate to the mesoderm surrounding the gut, and the trophosome develops49. Environmental bacteria are shown in purple.
For horizontally transmitted bacteria, symbiotic life is facultative: a free-living population serves as the inoculum for the symbiosis. Such free-living populations occur in soil37-39, marine shallow waters40-42 and the deep sea43. In some cases the free-living population is replenished by the release of symbionts from the host44,45.
Accumulation and entry
To establish an intimate association, the two free-living partners must physically contact each other, at the right time and in the right place, and must do so anew with each generation. Infection is often restricted to a specific life phase, such as post-settlement larvae in tubeworms, or juveniles in bivalves and squid. For example, the symbiont of the beard worm Siboglinum poseidoni can be ingested or taken up through the anus only during the larval phase46. By contrast, most microorganisms can access (and associate with) a functional gut throughout the life of the host, and infection of hornwort thalli and legume roots is continuous as long as the plants grow.
In some systems, the time frame for infection can be extended experimentally. In the squid Euprymna scolopes the speed of colonization of the light organ depends strongly on the density of the free-living symbiont Vibrio fischeri47. Similarly, infection of the gill epithelium of the lucinid clam Codakia orbicularis, which usually occurs after metamorphosis, can be artificially postponed by several months48. Whether such prolonged symbiosis competence is relevant in the natural environment remains unknown.
Infection can also be spatially limited. Stomata-like openings in hornwort thalli or pores on the ciliated appendages of the nascent squid light organ guide the prospective symbionts to defined areas inside the host. Even in the absence of special uptake structures, infection can be restricted to specific areas, such as the trunk region of tubeworm larvae49 or the gills (and sometimes part of the mantle epithelium) in bivalves45. In legumes, the susceptibility to colonization by rhizobia varies even within subsets of root hairs (reviewed in REF. 11).
One of the most widespread entry sites in animals is the oral opening. By accessing their hosts on ingested particles, microorganisms can colonize the gut epithelia. Freshly hatched fish and nymphs of the broad-headed insect Riptortus clavatus acquire their microbiota through the mouth from water50 or soil39, respectively. Once inside the gut, peristaltic movements can help the symbionts to contact the appropriate niche.
Other bacteria actively swim or glide, sometimes following chemical gradients. Legumes and hornworts are sessile and must inhabit a water-saturated environment for their symbionts to reach them. Hornworts even induce cyanobacteria to become motile by secreting hormogonium-inducing factors (HIFs) (reviewed in REF. 15). Following the action of HIFs, hormogonia enter stomata-like openings and glide into plant slime cavities. Free-living rhizobia have flagella and can also move towards plant roots by chemoattraction to compounds released by the plant root exudates, including various nutrients and host-specific flavonoids. Flavonoids can also induce rhizobial growth and, most importantly, the expression of symbiotically active genes such as nod genes (reviewed in REFS 51,52). The metagenome of the tubeworm symbiont Candidatus Endoriftia persephone displays remarkable dedication to chemoreception and motility, suggesting that the free-living counterparts of these endosymbionts can reach their respective hosts through chemotaxis53.
Given the turbulent nature of aquatic environments, it can be hazardous for a host looking for symbionts to rely exclusively on establishing chemoattractant gradients. Moreover, aquatic environments typically have low concentrations of potential symbionts54,55. Many aquatic hosts therefore actively pump water along their tissues, enhancing their chances of entrapping potential symbionts. Water currents generated by ingestion and egestion openings in bivalves facilitate encounters between prospective symbionts and the gill epithelium. For example, a squid hatchling captures planktonic V. fischeri only if it comes close to the ciliated appendages of the nascent light organ. This is promoted by mantle contractions and by cilia-generated microcurrents (reviewed in REF. 31).
Interpartner recognition
The initial physical encounter between partners typically occurs in host-secreted mucus. The mucus can cover plain surfaces (for example, the sticky developing tube surrounding tubeworm larvae49) or fill in the spaces among papillae (for example, in the giant rhubarb Gunnera spp.; reviewed in REF. 12), cilia (for example, in bivalves, beard worms and squid; reviewed in REFS 19,31) and villi (for example, in the guts of vertebrates; reviewed in REFS 34,35). Mucus quantity and quality can be subject to microbial control. Environmental bacteria stimulate the production of the mucus that covers the appendages of the squid nascent light organ. Similarly, vertebrate gut residents stimulate mucus shedding and modulate the gut's glycan composition.
Bacterial binding to host surfaces often involves proteinaceous surface appendages known as pili (or fimbriae). Loss-of-function mutations in the gene encoding Pila, the major component of type IV pili, affect the colonization competence of V. fischeri56. Azoarcus sp. also requires Pila to adhere to kallar grass (Leptochloa fusca), and Nostoc punctiforme uses Pila for chemotaxis and probably adherence (reviewed in REFS 10,15).
In mucus matrices, partners can select and attach to each other using their specific repertoires of surface sugars and sugar-binding proteins. Host surface sugars are present in the form of highly glycosylated proteins, whereas symbiont sugars are mainly exopolysaccharides (EPS) or lipopolysaccharides (LPS). Indeed, V. fischeri mutants lacking correctly modified LPS are colonization defective57. Examples of bacterial molecules that bind host sugars are rhizobial adhesins (known as ricadhesins) (reviewed in REF. 58) and the OmpU (V. fischeri59) and SuscD-like (Bacteroides thetaiotaomicron60) outer membrane proteins. The host molecules that can bind to symbiont sugars include LPS-binding protein on the surface of the developing light organ in the squid61, Mermaid on the surface of marine nematodes62 and codakine on the gills of lucinids63.
Bacterial autoaggregation is often a prerequisite of host attachment, and many of the bacterial surface molecules and structures mentioned above facilitate both processes. The ability of V. fischeri to specifically aggregate in the squid mucus depends on signalling by the RscS two-component regulation system, which controls production of EPS I by regulating the syp operon64-66. Moreover, mutations affecting the AinS–AinR quorum sensing system reduce the colonization speed in V. fischeri, probably owing to insufficient flagellar motility (reviewed in REF. 32). Along the same lines, in Sinorhizobium meliloti the exoS two-component regulatory system controls EPS I production and flagellum formation (reviewed in REF.13), and quorum sensing controls EPS II production and swarming motility67, 68.
Although the rule in horizontally transmitted symbioses is that interpartner recognition occurs in the host-secreted mucus, in most plant symbioses recognition starts at a distance. The binding of plant flavonoids to rhizobial nodD transcription factors results primarily in the synthesis and release of specific nod factors (reviewed in REFS 51,69). A high local concentration of the appropriate nod factor is required for the root hair to curl round a rhizobium. At this stage, ricadhesins mediate bacterial agglutination and binding to root hairs. Concomitantly, plant lectins mediate adhesion to specific rhizobial EPS (reviewed in REF. 70). These initial weak and reversible sugar–sugar and lectin–sugar interactions are followed by a tighter, irreversible binding that is mediated by bacterial cellulose fibrils (reviewed in REF. 11). Notably, nod factors are also required for the formation of S. meliloti biofilms71.
The journey to the symbiont housing organ
In digestive tract symbioses and in all marine ectosymbioses, the host mucus is also the final residence for the symbiont. However, the symbiont must migrate to the symbiont housing organ if it differs from the site of initial contact. In this situation, the symbiont must migrate either extracellularly in canals (such as hornwort slime cavities, Gunnera spp. stem gland channels and squid ducts), intercellularly (for example, rhizobia entering through cracks), through transcellular tunnels (for example, rhizobia inducing root hair curling) or both intercellularly and intracellularly (such as in tubeworms and kallar grass).
HIFs attract cyanobacteria to the slime cavities on the ventral side of hornwort thalli, and similar compounds probably attract cyanobacteria to the bottom of Gunnera spp. stem glands. V. fischeri reaches the deep crypts of the squid nascent light organ by flagellum-mediated chemotaxis towards diverse nutrient sources opposing the current that is generated by the ciliated epithelium lining the ducts. In Gunnera spp. the prospective symbionts swim against their positive phototactic behaviour and against the mucilage flow (reviewed in REFS 12,15,31).
Rhizobia that enter through cracks in the epidermis travel to the deep cortical layer in an intercellular infection thread, which is an organized line of infecting rhizobia with associated lignification of adjacent cell walls. The infection thread initiates from an infection pocket, which is generated by host cell programmed cell death (reviewed in REF. 72). However, migrating rhizobia are contained in a tubular invagination originating from the curled root hair cell (reviewed in REF. 11). The construction of this transcellular tunnel requires massive rearrangements of the plant cell cytoskeleton. As rhizobia en route to the deep cortical cells lack flagella, it was suggested that invasion progresses by bacterial proliferation at the infection thread tip, coupled to its constant extension13. This requires highly specific nod factors and, for indeterminate nodules, specific EPS.
The tubeworm symbiont Candidatus endoriftia persephone migrates from the epidermis to the symbiont housing organ, the trophosome, through mesoderm tissue, probably by entering and exiting all the cells it encounters (in small, infected larvae, symbionts have been found both intracellularly and intercellularly). The migrating symbionts are free in the cytoplasm, whereas later, in the trophosome, they are enclosed in vacuoles49. Also Azoarcus sp. can infect kallar grass by moving intracellularly and intercellularly, but it can only enter and colonize decaying or dead cells (reviewed in REF. 10).
In addition to physical obstacles, travelling symbionts must withstand the host humoral and cellular immune responses. Candidatus Endoriftia persephone possesses a wide repertoire of defence-associated genes, suggesting that it can withstand a vigorous host immune response53. Invading rhizobia are protected from oxidative stress (reactive oxygen and nitrogen species) in the infection thread by producing catalases, and can attenuate the plant immune response by producing LPS (reviewed in REF. 12). Furthermore, rhizobia are thought to avoid phagocytosis by immune cells using proteins secreted by a type III secretion system (T3SS)73. V. fischeri requires periplasmic catalase to survive oxidative stress while travelling from the pores to the light organ deep crypts74 and ompu to avoid phagocytosis by haemocytes59.
Once the rhizobia have reached the root cortex, the cyanobacteria have arrived at the bottom of the Gunnera spp. gland channels and Candidatus Endoriftia persephone has reached the prospective trophosome, they must accomplish a final task: internalization by host cells. In indeterminate nodules, each bacterium is endocytosed by a cortical cell in an individual unwalled membrane compartment that is derived from the infection thread. Each cortical cell can be infected by the single rhizobium that first entered the infection thread. Among the numerous bacterial factors that can affect symbiosome formation are LPS composition and tolerance to osmotic stress. The plant factors required for endocytosis include the Medicago truncatula NIP gene and wild-type expression levels of the leucine rich repeat domain-containing receptor DMI2 (also known as NORK). It remains unclear how cyanobacteria penetrate the thin walls of small dividing cells lining the gland channels, although mucilage pectolytic or cellulolytic activities might be involved (reviewed in REFS 12,13).
In conclusion, all horizontally transmitted symbioses require sophisticated molecular machineries to select specific symbionts from the environment. Most, if not all, involve sugar–lectin interactions and cellular surface structures, which can mediate symbiont–symbiont and host–symbiont attachment, and sometimes even help the partners to meet. Moreover, at each generation, the host and symbiont need to get accustomed to each other: the symbionts must combat the host immune response and, in turn, the host must not succumb to its symbionts.
Vertical transmission
In many vertically transmitted symbioses, there is no aposymbiotic phase and so the symbiotic association is permanent (BOXES 1,2; FIGS 3,4) (Supplementary information S1 (table). Uptake occurs in developing or fertilized eggs in some sponges, the gutless oligochaetes, bivalves, bryozoans, several insects and during sporulation in the water fern Azolla spp.. In some cases a long symbiotic phase follows a short aposymbiotic phase. This begins with the egg and may extend to various embryonic stages (in some sponges, Eisenia foetida earthworms, Trididemnum miniatum ascidians, and Acrytosiphon pisum and Brevicoryne brassicae aphids), to larvae still associated with the parent (in several ascidians and Glossina morsitans tsetse flies) or to juveniles developing in or outside the mother (Heterorhabditis bacteriophora nematodes and medical leeches (Hirudo spp.), respectively). Although all these associations are obligate for the host, the entomopathogenic nematode Steinernema carpocapsae can accomplish its entire life cycle aposymbiotically and only facultatively associates with its symbiont, Xenorhabdus nematophila, during the juvenile stage.
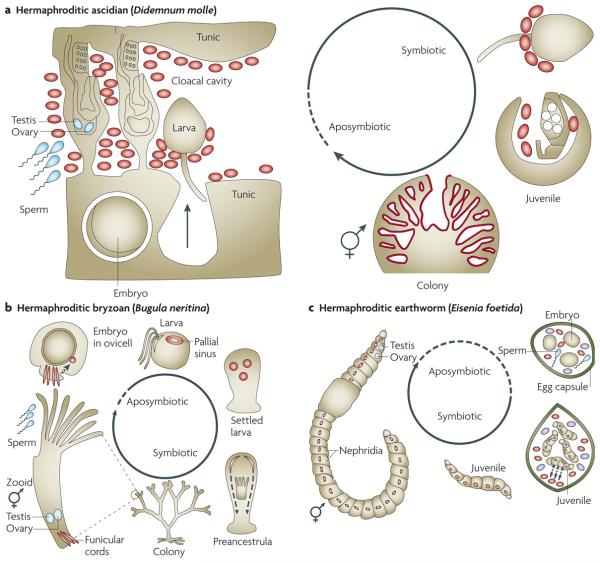
Figure 3. Simplified host life cycles of vertically transmitted extracellular endosymbionts.
a | In the colonial, hermaphroditic ascidian Didemnum molle the extracellular endosymbiont Prochloron didemni covers the tunic surface of the cloacal cavity. Sperm uptake into each zooid is followed by internal fertilization and embryonic development. The embryos migrate into the tunic and develop into larvae. When the larvae hatch into the cloacal cavity, they pick up the symbiont onto the posterior part of the body; larvae are then released from the cavity into the water column, settle after dispersal and undergo metamorphosis, during which the symbiont finally ends up on the surface of the cloacal cavity again88. b | The colonial, hermaphroditic bryozoan Bugula neritina harbours the extracellular endosymbiont Candidatus Endobugula sertula in the funicular cords of adult colonies and the pallial sinus of larvae. Sperm uptake and internal fertilization result in transfer of the zygote into the ovicell, in which the endosymbiont is most likely taken up through the funicular cords during embryonic development (not shown). The embryo develops into larvae which are then released and can disperse and settle. Following metamorphosis into preancestrula and ancestrula stages (first zooid), the symbionts migrate and end up in the funicular cords of the developing colony again94. c | The hermaphroditic earthworm Eisenia foetida harbours the extracellular endosymbiont Verminephrobacter eiseniae in the lumen of nephridial ampullae. During copulation and release of eggs and sperm, symbionts and albumin are released into the egg capsule, which is concurrently also infected with environmental bacteria (shown in purple). The egg is externally fertilized and develops into a juvenile in the egg capsule. During this time the symbionts are selected and taken up through a pore, they migrate through a canal to the ventral pore that leads into the nephridia, where they establish in the ampullae. The juveniles then hatch and develop into adults113,143.
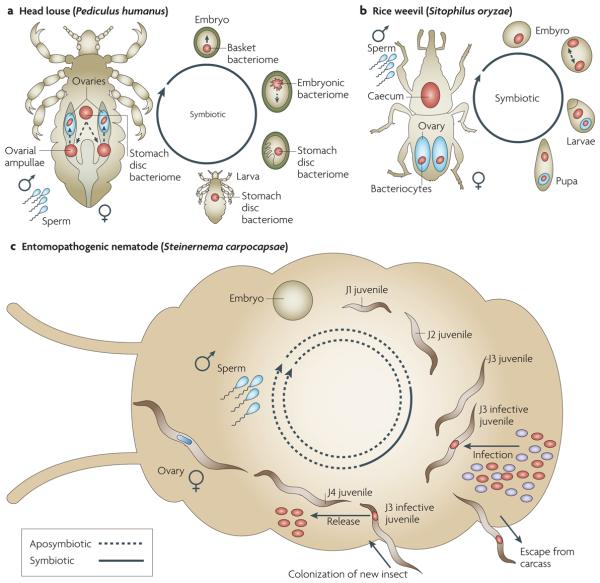
Figure 4. Simplified host life cycles of vertically or pseudovertically transmitted intracellular endosymbionts.
a | The head louse Pediculus humanus harbours the intracellular Candidatus Riesia pediculicola in the stomach disc and ovarial ampullae. Male and female lice reproduce through copulation and internal fertilization. The symbionts colonize the eggs through hydrophyles in eggshells and reside extracellularly in the periplasm. Individual eggs are laid in which embryonic development proceeds until the first instar nymphs hatch. During this development the bacteriome goes through three maturation stages (embryonic basket bacteriome with extracellular symbionts, embryonic bacteriome with intracellular symbionts and stomach disc bacteriome). After hatching and the development of two further instar nymph stages, in females symbionts are released and migrate to the oviducts to build a new bacteriome in the ovarial ampullae; from there, oocytes are infected and the male stomach disc bacteriome degenerates98,99,144. b | The rice weevil Sitophilus oryzae principal symbiont (SOPE) resides in the caeca and ovaries in females and in the caeca in males. Males and females reproduce through copulation and internal fertilization. Eggs containing endosymbionts are laid and sealed with a gelatinous plug. During early embryonic development, the SOPE population is split into two parts, one becoming associated with the primordial germ cells, which later constitute the intraovarian bacteriocytes that infect the next generation oocytes, and the other associates with the future bacteriome. Larvae develop, moult four times, pupate, and adults hatch; in young females symbionts in the caeca are reduced by unknown mechanisms so that in older females symbionts are found only in the ovaries145. c | Pseudovertical transmission of the entomopathogenic nematode Steinernema carpocapsae and its symbiont, Xenorhabdus nematophila. The male and female reproduce through copulation and internal fertilization, and the embryo develops indirectly, with four juvenile stages. Some juveniles that develop into the infective stage are colonized by symbionts entering the mouth and migrating through the pharynx to the vesicle (the gut lumen between two most anterior intestinal cells). Infective juveniles infected by the bacteria leave the insect cadaver and find a new host. Symbionts are released from the vesicle into a new insect host by passing through the intestine into the insect blood, where they reproduce and kill the insect. Symbionts within an insect cadaver originate from the infected nematode population; it is unknown how many nematodes infect a single insect (reviewed in REFS 21,146). This transmission mode has been termed pseudovertical because more than one nematode can be found within a single insect, and therefore the symbionts transmitted to the nematode progeny could come from the parent (vertical) or from the co-occurring nematodes in the insect (host switching). Environmental bacteria are shown in purple.
Vertically acquired symbionts are often transmitted through the female germ line (through the transovarial route) in animals with separate sexes (for example, in sponges) and hermaphrodites (for example, gutless oligochaetes). An example of biparental transfer is found in A. pisum and its facultative symbionts Candidatus Hamiltonella defensa, Candidatus Serratia symbiotica and Candidatus Regiella insecticola75. Furthermore, Candidatus Synechococcus spongiarum is present in ≤ 2% of the sperm of the sponge Chondrilla australiensis76. Although the Wolbachia spp. symbionts of Drosophila melanogaster (reviewed in REF. 77) and Anabaena azollae symbionts of the water fern78 also occur in the male germ line, there is no evidence of paternal transfer.
Vertical transmission is rarely the only means of symbiont transfer into the next generation. More often, phylogenetic evidence indicates that vertical acquisition is dominant, with occasional horizontal transfer within or between species or from the environment (BOX 2). For example, A. pisum females often mate with more than one male, so the progeny can acquire the symbionts from the ejaculate of several different males75. Intraspecific transfer was also proven among the parasitoid wasps Nasonia vitripennis79 and Trichogramma kaykai80. The rarer interspecific transfer naturally occurs between Trichogramma spp.81 and between sweet potato whiteflies and their parasitic wasps82. The examples below describe transmission of both exclusively and non-exclusively vertically transmitted symbionts.
Hanging onto the germ line
No symbiont translocation is needed when the symbiont is permanently present in the female germline stem cells, for example Wolbachia spp. (reviewed in REF. 77). This is achieved through an unknown mechanism that equally partitions Wolbachia spp. between the self-renewing stem cells and the differentiating daughter cells, which give rise to the oocytes. In early oogenesis, the prospective oocyte undergoes mitosis with incomplete cytokinesis, yielding 16 cells that are interconnected by cytoplasmic bridges. Fifteen of these cells give rise to the nurse cells, and the most posterior cell gives rise to the oocyte; Wolbachia spp. are segregated to the oocyte during this process by minus end-directed motors travelling on microtubules. An alternative infection route is from the heavily infected follicle cells that encase the developing oocyte. Later in oogenesis, the oocyte cytoskeleton is rearranged so that plus end-directed motors transport Wolbachia spp. to the oocyte's posterior side, guaranteeing symbiont integration into the future germ line. The cellularization of the embryo occurs after multiplication of the zygote nucleus into hundreds of nuclei and after these have migrated to the periphery. Because nuclei at the posterior pole are the first to be surrounded by plasma membranes, the first embryonic cells are Wolbachia-containing germ cells. Although posterior localization of Wolbachia spp. also occurs in mosquito and hymenopteran oocytes, some strains remain homogeneously distributed throughout oogenesis. In these cases, Wolbachia spp. associate with the nuclei so that the small proportion that eventually migrates towards the posterior pole will be incorporated in the future germ line. An insect cell line infected by Wolbachia spp. upregulates the expression of antioxidant proteins. Although this indicates that cultured host cells mount an oxidative stress response against Wolbachia spp., it is unclear how this endosymbiont combats this response83.
If the symbiont population is divided into a germ line and somatic population during early development, no transfer from the symbiont housing organ to the female germ line is necessary (reviewed in REF. 84). This is the case for the obligate symbionts of Sitophiluszeamais weevils, which reside in a bacteriome in the caecum and the ovary. When the fertilized eggs of S. zeamais start to differentiate, some symbionts populate the female gonad and others the bacteriome. When the symbionts must invade new bacteriomes, components of the T3SS are strongly overexpressed by the S. zeamais principal endosymbionts85.
The journey to the reproductive organ
Symbiont transfer from parent to progeny can vary depending on several parameters: which partner migrates (both partners, only the parent or only the symbiont); at which ontogenetic stage of the parent (embryo or adult) and progeny (developing and fertilized egg, embryo, larva or juvenile) development does migration occur; the distance from the symbiont housing organ to the reproductive organ; the mode of translocation (within bacteriocytes or free following release); and the site of egg infection (posterior pole or apolar).
The distance from the symbiont housing organ to the progeny can be covered by either or both migrating partners. The descent of eggs down the reproductive tract can be followed by external smearing with symbionts (in gutless oligochaetes86 and some insects; reviewed in REF. 26). In insects, this also requires symbiont transfer to pouches that are connected with the female genital openings. In addition, hatching larvae must feed on the symbionts. In some ascidians, instead of the eggs, it is the embryos or larvae (depending on the species) that migrate from the ovary through the tunic and are infected when entering the cloacal cavity, which is lined with extracellular immobile symbionts (Prochloron didemni)87-90.
Many cases of symbiont transfer from parent to progeny are known. In sponges, both symbionts and eggs are located in the extracellular mesohyl, which shortens the distance to cover. Which partner is responsible for getting together remains unknown. However, either the egg is infected before fertilization91 (and in one case the sperm is also infected76) or infection occurs during embryogenesis92,93. In aphids, bacteriocytes housing Buchnera aphidicola can also be found in the haemocoel, close to the ovaries, and are transferred from an adult to a sexual or parthenogenetic egg (reviewed in REF. 26).
Although transfer from the symbiont housing organ to the reproductive organ usually takes place in the adult host, it can also occur while the parent is still developing. In embryonic cockroaches, intact bacteriocytes migrate from the fat body to the closely opposed oocytes as soon as these have developed microvilli. Symbionts hang outside the oocytes, entrapped in the microvilli, until the cockroaches develop into adults and undergo ovulation. Only then are symbionts endocytosed by the oocytes26.
In other examples, the symbionts undertake a longer journey. In Bugula neritina bryozoans the extracellular endosymbiont is distributed in a network of channels that reach into zooids, which are specialized cells in which the embryos and larvae brood94. In shipworms and clams, endosymbionts must migrate from the gills to the ovary to infect the eggs; Solemya reidi symbionts have been detected in the clam ovary, encapsulated eggs and brooded larvae95. The eggs of the clam Calyptogena spp. are apparently infected through follicle cells, but the mechanism of symbiont transfer from the gills to the ovary remains enigmatic96,97.
A spectacular journey is undertaken inside the human louse, which harbours the intracellular Candidatus Riesia pediculicola in a gut-derived bacteriome98-100. During female development, symbionts exit the bacteriocytes through a hole and migrate directly to the lateral oviducts by moving posteriorly on the ventral surface of the gut. Because the symbionts lack flagella, they probably move by twitching or gliding. Subsequently, they enter the lateral oviduct cells by penetrating a fibrous mesh, which does not coat other oviduct regions. Haemocytes are also attracted to the lateral oviduct following moulting and are thought to repair the tissue damage caused by massive symbiont penetration; up to 200 symbionts can be transmitted in lice25.
Instead of the egg or embryo stage, it is the larvae of tsetse flies and the juveniles of entomopathogenic nematodes that are infected. Tsetse fly symbionts are transmitted to the larvae through infected milk secretions. Although Sodalis glossinidius resides in multiple fly tissues, including the milk gland, it is unknown how the symbiont Wigglesworthia glossinidia is transferred from the midgut mycetome to the lumen of the milk glands. S. glossinidius uses its T3SS to invade insect cells in vitro, as in its absence, S. glossinidius cannot be vertically transmitted, even when microinjected into the thorax of an adult fly85.
Shortly after colonizing an insect, entomopathogenic nematodes release their symbionts into the haemolymph, causing the death of their prey. As the progeny of the invading nematode feed on the dead insect, they ingest prospective symbionts. After entering and migrating through a specific group of gut cells, the symbiont of the nematode Heterorhabditis bacteriophora, Photorhabdus luminescens, infects and replicates within the neighbouring rectal gland cells. The eggs hatch internally, so that the mother is consumed by the developing infective juveniles. During the development of infective juveniles, the infected rectal glands of the adult break open and release symbionts into the mother's body cavity. Each infective juvenile is then colonized by a single bacterium that attaches to the pre-intestinal valve cell101. P. luminescens can use a T3SS to secrete YopT, a homologue of a Yersinia pestis protein that has antiphagocytic activity, but it is unclear whether this secretion system is necessary for nematode colonization102. Moreover, a correctly modified LPS on the surface of P. luminescens might confer resistance to the nematode humoral defences (reviewed in REF. 22). In S. carpocapsae (reviewed in REF. 21), after a few rounds of aposymbiotic reproduction in the insect carcass, one or two X. nematophila symbionts colonize the lumen at the anterior end of the infected juvenile gut between two epithelial cells (known as the vesicle). The vesicle contains a group of untethered spheres that probably evolved to optimize presentation of sugar-rich mucus103.
Aeromonas veronii, the main symbiont of the digestive tract of the medical leech, must be released to be taken up by the cocoon within 24 hours of deposition104. Because symbionts are undetectable in the reproductive organs and on the body surface but are found in the oral opening, the cocoon fluid is probably infected by oral smearing105. Proteins secreted by a T3SS have been implicated in the avoidance of phagocytosis by leech-associated A. veronii, and its LPS seems to confer resistance to the complement system that is present in the blood meal106.
At the end of their journey, vertically transmitted intracellular symbionts are internalized by the host cells by endocytosis (for example, in the sponge Halisarca dujardini93), by fusion of symbiont housing nurse cells with eggs (for example, in the sponge Chrondrilla australiensis107) or by the formation of transient collagen-like connections between nurse cells and eggs (for example, in the sponge Hippospongia lachine92). In the sweet potato whitefly Bemisia tabaci, however, intact bacteriocytes are directly enclosed in the egg108. B. aphidicola is the best-described example of this internalization109. Invasion of the A. pisum aphid embryo by B. aphidicola varies depending on its sexual or parthenogenetic origin. If the embryo originates from sexual reproduction, the posterior follicle cells lengthen along their apical–basal axis just before symbiont invasion. The bacteria are released by the bacteriocytes and probably enter the embryo through multiple, filamentous actin-reinforced channels in the posterior follicular epithelium. At this point, the prospective bacteriocyte nuclei migrate to the bacterial mass and form a bacteriome. By contrast, to enter a parthenogenetic aphid embryo, symbionts must penetrate enlarged posterior follicle cells. A channel appears between the posterior follicle cells, allowing bacteria to flow into the posterior of the embryo. Bacterial transmission apparently involves the fusion of a membrane-bound bacterial package with the posterior follicular epithelium. Because the most posterior nuclei are the only embryonic nuclei, they can associate with the bacteria and give rise, on cellularization, to symbiont-containing bacteriocytes. The number of B. aphidicola transmitted to each egg varies between 850 and 8,000 bacteria among different aphid taxa110.
Interpartner recognition
As all symbionts that are strictly vertically transmitted are obligate symbionts and do not need to be selected from an environmental population, surface molecules for specific interpartner recognition are not mandatory. Given the pivotal role of lectins in horizontally transmitted symbioses, it is remarkable that the Azolla spp.–Anabaena spp. system is the only vertically transmitted symbiosis in which lectins are known to be involved (reviewed in REFS 12,15). Nevertheless, facultative symbionts (for example, Wolbachia spp. and P. luminescens) and symbionts that confront environmental bacteria (for example, Verminephrobacter eiseniae and X. nematophila) display surface structures and molecules that are similar to those identified in horizontally transmitted symbionts.
Wolbachia spp. outer membrane proteins (Wsp) exhibit great variability between strains and might function in mediating infection of specific host cells, which can, in turn, infect the oocytes. Alternatively, Wsp might inhibit apoptosis of host cells by binding Toll-like receptor 2 (reviewed in REF. 77).
The P. luminescens pbgPE operon is required for correct LPS modification and successful nematode colonization. As the P. luminescens genome is predicted to encode different fimbriae, these could allow attachment to the surface of different digestive tract cells. Because fimbria-encoding loci vary among P. luminescens isolates, the molecular signature of certain fimbriae might also mediate acquisition of specific isolates (reviewed in REF. 22).
The X. nematophila nilABC genes are genetically linked, coordinately regulated and encode membrane proteins that are essential for colonizing the nematode host111. These three proteins function together to mediate attachment to the sugar-rich host mucus. Intriguingly, nilB homologues are present in pathogens that colonize mucosa (reviewed in REF. 21). In addition to nil genes, mrxA, which encodes the structural subunit of type I fimbriae, is also required for successful nematode colonization112.
Although the role of quorum sensing in vertically transmitted symbioses remains elusive, successful X. nematophila, and probably P. luminescens, colonization requires a global transcriptional change in response to environmental signals (reviewed in REF. 21).
The journey to the symbiont housing organ
As intricate as the journey to the reproductive organ can be, some vertically transmitted symbionts must migrate further, to the symbiont housing organ of the progeny. After complete cellularization, the A. pisum embryo undergoes a series of morphogenetic movements that include germ band extension, twisting, flipping and retraction. Although these movements are so radical that the dorsal–ventral and the anterior–posterior axes are inverted twice, the symbiont-containing bacteriocytes remain tightly associated with the germ cells in the dorsal abdominal region, close to the ovaries109.
Aphid bacteriocytes are at the mercy of harsh developmental movements, whereas V. eiseniae acts on its way to earthworm nephridia: it enters the embryo only when segmentation has already started and recruitment canals are available. After gathering on the dorsal side of a subepithelial canal, it migrates down to the ventral side and reaches the nephridiopore, where it remains for some time before entering the nephridium and proceeding to its definitive residence, the ampulla. Although other, less abundant bacterial phylotypes are present in the canal, they do not migrate into the open nephridial pore. Because both nephridial development and symbiont colonization proceed segment after segment from anterior to posterior, one hypothesis is that as the recruitment canals sequentially mature, they chemo-attract prospective symbionts113. Although the leech bladder symbiont Ochrobactrum sp. was detected in the cocoons immediately following deposition, it is not known how it is directly transmitted to the embryo104.
An unusual strategy is used by the symbionts of S. reidi to migrate to the progeny's gills. Bacteria located in test cells surrounding the embryo are ingested and transported into the foregut, and degradation in the digestive system positions them in the perivisceral cavity114,115. It is not clear how they then reach the developing gills.
In summary, hosts that can pass on their symbionts by vertical transmission have evolved strategies to deeply integrate the symbionts in their reproductive and developmental biology. Nevertheless, the molecular mechanisms underpinning this integration are not merely host directed. Instead, components of bacterial origin, such as the T3SS, are emerging as conserved requirements.
Conclusions
This Review illustrates how some symbiotic plants and animals have retained the ancestral, horizontal transmission mode throughout evolution, whereas others have opted for the vertical route and have become capable of integrating the symbionts, usually into the female germ line. However, some hosts have kept one foot in both camps: environmental acquisition has been maintained or host-switching mechanisms have evolved, so the mode of transmission is mixed. More rarely — and unquestionably demonstrated only for some insects — partners have ‘unlearned’ all the transfer strategies except for vertical transmission.
The integration of microbial symbionts into the reproductive and developmental processes of their hosts has many manifestations. Regardless of the kingdom to which the host belongs, each transmission strategy involves costs that are difficult to calculate. This complicates predicting how the progeny will inherit microbial partners. Although symbiont acquisition in a hornwort and a squid are strikingly similar, the horizontal and vertical transmission modes, strictly speaking, share few features. The transmission of V. fischeri and B. aphidicola clearly rely on antithetic molecular and anatomical adaptations. However, both paths can lead to specific, obligate and long-lived symbioses.
Horizontal acquisition typically takes place after progeny dispersal or dormancy associated with major developmental morphological changes (for example, after metamorphosis). A mucus interface often facilitates symbiont attraction, accumulation and recognition. It can also provide the settings for initial symbiont selection. Once the symbionts have gained entry and reached their final residence, the selection stringency often increases. By contrast, strict vertical transmission occurs before the progeny leaves the mother. In a sterile host, heritable symbionts face no competition with environmental bacteria. Although recognition and selection represent ancillary issues here, two symbiont translocations must be finely choreographed: from the symbiont housing organ of the parent to the transmission site, and from this site to the symbiont housing organ in the progeny.
Whether beneficial or pathogenic, environmentally acquired microorganisms attach to host-secreted mucus and exploit similar invasion mechanisms, without succumbing to host immune responses. Oxidative stress is a common immune response in hosts with horizontally transmitted symbioses. In some cases, such a defence strategy can even promote symbiosis establishment. Bacterial strategies to resist host immune defences include secretion systems and appropriately modified LPS. Remarkably, symbionts exposed to a non-sterile environment, whether horizontally or vertically transmitted, display similar surface structures to contact their hosts.
Symbionts that are exclusively transmitted through the vertical route resemble organelles more than beneficial symbionts or pathogens acquired from the environment. They lack genes for motility and environmental stress responses and, because they are enslaved by their hosts, they only retain genes that supply them with essential dietary requirements.
Our understanding of transmission paths does not yet extend to their causes. Theoretical evolutionary models could direct our attention to crucial factors linked with, or restricted to, one or the other mode of transmission. Moreover, they could help to identify future directions for symbiosis research. At the same time, we will greatly profit from integrating our knowledge on the immense diversity of symbioses and their countless transmission strategies into an ecological framework.
Supplementary Material
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) grant P20282-B17 (M.B.) and the Austrian Research Promotion Agency (FFG) grant 814324 (S.B.). We particularly thank D. Distel, H. Felbeck, H. Goodrich-Blair, Y. Gottlieb, J. Graf, A.Heddi, U. Hentschel, J. A. Ott, Robert C. Vrijenhoek and W. Miller for insightful discussions and valuable comments on the manuscript, and S. Davidson, M. Haygood, E. Hirose, M. J. McFall-Ngai and K. Sharp for their help with some figures. We also thank S. Espada Hinojosa and I. Kolar for their assistance with the literature and M. Stachowitsch for editorial work. Several original papers on transmission were not cited owing to space limitations.
Glossary
- Pelagic dispersal
The spreading of an organism, usually its larval stage, in the water column.
- Sporophyte
The diploid generation in the life cycle of the hornwort, which develops from the zygote and produces spores.
- Thallus
Undifferentiated vegetative tissue in hornworts that has a circular or ribbon-like arrangement.
- Hormogonium
A short, motile filament that lacks heterocysts. Hormogonia provide a means of dispersal for otherwise immotile cyanobacteria.
- Flavonoid
A 2-phenyl-1,4-benzopyrone derivative, produced by plants, that serves as a defence and signalling compound.
- Swarming motility
Rapid and coordinated movement of a bacterial population across solid or semi-solid surfaces that is powered by numerous flagella.
- Indeterminate nodule
A nodule formed by plants of some clades of legumes that develops a continuously growing nodule meristem at the distal end and has zones of tissue at different stages of development.
- Trophosome
Symbiont-housing organ in siboglinid tubeworms, of mesodermal origin in Vestimentifera, and endodermal origin in Frenulata.
- Symbiosome
Host membrane surrounding the symbionts
- Nurse cell
A polytenic germline cell in insects that contributes to the development of the oocyte, producing the bulk of its cytoplasm and multiple nuclei.
- Bacteriome
A specialized organ containing host cells (bacteriocytes), which house endosymbiotic bacteria.
- Ontogenetic stage
Life cycle phases in the development from the fertilized egg to the adult.
- Mesohyl
A proteinaceous gelatinous matrix between the epidermis and gastrodermis of cnidarians.
- Haemocoel
The space between the organs through which haemolymph circulates in arthropods.
- Parthenogenetic egg
An unfertilized egg that develops into a new individual.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
Entrez Genome Project: http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj Bacteroides thetaiotaomicron| Buchnera aphidicola | Candidatus Endoriftia persephone | Candidatus Hamiltonella defensa | Candidatus Regiella insecticola | Candidatus Riesia pediculicola | Candidatus Serratia symbiotica | Nostoc punctiforme | Photorhabdus luminescens | Prochloron didemni | Sinorhizobium meliloti | Sodalis glossinidius | Vibrio fischeri | Wigglesworthia glossinidia | Xenorhabdus nematophila
FURTHER INFORMATION
Monica Bright's homepage: http://www.hydrothermalvent.com
Silvia Bulgheresi's homepage: http://www.univie.ac.at/shallow-water-symbiosis/p_bulgh.html
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
References
- 1.Ewald PW. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 1987;503:295–306. doi: 10.1111/j.1749-6632.1987.tb40616.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamura N. Vertical transmission and evolution of mutualism from parasitism. Theor. Popul. Biol. 1993;44:95–109. [Google Scholar]
- 3.Lipsitsch M, Siller S, Nowak MA. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution. 1996;50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamura N. Evolution of mutualistic symbiosis: a differential equation model. Res. Popul. Ecol. 1996;38:211–218. [Google Scholar]
- 5.Genkai-Kato M, Yamamura N. Evolution of mutualistic symbiosis without vertical transmission. Theor. Popul. Biol. 1999;55:309–323. doi: 10.1006/tpbi.1998.1407. [DOI] [PubMed] [Google Scholar]
- 6.Sharp KH, Eam B, Faulkner DJ, Haygood MG. Vertical transmission of diverse microbes in the tropical sponge Corticium sp. Appl. Environ. Microbiol. 2007;73:622–629. doi: 10.1128/AEM.01493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steger D, et al. Diversity and mode of transmission of ammonia-oxidizing archaea in marine sponges. Environ. Microbiol. 2008;10:1087–1094. doi: 10.1111/j.1462-2920.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 8.de Bary A. Die Entstehung der Symbiose. Verlag von Karl, J. Trübner; Strassburg: 1879. [Google Scholar]
- 9.McFall-Ngai MJ. Unseen forces: the influence of bacteria on animal development. Dev. Biol. 2002;242:1–14. doi: 10.1006/dbio.2001.0522. [DOI] [PubMed] [Google Scholar]
- 10.Hurek T, Reinhold-Hurek B. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J. Biotechnol. 2003;106:169–178. doi: 10.1016/j.jbiotec.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Gage DJ. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 2004;68:280–300. doi: 10.1128/MMBR.68.2.280-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams DG, Bergman B, Nierzwicki-Bauer SA, Rai AN, Schüβler A. The Prokaryotes. New York; Springer: 2006. pp. 331–363. [This book chapter provides an excellent overview of plant–cyanobacteria symbioses.] [Google Scholar]
- 13.Jones KJ, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nature Rev. Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [An excellent review of legume–rhizobia symbiosis establishment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usher KM, Bergman B, Raven JA. Exploring cyanobacterial mutualisms. 2007;38:255–273. [Google Scholar]
- 15.Adams DG, Duggan PS. Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 2008;59:1047–1058. doi: 10.1093/jxb/ern005. [DOI] [PubMed] [Google Scholar]
- 16.Oldroyd GED, Downie JA. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007;71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usher KM. The ecology and phylogeny of cyanobacterial symbionts in sponges. Mar. Ecol. 2008;29:178–192. doi: 10.1007/s00248-003-1062-3. [DOI] [PubMed] [Google Scholar]
- 19.Dubilier N, Bergin C, Lott C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nature Rev. Microbiol. 2008;6:725–740. doi: 10.1038/nrmicro1992. [A scrupulous account of marine chemosynthetic symbioses.] [DOI] [PubMed] [Google Scholar]
- 20.Vrijenhoek RC. In: Topics in Geobiology. The Vent and Sea Biota. Kiel S, editor. Springer; Berlin, Germany: (in the press) [Google Scholar]
- 21.Herbert EE, Goodrich-Blair H. Friend and foe: the two faces of Xenorhabdus nematophila. Nature Rev. Microbiol. 2007;5:634–646. doi: 10.1038/nrmicro1706. [An outstanding summary of the Steinernema–Xenorhabdus symbiosis.] [DOI] [PubMed] [Google Scholar]
- 22.Clarke DJ. Photorhabdus: a model for the analysis of pathogenicity and mutualism. Cell. Microbiol. 2008;10:2159–2167. doi: 10.1111/j.1462-5822.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 23.Bright M, Giere O. Microbial symbiosis in Annelida. Symbiosis. 2005;38:1–45. [Google Scholar]
- 24.Graf J, Kikuchi Y, Rio RVM. Leeches and their microbiota: naturally simple symbiosis models. Trends Microbiol. 2006;14:365–371. doi: 10.1016/j.tim.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Buchner P. Endosymbiosis of Animals with Plant Microorganisms. Wiley & Sons; New York: 1965. [A seminal and exhaustive book on insect symbioses.] [Google Scholar]
- 26.Douglas AE. Mycetocyte symbiosis in insects. Biol. Rev. Camb. Philos. Soc. 1989;64:409–434. doi: 10.1111/j.1469-185x.1989.tb00682.x. [This is a fine guide to the overwhelming plethora of transmission modes of insect symbionts.] [DOI] [PubMed] [Google Scholar]
- 27.Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 28.Dale C, Moran NA. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126:453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 30.Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote–animal symbioses. Nature Rev. Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 31.Nyholm SV, McFall-Ngai MJ. The winnowing: establishing the squid–Vibrio symbiosis. Nature Rev. Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [A compelling overview of the establishment of the squid–Vibrio spp. symbiosis.] [DOI] [PubMed] [Google Scholar]
- 32.Visick KL, Ruby EG. Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 2006;9:632–638. doi: 10.1016/j.mib.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 34.Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nature Immunol. 2004;5:569–573. doi: 10.1038/ni1079. [DOI] [PubMed] [Google Scholar]
- 35.Cheesman SE, Guillemin K. We know you are in there: conversing with the indigenous gut microbiota. Res. Microbiol. 2007;158:2–9. doi: 10.1016/j.resmic.2006.10.005. [This review ponders the parallels between vertebrate microbial mutualisms and the squid–Vibrio spp. symbiosis.] [DOI] [PubMed] [Google Scholar]
- 36.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Rev. Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West N, Adams DG. Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site. Appl. Environ. Microbiol. 1997;63:4479–4484. doi: 10.1128/aem.63.11.4479-4484.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa JL, Paulsrud P, Rikkinen J, Lindblad P. Genetic diversity of Nostoc symbionts endophytically associated with two bryophyte species. Appl. Environ. Microbiol. 2001;67:4393–4396. doi: 10.1128/AEM.67.9.4393-4396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikuchi Y, Hosokawa T, Fukatsu T. Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 2007;73:4308–4316. doi: 10.1128/AEM.00067-07. [The first report of a horizontally transmitted insect symbiont.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee K-H, Ruby EG. Detection of the light organ symbiont, Vibrio fischeri, in hawaiian seawater by using lux gene probes. Appl. Environ. Microbiol. 1992;58:942–947. doi: 10.1128/aem.58.3.942-947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gros O, Liberge M, Heddi A, Khatchadourian C, Felbeck H. Detection of the free-living forms of sulfide-oxidizing gill endosymbionts in the lucinid habitat (Thalassia testdinum environment) Appl. Environ. Microbiol. 2003;69:6264–6267. doi: 10.1128/AEM.69.10.6264-6267.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aida M, et al. Distribution and population of free-living cells related to endosymbiont A harboured in Oligobrachia mashikoi (a siboglinid polychaete) inhabiting Tsukumo Bay. Microbes Environ. 2008;23:81–88. doi: 10.1264/jsme2.23.81. [DOI] [PubMed] [Google Scholar]
- 43.Harmer TL, et al. Free-living tube worm endosymbionts found at deep-sea vents. Appl. Environ. Microbiol. 2008;74:3895–3898. doi: 10.1128/AEM.02470-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- 45.Salerno JL, et al. Characterization of symbiont populations in life-history stages of mussels from chemosynthetic environments. Biol. Bull. 2005;208:145–155. doi: 10.2307/3593123. [DOI] [PubMed] [Google Scholar]
- 46.Callsen-Cencic P, Flügel HJ. Larval development and the formation of the gut of Siboglinum poseidoni Flügel & Langhof (Pogonophora, Perviata). Evidence of protostomian affinity. Sarsia. 1995;80:73–89. [Google Scholar]
- 47.McCann J, Stabb EV, Milikan DS, Ruby EG. Population effects of Vibrio fischeri during infection of Euprymna scolopes. Appl. Environ. Microbiol. 2003;69:5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gros O, Frenkiel L, Moueza M. Gill filament differentiation and experimental colonization by symbiotic bacteria in aposymbiotic juveniles of Codakia orbicuaris (Bivalvia: Lucinidae) Invertebr. Reprod. Dev. 1998;34:219–231. [Google Scholar]
- 49.Nussbaumer AD, Fisher CR, Bright M. Horizontal endosymbiont transmission in hydrothermal vent tubeworms. Nature. 2006;441:345–348. doi: 10.1038/nature04793. [A groundbreaking study of the acquisition of vestimentiferan symbionts.] [DOI] [PubMed] [Google Scholar]
- 50.Bates JM, et al. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 2006;297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Cooper JE. Multiple responses of rhizobia to flavonoids during legume root infection. Adv. Bot. Res. 2004;41:1–62. [Google Scholar]
- 52.Brencic A, Winans S. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol. Mol. Biol. Rev. 2005;69:155–194. doi: 10.1128/MMBR.69.1.155-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robidart JC, et al. Metabolic versatility of the Riftia pachyptila endosymbiont revealed through metagenomics. Environ. Microbiol. 2008;10:727–737. doi: 10.1111/j.1462-2920.2007.01496.x. [DOI] [PubMed] [Google Scholar]
- 54.Gros O, Darrasse A, Durand P, Frenkiel L, Moueza M. Environmental transmission of a sulfur-oxidizing bacterial gill endosymbiont in the tropical lucinid bivalve Codakia orbicularis. Appl. Environ. Microbiol. 1996;62:2324–2330. doi: 10.1128/aem.62.7.2324-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruby EG, Lee K-H. The Vibrio fischeri-Euprymna scolopes light orga. association: current ecological paradigms. Appl. Environ. Microbiol. 1998;64:805–812. doi: 10.1128/aem.64.3.805-812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stabb EV, Ruby EG. Contribution of pilA to competitive colonization of the squid Euprymna scolopes by Vibrio fischeri. Appl. Environ. Microbiol. 2003;69:820–826. doi: 10.1128/AEM.69.2.820-826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adin DM, et al. Characterization of htrB and msbB mutants of the light organ symbiont Vibrio fischeri. Appl. Environ. Microbiol. 2007;74:633–644. doi: 10.1128/AEM.02138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fauvart M, Michiels J. Rhizobial secreted proteins as determinants of host specificity in the rhizobium-legume symbiosis. FEMS Microbiol. Lett. 2008;285:1–9. doi: 10.1111/j.1574-6968.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 59.Aeckersberg F, Lupp C, Feliciano B, Ruby EG. Vibrio fischeri outer membrane protein OmpU plays a role in normal symbiotic colonization. J. Bacteriol. 2001;183:6590–6597. doi: 10.1128/JB.183.22.6590-6597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, et al. A genomic view of the human–Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 61.Chun CK, et al. Effects of colonization, luminescence, and autoinducer on host transcription during development of the squid-vibrio association. Proc. Natl Acad. Sci. USA. 2008;105:11323–11328. doi: 10.1073/pnas.0802369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bulgheresi S, Schabussova I, Mullin NP, Maizels RM, Ott JA. A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 2006;72:2950–2956. doi: 10.1128/AEM.72.4.2950-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gourdine JP, Smith-Ravin EJ. Analysis of a cDNA-derived sequence of a novel mannose-binding lectin, codakine, from the tropical clam Codakia orbicularis. Fish Shellfish Immunol. 2007;22:498–509. doi: 10.1016/j.fsi.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Yip ES, Grublesky BT, Hussa EA, Visick KL. A novel, conserved cluster of genes promotes symbiotic colonization and σ54-dependent biofilm formation by Vibrio fischeri. Mol. Microbiol. 2005;57:1485–1498. doi: 10.1111/j.1365-2958.2005.04784.x. [DOI] [PubMed] [Google Scholar]
- 65.Hussa EA, O'Shea TM, Darnell CL, Ruby EG, Visick KL. Two-component response regulators of Vibrio fischeri: identification, mutagenesis, and characterization. J. Bacteriol. 2007;189:5825–5838. doi: 10.1128/JB.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandel MJ, Wollenberg MS, Stabb EV, Visick KL, Ruby EG. A single regulatory gene is sufficient to alter bacterial host range. Nature. 2009;458:215–218. doi: 10.1038/nature07660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoang HH, Becker A, Gonzalez JE. The LuxR homolog ExpR, in combination with the Sin quorum sensing system, plays a central role in Sinorhizobium meliloti gene expression. J. Bacteriol. 2004;186:5460–5472. doi: 10.1128/JB.186.16.5460-5472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao M, et al. sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 2005;187:7931–7944. doi: 10.1128/JB.187.23.7931-7944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper JE. Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J. Appl. Microbiol. 2007;103:1355–1365. doi: 10.1111/j.1365-2672.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- 70.De Hoff PL, Brill LM, Hirsch AM. Plant lectins: the ties that bind in root symbiosis and plant defense. Mol. Genet. Genomics. 2009;282:1–15. doi: 10.1007/s00438-009-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujishige NA, et al. Rhizobium common nod genes are required for biofilm formation. Mol. Microbiol. 2008;67:504–515. doi: 10.1111/j.1365-2958.2007.06064.x. [DOI] [PubMed] [Google Scholar]
- 72.Goormachtig S, Capoen W, Holsters M. Rhizobium infection: lessons from the versatile nodulation behaviour of water-tolerant legumes. Trends Plant Sci. 2004;9:518–522. doi: 10.1016/j.tplants.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Bartsev A, et al. NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions. Plant Physiol. 2004;134:871–879. doi: 10.1104/pp.103.031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ. NO means ‘yes’ in the squid-vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cell. Microbiol. 2004;6:1139–1151. doi: 10.1111/j.1462-5822.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 75.Moran NA, Dunbar HE. Sexual acquisition of beneficial symbionts in aphids. Proc. Natl Acad. Sci. USA. 2006;103:12803–12806. doi: 10.1073/pnas.0605772103. [This is the first report of intraspecific symbiont transfer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Usher KM, Sutton DC, Toze S, Kuo J, Fromont J. Inter-generational transmission of microbial symbionts in the marine sponge Chondrilla australiensis (Demospongiae) Mar. Freshw. Res. 2005;56:125–131. [Google Scholar]
- 77.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 2008;42:1–25. doi: 10.1146/annurev.genet.41.110306.130354. [A must-read review on Wolbachia spp. transmission and interactions with its host.] [DOI] [PubMed] [Google Scholar]
- 78.Perkins SK, Peters GA. The Azolla-Anabaena symbiosis: endophyte continuity in the Azolla life-cycle is facilitated by epidermal trichomes. I. Partitioning of the endophytic Anabaena into developing sporocarps. New Phytol. 1993;123:53–64. [Google Scholar]
- 79.Werren JH, Skinner SW, Huger AM. Male-killing bacteria in a parasitic wasp. Science. 1986;231:990–992. doi: 10.1126/science.3945814. [DOI] [PubMed] [Google Scholar]
- 80.Huigens ME, et al. Infectious parthenogenesis. Nature. 2000;405:178–179. doi: 10.1038/35012066. [DOI] [PubMed] [Google Scholar]
- 81.Huigens ME, de Almeida RP, Boons PA, Luck RF, Stouthamer R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. Biol. Sci. 2004;271:509–515. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiel E, et al. Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS ONE. 2009;4:e4767. doi: 10.1371/journal.pone.0004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brennan LJ, Keddie BA, Braig HR, Harris HL. The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS ONE. 2008;3:e2083. doi: 10.1371/journal.pone.0002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frank SA. Host control of symbiont transmission: the separation of symbionts into germ and soma. Am. Nat. 1996;148:1113–1124. [Google Scholar]
- 85.Dale C, Plague GR, Wang B, Ochman H, Moran NA. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc. Natl Acad. Sci. USA. 2002;99:12397–12402. doi: 10.1073/pnas.182213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giere O, Langheld C. Structural organisation, transfer and biological fate of endosymbiotic bacteria in gutless oligochaetes. Mar. Biol. 1987;93:641–650. [Google Scholar]
- 87.Hirose E. Plant rake and algal pouch of the larvae in the tropical ascidian Diplosoma similis: an adaptation for vertical transmission of photosynthetic symbionts Prochloron sp. Zool. Sci. 2000;17:233–240. [An exquisite morphological study of ascidian adaptations to symbiont vertical transmission.] [Google Scholar]
- 88.Hirose E, Fukuda T. Vertical transmission of photosymbionts in the colonial ascidian Didemnum molle: the larval tunic prevents symbionts from attaching to the anterior part of larvae. Zool. Sci. 2006;23:669–674. doi: 10.2108/zsj.23.669. [DOI] [PubMed] [Google Scholar]
- 89.Hirose E, Adachi R, Kuze K. Sexual reproduction of the Prochloron-bearing ascidians, Trididemnum cyclops and Lissoclinum bistratum, in subtropical waters: seasonality and vertical transmission of photosymbionts. J. Mar. Biolog. Assoc. UK. 2006;86:175–179. [Google Scholar]
- 90.Hirose E, Hirose M. Morphological process of vertical transmission of photosymbionts in the colonial ascidian Trididemnum miniatum Kott, 1977. Mar. Biol. 2007;150:359–367. [Google Scholar]
- 91.Ereskovsky AV, Boury-Esnault N. Cleavage pattern in Oscarella species (Porifera, Demospongiae, Homoscleromorpha): transmission of maternal cells and symbiotic bacteria. J. Nat. Hist. 2002;36:1761–1775. [Google Scholar]
- 92.Kaye HR. Sexual reproduction in four Caribbean commercial sponges. II. Oogenesis and transfer of bacterial symbionts. Invertebr. Reprod. Dev. 1991;19:13–24. [Google Scholar]
- 93.Ereskovsky AV, Gonobobleva E, Vishnyakov A. Morphological evidence for vertical transmission of symbiotic bacteria in the viviparous sponge Halisarca dujardini Johnston (Porifera, Demospongiae, Halisarcida) Mar. Biol. 2005;146:869–875. [Google Scholar]
- 94.Sharp KH, Davidson SK, Haygood MG. Localization of ‘Candidatus Endobugula sertula’ and the bryostatins throughout the life cycle of the bryozoan Bugula neritina. ISME J. 2007;1:693–702. doi: 10.1038/ismej.2007.78. [A first-rate description of the transmission of a bryozoan symbiont.] [DOI] [PubMed] [Google Scholar]
- 95.Cary SC. Vertical transmission of a chemoautotrophic symbiont in the protobranch bivalve, Solemya reidi. Mol. Marine Biol. Biotechnol. 1994;3:121–130. [PubMed] [Google Scholar]
- 96.Cary SC, Giovannoni SJ. Transovarial inheritance of endosymbiotic bacteria in clams inhabiting deep-sea hydrothermal vents and cold seeps. Proc. Natl Acad. Sci. USA. 1993;90:5695–5699. doi: 10.1073/pnas.90.12.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Endow K, Ohta S. Occurrence of bacteria in the primary oocytes of vesicomyid clam Calyptogena soyoae. Mar. Ecol. Prog. Ser. 1990;64:309–311. [Google Scholar]
- 98.Eberle MW, McLean DL. Initiation and orientation of the symbiote migration in the human body louse Pediculus humanus L. J. Insect Physiol. 1982;28:417–422. [Google Scholar]
- 99.Eberle MW, McLean DL. Observation of symbiote migration in human body lice with scanning and transmission electron microscopy. Can. J. Microbiol. 1983;29:755–762. doi: 10.1139/m83-123. [This paper provides some marvellous micrographs of the louse symbionts on their way to the ovaries.] [DOI] [PubMed] [Google Scholar]
- 100.Sasaki-Fukatsu K, et al. Symbiotic bacteria associated with stomach discs of human lice. Appl. Environ. Microbiol. 2006;72:7349–7352. doi: 10.1128/AEM.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ciche TA, Kim K-S, Kaufmann-Daszczuk B. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl. Environ. Microbiol. 2008;74:2275–2287. doi: 10.1128/AEM.02646-07. [An accurate description of a peculiar and unexpected vertical transmission route.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brugirard-Ricaud K, et al. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell. Microbiol. 2005;7:363–371. doi: 10.1111/j.1462-5822.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 103.Martens EC, Goodrich-Blair H. The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell. Microbiol. 2005;7:1723–1735. doi: 10.1111/j.1462-5822.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 104.Rio RV, Maltz M, McCormick B, Reiss A, Graf J. Symbiont succession during the embryonic development of the european medicinal leech, Hirudo verbana. Appl. Environ. Microbiol. 2009;5:6890–6895. doi: 10.1128/AEM.01129-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Büsing K-H, Döll W, Freytag K. Die Bakterienflora der medizinischen medizinischen Blutegel. Arch. Mikrobiol. 1953;19:52–86. [PubMed] [Google Scholar]
- 106.Silver AC, et al. Interaction between innate immune cells and a bacterial type III secretion system in mutualistic and pathogenic associations. Proc. Natl Acad. Sci. USA. 2007;104:9481–9486. doi: 10.1073/pnas.0700286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Usher KM, Kuo J, Fromont J, Sutton DC. Vertical transmission of cyanobacterial symbionts in the marine sponge Chondrilla australiensis (Demospongiae) Hydrobiologia. 2001;461:15–23. [Google Scholar]
- 108.Gottlieb Y, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 2008;22:2591–2599. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- 109.Miura T, et al. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea) J. Exp. Zool. 2003;295B:59–81. doi: 10.1002/jez.b.3. [DOI] [PubMed] [Google Scholar]
- 110.Mira A, Moran NA. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 2002;44:137–143. doi: 10.1007/s00248-002-0012-9. [DOI] [PubMed] [Google Scholar]
- 111.Cowles CE, Goodrich-Blair H. The Xenorhabdus nematophila nilABC genes confer the ability of Xenorhabdus spp. to colonize Steinernema carpocapsae nematodes. J. Bacteriol. 2008;190:4121–4128. doi: 10.1128/JB.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chandra H, Khandelwal P, Khattrl A, Banerjee N. Type 1 fimbriae of insecticidal bacterium Xenorhabdus nematophila is necessary for growth and colonization of its symbiotic host nematode Steinernema carpocapsiae. Environ. Microbiol. 2008;10:1285–1295. doi: 10.1111/j.1462-2920.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 113.Davidson SK, Stahl DA. Selective recruitment of bacteria during embryogenesis of an earthworm. ISME J. 2008;2:510–518. doi: 10.1038/ismej.2008.16. [This is a high quality account of the symbiont journey to the nephridia of developing earthworms.] [DOI] [PubMed] [Google Scholar]
- 114.Gustafson RG, Reid RGB. Larval and post-larval morphogenesis in the gutless protobranch bivalve Solemya reidi (Cryptodonta: Solemyidae) Mar. Biol. 1988;97:373–387. [Google Scholar]
- 115.Gustafson RG, Reid RGB. Association of bacteria with larvae of the gutless protobranch bivalve Solemya reidi (Cryptodonta: Solemyidae) Mar. Biol. 1988;97:389–401. [Google Scholar]
- 116.Ohta T. Slightly deleterious mutant substitutions in evolution. Nature. 1973;246:96–98. doi: 10.1038/246096a0. [DOI] [PubMed] [Google Scholar]
- 117.Moran NA. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl Acad. Sci. USA. 1996;93:2873–2878. doi: 10.1073/pnas.93.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Funk DJ, Wernegreen JJ, Moran NA. Intraspecific variation in symbiont genomes: bottlenecks and the Aphid-Buchnera association. Genetics. 2001;157:477–489. doi: 10.1093/genetics/157.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 120.Rio RVM, Lefevre C, Heddi A, Aksoy S. Comparative genomics of insect-symbiotic bacteria: influence of host environment on microbial genome composition. Appl. Environ. Microbiol. 2003;69:6825–6832. doi: 10.1128/AEM.69.11.6825-6832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woyke T, et al. Symbiosis insights through metagenomic analysis of a microbial consortium. Nature. 2006;443:950–955. doi: 10.1038/nature05192. [DOI] [PubMed] [Google Scholar]
- 122.Charles H, Heddi A, Guillaud J, Nardon C, Nardon P. A molecular aspect of symbiotic interactions between the weevil Sitophilus oryzae and its endosymbiotic bacteria: over-expression of a chaperonin. Biochem. Biophys. Res. 1997;239:769–774. doi: 10.1006/bbrc.1997.7552. [DOI] [PubMed] [Google Scholar]
- 123.Plague GR, Dunbar HE, Tran PL, Moran NA. Extensive proliferation of transposable elements in heritable bacterial symbionts. J. Bacteriol. 2008;190:777–779. doi: 10.1128/JB.01082-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mira A, Ochman H, Moran NA. Deletional bias and the evolution of bacterial genomes. Trends Genet. 2001;17:589–596. doi: 10.1016/s0168-9525(01)02447-7. [DOI] [PubMed] [Google Scholar]
- 125.Moran NA, Plague GR. Genomic changes following host restriction in bacteria. Curr. Opin. Genet. Dev. 2004;14:627–633. doi: 10.1016/j.gde.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Gros O, Liberge M, Felbeck H. Interspecific infection of aposymbiotic juveniles of Codakia orbicularis by various tropical lucinid gill-endosymbionts. Mar. Biol. 2003;142:57–66. [Google Scholar]
- 127.Kikuchi Y, Meng XY, Fukatsu T. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae) Appl. Environ. Microbiol. 2005;71:4035–4043. doi: 10.1128/AEM.71.7.4035-4043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nishiguchi MK, Ruby EG, McFall-Ngai MJ. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-Vibrio symbioses. Appl. Environ. Microbiol. 1998;64:3209–3213. doi: 10.1128/aem.64.9.3209-3213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Won Y-J, Jones WJ, Vrijenhoek RC. Absence of cospeciation between deep-sea mytilids and their thiotrophic endosymbionts. J. Shellfish Res. 2008;27:129–138. [Google Scholar]
- 130.Goffredi SK, Hurtado LA, Hallam S, Vrijenhoek RC. Evolutionary relationships of deep-sea vent and cold seep clams (Mollusca: Vesicomyidae) of the “pacifica/lepta” species complex. Mar. Biol. 2003;142:311–320. [Google Scholar]
- 131.Hurtado LA, Mateos M, Lutz RA, Vrijenhoek RC. Coupling of bacterial endosymbiont and host mitochondrial genomes in the hydrothermal vent clam Calyptogena magnifica. Appl. Environ. Microbiol. 2003;69:2058–2064. doi: 10.1128/AEM.69.4.2058-2064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stewart FJ, Young CR, Cavanaugh CM. Lateral symbiont acquisition in a maternally transmitted chemosynthetic clam endosymbiosis. Mol. Biol. Evol. 2008;25:673–687. doi: 10.1093/molbev/msn010. [DOI] [PubMed] [Google Scholar]
- 133.Allen JM, Reed DL, Perotti MA, Braig HR. Evolutionary relationships of “Candidatus Riesia spp.”, endosymbiotic Enterobacteriaceae living within hematophagous primate lice. Appl. Environ. Microbiol. 2007;73:1659–1664. doi: 10.1128/AEM.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schramm A, et al. Acidovorax-like symbionts in the nephridia of earthworms. Environ. Microbiol. 2003;5:804–809. doi: 10.1046/j.1462-2920.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- 135.Shoemaker DD, et al. The distribution of Wolbachia in fig wasps: correlations with host phylogeny, ecology and population structure. Proc. R. Soc. Lond., B, Biol. Sci. 2002;269:2257–2267. doi: 10.1098/rspb.2002.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kikuchi Y, Fukatsu T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl. Environ. Microbiol. 2003;69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reuter M, Keller L. High levels of multiple Wolbachia infection and recombination in the ant Formica exsecta. Mol. Biol. Evol. 2003;20:748–753. doi: 10.1093/molbev/msg082. [DOI] [PubMed] [Google Scholar]
- 138.Münchhoff J, et al. Host specificity and phylogeography of the prochlorophyte Prochloron sp., an obligate symbiont in didemnid ascidians. Environ. Microbiol. 2007;9:890–899. doi: 10.1111/j.1462-2920.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 139.Blazejak A, Kuever J, Erseus C, Amann R, Dubilier N. Phylogeny of 16S rRNA, ribulose 1, 5-bisphosphate carboxylase/oxygenase, and adenosine 5′-phosphosulfate reductase genes from gamma- and alphaproteobacterial symbionts in gutless marine worms (Oligochaeta) from Bermuda and the Bahamas. Appl. Environ. Microbiol. 2006;72:5527–5536. doi: 10.1128/AEM.02441-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim-Fong GE, Regali LA, Haygood MG. Evolutionary relationships of “Candidatus Endobugula” bacterial symbionts and their Bugula bryozoan hosts. Appl. Environ. Microbiol. 2008;72:3605–3609. doi: 10.1128/AEM.02798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krueger DM, Gustafson RG, Cavanaugh CM. Vertical transmission of chemoautotrophic symbionts in the bivalve Solemya velum (Bivalvia: Protobranchia) Biol. Bull. 1996;190:195–202. doi: 10.2307/1542539. [DOI] [PubMed] [Google Scholar]
- 142.Schmitt S, Angermeier H, Schiller R, Lindquist N, Hentschel U. Molecular microbial diversity survey of sponge reproductive stages and mechanistic insights into vertical transmission of microbial symbionts. Appl. Environ. Microbiol. 2008;74:7694–7708. doi: 10.1128/AEM.00878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davidson SK, Stahl DA. Transmission of nephridial bacteria of the earthworm Eisenia fetida. Appl. Environ. Microbiol. 2006;72:769–775. doi: 10.1128/AEM.72.1.769-775.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Perotti MA, Allen JM, Reed DL, Braig HR. Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J. 2007;21:1058–1066. doi: 10.1096/fj.06-6808com. [DOI] [PubMed] [Google Scholar]
- 145.Nardon P. Contribution à l'ètude des symbiotes ovaries de Sitophilus sasakii: localisation, histochimie et ultrastructure chez la femelle adults. C. R. Acad. Sci. III, Sci. 1971;272:2975–2978. [Google Scholar]
- 146.Meeks JC, et al. An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium. Photosyn. Res. 2001;70:85–106. doi: 10.1023/A:1013840025518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.