Abstract
Although strong positive association between alcoholism and depression is a common epidemiological observance, the causal relationship and the neurobiological substrates of such observations are far from clear. We have reported that chronic daily exposure to a relatively high dose of alcohol in rats can induce or exacerbate an already existing depressive-like behavior (Pharm Biochem Behav 91:97-103, 2008). Moreover, these effects of alcohol were blocked by pretreatment with desipramine, a tricyclic antidepressant, implicating a role for the biogenic amines in this type of depressive symptoms. In order to further delineate the involvement of specific neurotransmitters in alcohol-induced depressive symptoms, we examined the concentrations of norepinephrine (NE), dopamine (DA) and serotonin (5-HT) in the frontal cortex and hippocampus following alcohol administration as well as pretreatment with two antidepressants, nomifensine and imipramine selective NE/DA and NE/5-HT uptake inhibitors, respectively. Adult female Wistar and Wistar-Kyoto (WKY) rats were exposed to alcohol via inhalation chambers (3 hr/day for 10 days) to achieve daily blood alcohol concentration of approximately 150 mg%. On day 11, the animals were evaluated for general locomotor activity (LCA) and performance in the forced swim test (FST), followed by neurochemical analyses. As expected WKY had lower LCA and higher immobile FST compared to Wistar rats. WKY rats also had lower levels of all three biogenic amines compared to Wistar rats in both areas. However, only cortical NE was reduced in both strains following alcohol administration. Treatments with nomifensine and imipramine blocked the behavioral and most of the neurochemical deficits caused by alcohol in both strains. These results implicate cortical NE as a major player in alcohol-induced depression. Moreover, it is suggested that selective NE uptake inhibitors may be of particular therapeutic potential in co-morbid condition of alcoholism and depression.
Keywords: Alcohol, Depression, Monoamines, Norepinephrine uptake inhibitor, Imipramine, Nomifensine, Animal Model, WKY Rats, Hippocampus, Frontal Cortex
Introduction
A significant co-morbid expression of alcoholism and depression, particularly in women is evident in epidemiological studies (Dixit and Crum, 2000; Rodgers et al., 2000; Spak et al., 2000). This dual diagnosis is an important clinical assessment since treatment outcome for either condition, if considered alone, may not be fully adequate. Interestingly, pharmacologic treatment of the depressive symptoms results in better treatment outcome for alcoholism (Kessler et al., 1997; Schuckit et al., 1997). Likewise, treatment of primary alcoholism results in rapid reduction in depressive symptom (Brown and Schuckit, 1988). Indeed, 80% of alcoholic patients with major depression no longer present depressive symptoms after 2 wks of sobriety (Dackis et al., 1986). However, if unmanaged, depressive symptom especially during alcohol withdrawal can lead to relapse and increased alcohol intake (Johanson and Fischman, 1989; Hodgins et al., 1995; Schulteis, et al., 1995; Dixit and Crum, 2000).
Positive association between depressive symptom and voluntary alcohol intake has also been observed in animal models of depression and alcoholism (Overstreet, et al., 1992; Paré et al., 1999). Thus, Wistar-Kyoto (WKY) rats, considered a putative animal model of depression voluntarily consume more alcohol than their control counterpart Wistar rats or Sprague-Dawley rats (Paré et al., 1999; Jiao et al., 2006). The Fawn-Hooded (FH) rats which exhibit both high alcohol intake and depressive characteristics may serve as a model of human alcoholism-depression co-morbid condition (Rezvani et al., 2002, 2007). Although various theories have attempted to explain the association between alcoholism and depression, it appears that complex factors including genetic predisposition and alterations in neurochemical substrates by high alcohol level may contribute to such co-morbidity (Merikangas and Gelernter, 1990; Nurnberger and Berrettini, 1998; Rezvani et al., 2002, 2007; Ovestreet et al., 2005, 2008).
Neuronal systems that are involved in mood regulation and are primary targets for the development of antidepressants include the serotonergic and noradrenergic systems (Rozas, 2009). Results of various studies also support a role for the dopaminergic system in depression, particularly in conjunction with psychomotor impairment (Clausius et al., 2009). In some instances dopaminergic agents have been used to manage depression when conventional antidepressant treatments fail (Willner, 1997). Involvement of these biogenic amines in alcoholism is also amply documented (Ratsma et al., 2002; Tupala and Tiihonen, 2004). We have observed that chronic administration of a relatively high alcohol dose induces depressive-like behavior in Wistar rats and exacerbates the depressive characteristics of the WKY rats as evidenced by exaggerated immobility in the forced swim test (Getachew et al., 2008). Moreover, treatment with the tricyclic antidepressant desipramine, a selective norepinephrine/serotonin reuptake inhibitor, normalizes the depressive-like behavior of the WKY rats and effectively blocks the “depressogenic” effects of alcohol in both strains (Getachew et al., 2008). It is of relevance to note that WKY rats which respond well to tricyclic antidepressants for normalization of their depressive characteristic do not respond to selective serotonin reuptake inhibitors (SSRI) such as fluoxetine or paroxetine and hence may be considered as a model of treatment resistant depression (Griebel et al., 1994; Lahmame et al., 1997; Lopez-Rubalcaava and Lucki, 2000; Tejani-Butt et al., 2003).
Although the results of our previous study with desipramine (Getachew et al., 2008) strongly implicate NE or 5-HT in alcohol-induced depressive behavior in both Wistar and WKY rats, they do not rule out a possible role for DA. Furthermore, the contribution from individual neurotransmitter and/or brain region in these effects of alcohol is not evident. Thus, this study was designed to further delineate the involvement of specific neurotransmitters and brain regions in alcohol-induced depression and its normalization by clinically useful antidepressants. Since ample evidence provided by clinical imaging and pharmacological studies implicate the frontal cortex and the hippocampus as two primary areas involved in mood regulation and response to antidepressants, we concentrated on these two areas (Caldecott-Hazard et al. 1991; Sheline et al 1996; Mayberg 1994; Drevets et al., 1997; Bremner et al 2002; Schmidt and Duman, 2007).
We selected two compounds (imipramine and nomifensine) which possess relatively similar affinity for blocking the norepinephrine transporter (NET), but have distinct affinity for blocking the 5-HT transporter. Our hypotheses were: 1) WKY rats will have lower levels of biogenic amines (particularly NE) in frontal cortex and hippocampus compared to Wistar rats, 2) Alcohol-induced depressive-like behavior will be associated with a reduction of biogenic amines (NE, DA and 5-HT) in both strains. 3) Both imipramine and nomifensine will normalize the behavioral and neurochemical deficits induced by chronic alcohol exposure.
Materials and Method
Animals
Age matched adult female WKY and Wistar (WIS) rats (Harlan, Indianapolis, IN) were used throughout the study. Animals were housed in groups of four in standard polypropylene shoebox cages (42 × 20.5 × 20 cm) on hardwood chip bedding (alpha-dry) in a room designated for female rats. Throughout the experiment animals had free access to food (Harlan Tek Lab) and water. The room was maintained at 24-26 °C at 55-66% relative humidity, on a 12-h reversed light/dark cycle (lights on at 1900 hr). All experiments were carried out in accordance with NIH guidelines as approved by the Institutional Animal Care and Use Committee.
To acclimatize the subjects to housing conditions, animals arrived one week prior to testing. During this period, they were handled once daily in order to minimize any stress effects that might result from routine handling. The animals were weighed on day 1 and day 10 of the study. All behavioral testing were carried out in the early portion of the dark phase (active behavioral phase for rats) between 9:00 A.M. and 12:00 P.M using a red light as source of illumination.
Ethanol (EtOH) Exposure
Inhalation chambers were used to expose the animals to EtOH (La Jolla Alcohol Research Inc., La Jolla, CA). Briefly, 95% EtOH was pumped at regulated rate from 5 gallon reservoir via a peristaltic pump to a 5000 ml Erlenmeyer vacuum flask that was kept on a warming tray (52 °C). EtOH was then volatilized and mixed with pressurized air. The flow of this mixture was controlled by a pressure gauge as it was delivered to individual chambers. The control group received only air via exactly similar system (Getachew et al., 2008). The inhalation chamber has the advantage of easily achieving and maintaining the targeted blood alcohol level (Kliethermes et al., 2004). Moreover, the variability in the EtOH concentration between similarly controlled chambers is minimal (Lee et al., 2000).
USP 200 proof EtOH was purchased from VWR Scientific Products (Bridgeport, NJ) and was diluted down with distilled water to 95% ethanol v/v.
Drug Treatments
Impiramine HCl (Imip), and nomifensine maleate (Nomi) were purchased from Sigma-Aldrich Co. (St. Louis, MO). The selection of these drugs was based on their similar affinity for NE transporter but distinct affinity for 5-HT transporter. Thus, both Imip and Nomi block the NE transporter with inhibition constant (Ki) of 37 and 15.6 nanomoles respectively, whereas the Ki values for 5-HT transporter are (Imip: 1.41 nmoles, Nomi: 1,000 nmoles) (Owens et al., 1997; Baldessarini, 2006).
Drugs were dissolved in saline and were injected intraperitoneally (i.p.) (10 mg/kg in volume of 1ml/kg). The selection of dosages was based on previous studies showing effectiveness of the drug doses as antidepressant or against alcohol consumption in WKY rats (Lahmame et al., 1997; Paré et al 1999; Jiao et al., 2006). Control animals were injected with saline.
Experimental Protocol
EtOH-naive female WKY and WIS rats were randomly divided and placed in either EtOH vapor or air inhalation chambers (4 rats/chamber) 3 hrs daily for ten days. We used the same parameters as in our previous protocol (e.g. air pressure ≈5 psi, airflow rate ≈ 15-20, drip rate = 60 ml/hr) to achieve the target blood alcohol concentration (BAC) of approximately 150 mg% which is equivalent to relatively heavy moderate consumption in humans (Getachew et al., 2008). The BAC was determined in each experimental protocol to ensure the achievement of the desired level (see below).
Each experimental protocol consisted of 4 groups, 8 animals/group including both WIS and WKY rats. In the first set of experiments, to determine the effects of alcohol in the two strains, animals were exposed to alcohol or air for 10 days, and 18-20 hr after the last exposure were assessed for the behavioral parameters.
In subsequent sets of experiments, to determine the effects of antidepressants on alcohol-induced changes, new groups of WIS and WKY rats were exposed to alcohol daily as in the first experiment, but each day following alcohol exposure they were treated with saline or an antidepressant. Thus, in the second set of experiments the effects of impiramine and in the third set of experiments the effects of nomifensine was evaluated. As in the first experiment, animals were evaluated for the behavioral tests 18-20 hr after the last exposure. Moreover, following 2 hrs of rest in their home cage, these animals were sacrificed for neurochemical evaluation.
Blood alcohol determination
To determine the BAC at various time points, a separate group of WKY and WIS rats (4 rats/group) was exposed to EtOH under identical conditions. Blood from these animals was sampled by tail bleed technique every three days immediately after the end of EtOH exposure. Briefly, tail blood (0.4 ml) was collected in tubes coated with 0.2M ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich CO., St. Louis, MO) and centrifuged for 5 min at 1500g at 4°C. The plasma was extracted and BAC was assayed by injecting 5μL plasma into GM7 Micro-Stat Analyzer (Analox Instruments Ltd., Lunenburg, MA).
Locomotor Activity (LCA) Monitoring
On day 11, at least 1 hr before behavioral tests, animals were moved from the housing units to the testing room in their home cages. Open-field LCA was measured for ten minutes in each animal immediately preceding the forced swim test. An open-field activity monitoring cage (27 × 27 × 20.3 cm, Med Associates, Inc., St. Albans, VT) was used to assess activity. Ambulatory counts representing the number of infrared beam interruptions were recorded.
Forced Swim Test (FST)
The method of Porsolt et al. (1977) with modification by Detke et al. (1995) was used to assess the immobility of the rats as a measure of their helplessness or depressive-like behavior (Porsolt et al., 1977; Detke, 1995). Rats were placed individually in a round Pyrex cylinder pool measuring 17 cm in diameter and 60 cm in height for 5 min. The cylinder was filled with 30 cm water (25±1 °C) to ensure that animals could not touch the bottom of the container with their hind paws or their tails (Lucki, 1997). The FST activity was video recorded for each animal for subsequent analysis. The rat was removed after 5 min, dried, and placed in its home cage. A time sampling scoring technique was used whereby the predominant behavior in each 5-s period of the 300-s test was recorded. Inactivity (immobility) and activity (swimming) were distinguished as mutually exclusive behavioral states. Swimming behavior was defined as movement (usually horizontal) throughout the cylinder. Immobility was defined when no additional activity was observed other than that required to keep the rat's head above the water (Getachew et al., 2008).
Brain Dissection and Monoamine Level Determination
Two hrs following the behavioral testing, animals were sacrificed and their brains were removed and stored at -80°C. Frozen brain tissues were thawed on ice and frontal cortex and hippocampus (bilateral) were dissected alternating between strains and treatment groups as described previously (Tizabi et al., 1999, 2001). The dissected regions were placed in 0.1 N HCl and were sonicated for 10 seconds. Twenty microliters of homogenate was extracted for protein determination. The rest of the homogenate was centrifuged at 16 000×g for 15 min at 4 °C. The supernatant was then transferred into 0.2 micron filter centrifuge tubes and re-centrifuged. The supernatant was removed and analyzed by reverse phase high performance liquid chromatography (RP-HPLC) using LCEC analyzer (LC22C, Bioanalytical Systems, Indianapolis, IN) and electrochemical detector to determine the concentration of DA, NE and 5-HT. The degassed mobile phase, 2% solution of acetonitrile containing 0.6% tetrahydrofuran, 0.1% diethylamine, 0.025 mM EDTA, 2.3 mM 1-octane-sulfonic acid (final pH 3.1) was delivered at a flow rate of 1300 μl/min. The mobile phase and the supernatant were filtered through a Millipore type HA 0.22 μm membrane and samples were injected directly into Rheodyne 7125, 5μL loop with backflow pressure 3000-3500 psi. The liquid chromatography system was equipped with ODS column (1×125mm) and electrochemical detector (Bioanalytical Systems, Indianapolis, IN). Concentrations of unknown peaks were generated automatically by Chromatography software package. Calculations of unknowns were performed using built-in internal standard method. In this system, optical densities were automatically converted into microgram units derived from a standard curve. Protein analysis was performed by Peirce protein assay using BCA reagent. Measurements of monoamines were expressed as nanogram substrate/microgram of protein (ng/ug Pr).
Statistical Analysis
All data were analyzed using Two-way analysis of variance (ANOVA), followed by Tukey's post hoc test when significant main effects were indicated. In instances where only two groups needed to be compared, the student's t test was applied. All analyses were two-tailed and P<0.05 was considered significant. It is important to note that in the experimental design the effect of each drug was evaluated separately. Thus, independent statistical analysis was applied for each experiment. In depicting the results, however, the effects of various treatments are combined in a single graph for simplicity of visual comparison.
Results
I. Body Weight
Wistar rats of the same age tend to be significantly heavier than WKY rats (WIS = 260 ± 2 gm, WKY= 213 ± 3 gm, at the beginning of the experiment). The difference in weight remained consistent throughout the experiment period. Neither alcohol, nor any drug treatment affected the weight in these animals.
II. Blood alcohol concentration
Blood alcohol concentration (BAC) for both Wistar and WKY rats, at all time points ranged between 140 to 158 mg% without any significant difference between the two strains.
III. Forced Swim Test (FST)
As depicted in Figure 1A, WKY rats showed significant immobility in the FST (approximately 83% more) compared to WIS rats F(1,28)=7.8, P<0.05. Alcohol exposure resulted in an increase in immobility in both WIS (approx 50%, P<0.01) and WKY (approx 33%, P<0.05) rats. Saline treatment had no effect on immobility (data not shown). Treatment with impiramine or nomifensine totally blocked alcohol effects.
Fig. 1.
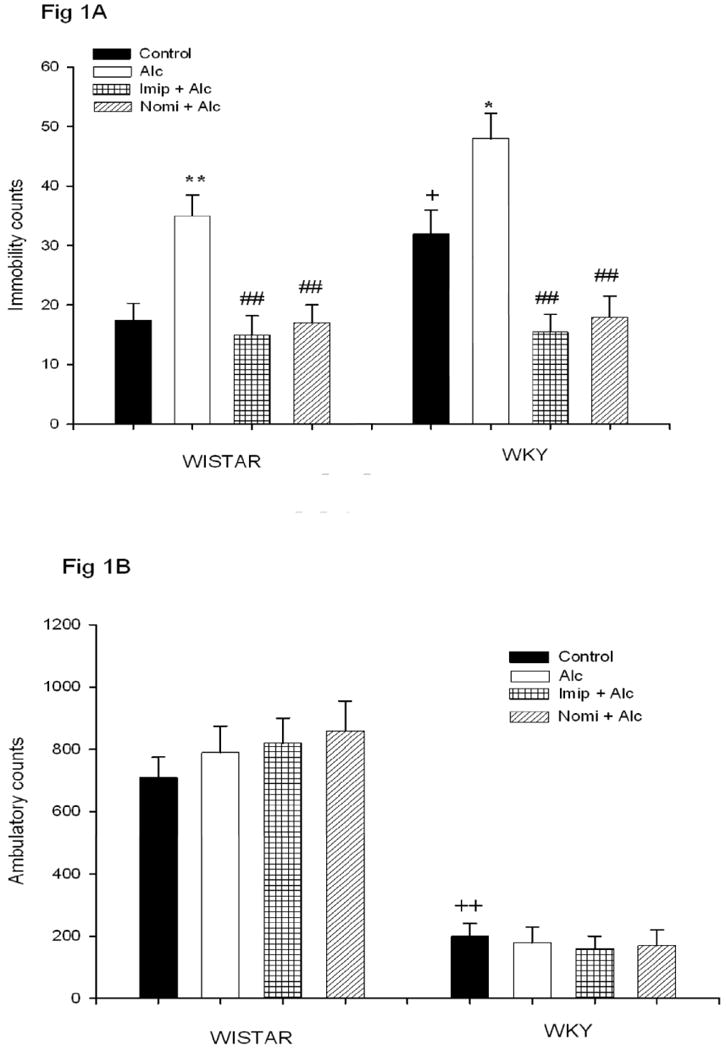
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in immobility in the FST (1A) and LCA (1B) in WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were tested for LCA and FST 18-20 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. *P<0.05, **P<0.01 compared to control. ## P<0.01 compared to alcohol (Alc). +P<0.05, ++P<0.01 compared to Wistar. N= 8/group.
IV. Locomotor Activity (LCA)
Figure 1B depicts open field LCA in WIS and WKY rats. As expected, WKY showed significantly less locomotion (approx 72% less) compared to WIS rats F(1,28) = 59, P<0.01. Neither alcohol nor any drug treatment affected the LCA in either strain, suggesting that the effect on the FST is independent of general locomotor activity.
V. Frontal Cortex Monoamines
A. Norepinephrine
Figure 2 depicts the frontal cortex NE concentration following alcohol and antidepressant treatment. Since saline treatment was not different from the control, it is not included in the graph. EtOH exposure resulted in a significant decrease in NE concentration in both WIS (approx 45%) and WKY (approx 42%) rats F(1, 28) = 9.4, P<0.05. Interestingly, WKY rats had lower basal NE level (approx 30%, P<0.05) in this area compared to WIS rats. Treatment with either imipramine or nomifensine reversed alcohol-induced reduction in NE levels in both strains. Curiously, nomifensine elevated the NE level above control levels (approx 80%) in WKY rats only F(1,28) = 13.77 P<0.01, suggestive of this strain's sensitivity to nomifensine compared to WIS rats.
Fig. 2.
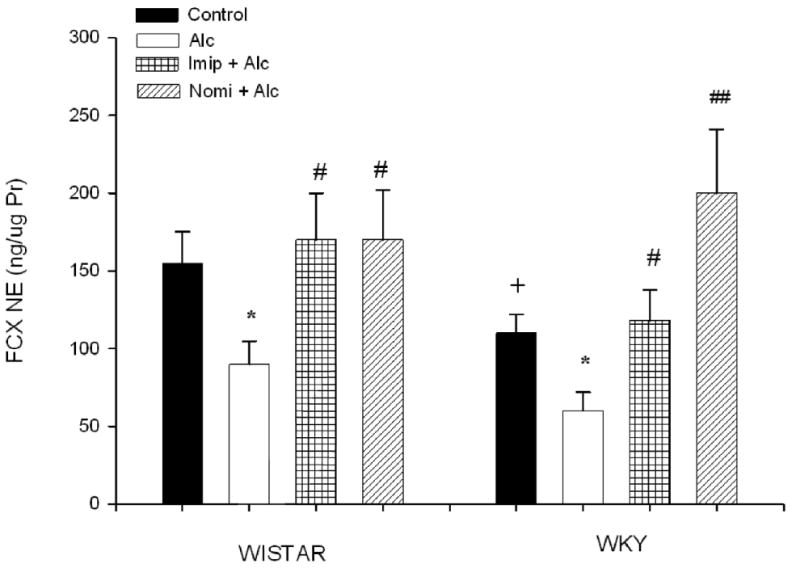
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in NE levels in the frontal cortex of WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were sacrificed 20-22 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. *P<0.05 compared to control. # P<0.05, ## P<0.01 compared to alcohol (Alc). +P<0.05 compared to Wistar. N= 8/group.
B. Dopamine
Figure 3 depicts the frontal cortex DA concentration following alcohol and antidepressant treatment. EtOH exposure resulted in a significant decrease (approx 65%) in DA concentration in WIS rats only F(1, 28) = 14.91, P<0.01. Nomifensine treatment resulted in an increase in cortical DA level in both strains (approx 180% in WIS and 300% in WKY rats, P<0.01), not a surprise finding, given that nomifensine has a significant effect on blocking DA transporter as well (Baldessarini, table 17-2, 11th edition, 2006). The higher increase in DA in WKY rats suggests a higher sensitivity of this strain to nomifensine than WIS rats. DA level in the frontal cortex was also lower in WKY (approx 36%, P<0.05) compared to WIS rats.
Fig. 3.
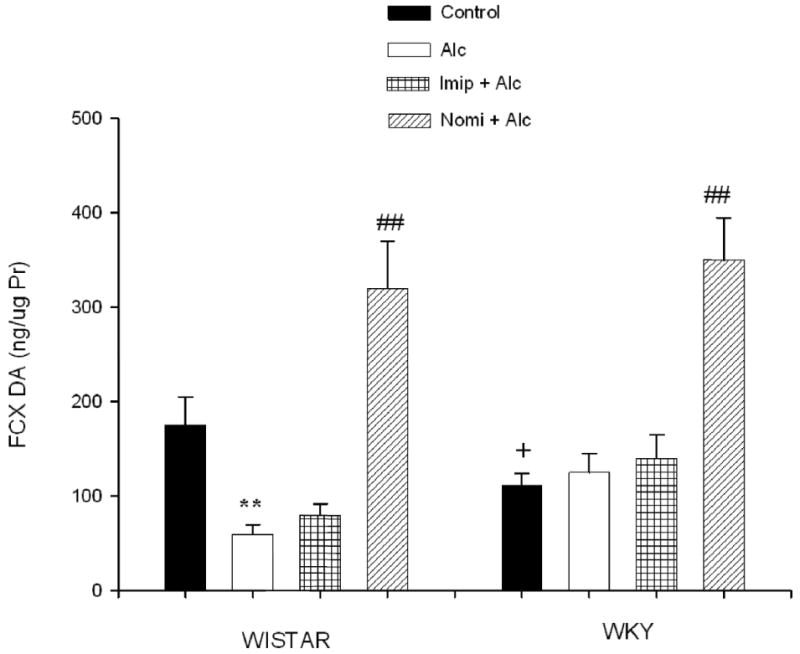
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in DA levels in the frontal cortex of WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were sacrificed 20-22 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. **P<0.01 compared to control. ## P<0.01 compared to alcohol (Alc). +P<0.05 compared to Wistar. N= 8/group.
C. Serotonin
Figure 4 depicts the frontal cortex 5-HT concentration following alcohol and antidepressant treatment. EtOH exposure resulted in a significant decrease in 5-HT concentration in WIS rats only (approx 60%, P<0.01), similar to what was seen with DA. In this case, however, treatment with imipramine resulted in significant increase in 5-HT levels in both strains (approx 46% in WIS and 350% in WKY rats), F(1, 28) = 12,02, P<0.01, a result consistent with impiramine's effect on 5-HT transporter. WKY rats appear to have higher sensitivity to imipramine than WIS rats. WKY rats exhibited a lower (approx 62%, P<0.01) basal cortical 5-HT compared to WIS rats.
Fig. 4.
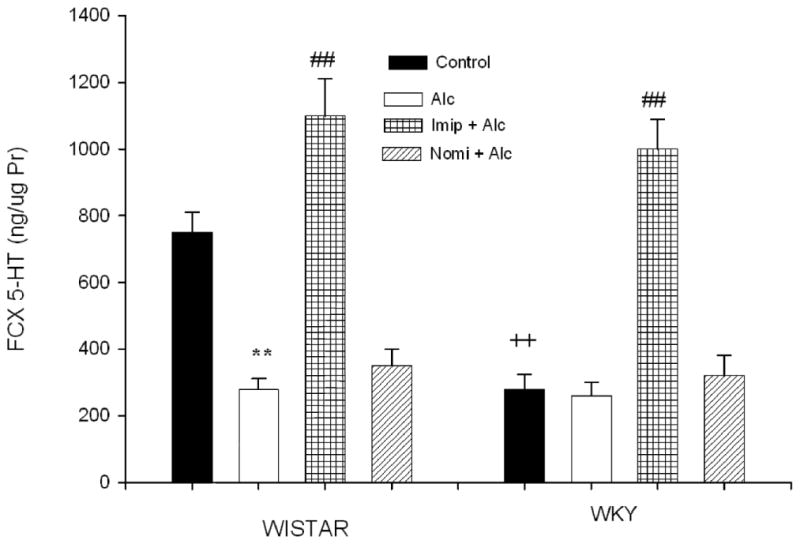
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in 5-HT levels in the frontal cortex of WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were sacrificed 20-22 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. **P<0.01 compared to control. ## P<0.01 compared to alcohol (Alc). ++P<0.01 compared to Wistar. N= 8/group.
VI. Hippocampal Monoamines
A Norepinephrine
Figure 5 depicts the hippocampal NE concentration following alcohol and antidepressant treatment. EtOH exposure resulted in a significant decrease (approx 60%) in NE concentration in Wistar rats only F(1, 28) = 17.67, P<0.01. WKY rats had lower basal NE level (approx 62%, P<0.05) in this area compared to Wistar rats. Treatment with either imipramine or nomifensine reversed alcohol-induced reduction in NE levels in Wistar rats. Both drugs caused an increase in hippocampal NE in WKY rats F(1, 28) = 9.82, P<0.05, consistent with their high affinity to block NE transporter.
Fig. 5.
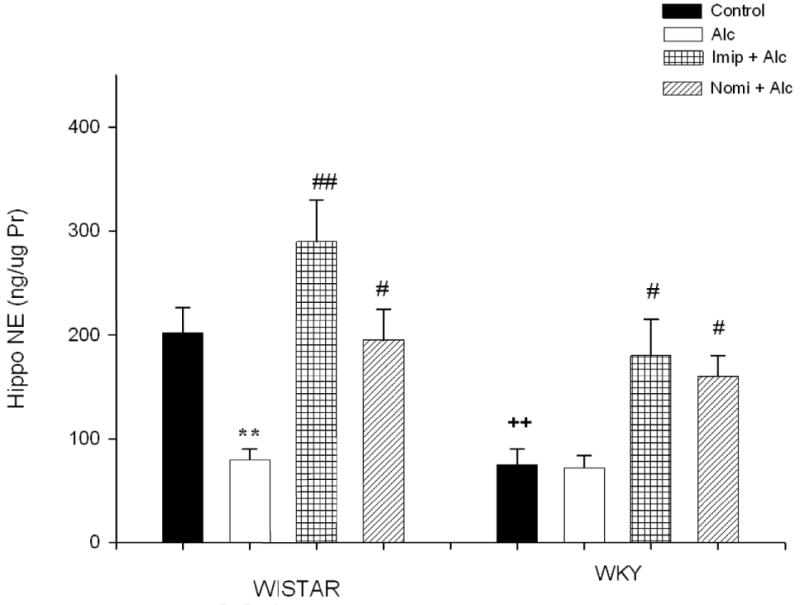
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in NE levels in the hippocampus of WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were sacrificed 20-22 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. **P<0.01 compared to control. c ## P<0.01 compared to alcohol (Alc). ++P<0.01 compared to Wistar. N= 8/group.
B. Dopamine
Figure 6 depicts the hippocampal DA concentration following alcohol and antidepressant treatment. EtOH exposure resulted in a significant decrease (approx 43%) in DA concentration in WIS rats only F(1, 28) = 4.89, P<0.05. Treatment with nomifensine not only reversed alcohol-induced reduction in DA levels, but also caused an elevation (approx 54%) of this transmitter above control rats. Nomifensine also resulted in an increase in hippocampal DA concentration in WKY rats F(1, 28) = 12.66, P<0.01 which had a lower basal DA concentration (approx 70%, P<0.01) compared to WIS rats.
Fig. 6.
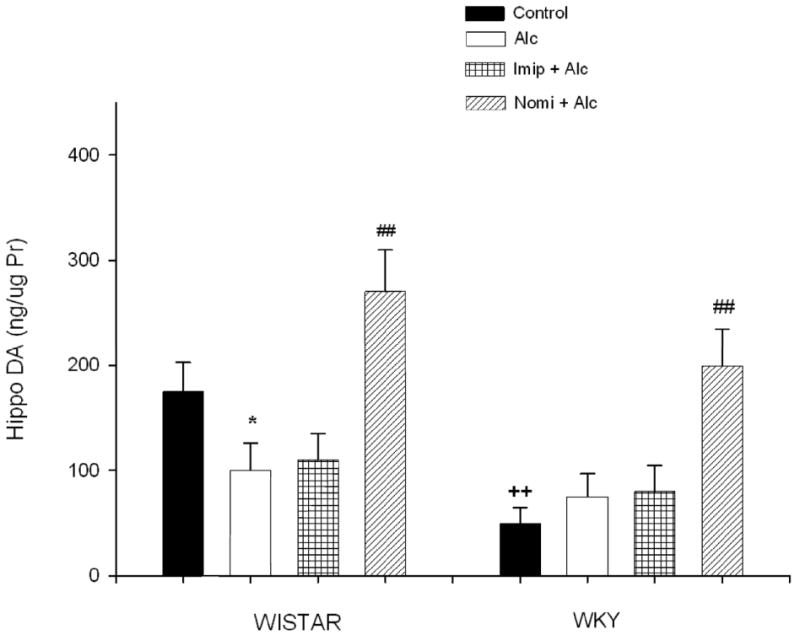
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in DA levels in the hippocampus of WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were sacrificed 20-22 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. *P<0.05 compared to control. ## P<0.01 compared to alcohol (Alc). ++P<0.01 compared to Wistar. N= 8/group.
C. Serotonin
Figure 7 depicts the hippocampal 5-HT concentration following alcohol and antidepressant treatment. EtOH exposure resulted in a significant decrease (approx 70%) in 5-HT concentration in WIS rats only F(1, 28) = 14.38, P<0.01. As seen in the frontal cortex, only treatment with imipramine reversed alcohol-induced reduction in 5-HT levels. Imipramine also caused a significant increase in 5-HT levels in WKY rats F(1, 28) = 5.89, P<0.05 which had a significantly lower (approx 61%, P<0.01) basal 5-HT concentration compared to WIS rats.
Fig. 7.
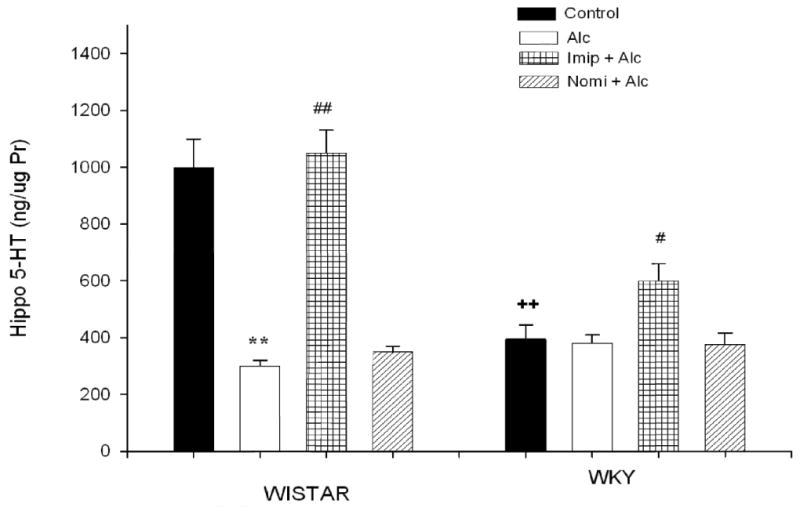
Effects of daily treatment with imipramine (Imip) and nomifensine (Nomi) on alcohol-induced changes in 5-HT levels in the hippocampus of WIS and WKY rats. Animals were exposed daily (3hr) to alcohol vapor for 10 days. Drugs were administered daily after alcohol exposure. Animals were sacrificed 20-22 hr after the last treatment. The effect of each drug was evaluated separately. Values are mean ± SEM. **P<0.01 compared to control. #P<0.05, ## P<0.01 compared to alcohol (Alc). ++P<0.01 compared to Wistar. N= 8/group.
Discussion
The results of this study while confirming the “depressogenic” effect of a relatively high chronic alcohol dose also provide some clue to the neurobiological substrate for this phenomenon. It appears that NE transmission in the frontal cortex is a major player in this regard. This contention is based on the observation that variation in frontal cortex NE concentrations was the most consistent finding in relation to alcohol and depressive-like behavior in the model studied. Thus, cortical NE was the only biogenic amine that was reduced in response to alcohol in both WKY and WIS rats. This reduction in cortical NE was associated with exacerbation of the depressive-like characteristic in WKY rats and induction of depressive-like behavior in WIS rats by alcohol. Moreover, treatment with clinically effective antidepressants such as impiramine and nomifensine both of which have a relatively high affinity for NE transporter, completely reversed the depressogenic effects of alcohol in both strains.
Although only cortical NE was reduced by alcohol in both strains, distinctive effects of alcohol on DA and 5-HT was also observed in the cortex and hippocampus of WIS rats. The reduction in these neurotransmitters induced by alcohol was normalized or even elevated above control levels by drug treatments. For example, impiramine which also inhibits 5-HT transporter caused an increase in both cortical and hippocampal 5-HT levels beyond control levels, whereas nomifensine which also inhibits DA transporter caused an increase in DA level beyond control level in both regions. In addition, WKY rats showed consistent reduction of all three biogenic amines in both areas compared to WIS rats. Thus, the depressive-like characteristic of WKY rats might also be influenced by neurotransmitters other than NE. However, since the depressive-like behavior of the WKY rats is unresponsive to selective serotonin reuptake inhibitors (Griebel et al., 1994; Lahmame et al., 1997; Lopez-Rubalcaava and Lucki, 2000; Tejani-Butt et al., 2003), it is unlikely that the observed differences in serotonin play a significant role in the depressive behavior of WKY rats. Dopamine, on the other hand, may be playing an important role in observed behavioral differences between WIS and WKY rats, particularly in regard to general locomotor activity as a role for DA in locomotion is well established (Sullivan and Brake, 2003). Moreover, since nomifensine blocks the DA transporter with near equal potency as it blocks NE transporter, some of the observed effects of this drug on reversal of immobility in both strains may be due to increases in DA in the cortex and the hippocampus. Clearly, further studies on the role of DA and specific sites of action in regard to depressive-like behavior are warranted.
The results of this study are also in agreement with previous reports implicating the biogenic amines including NE in negative mood states associated with alcoholism. Ample evidence suggests dysfunctions in monoaminergic input to the prefrontal cortex and other brain regions including hippocampus are important not only in inherent depressive-like characteristics, but also in alcohol-related mood dysregulation (Ballenger et al., 1979; Caldecott-Hazard et al. 1991; Heinz et al., 1998; Laine et al., 1999). Thus, preclinical pharmacological studies and clinical imaging results suggest that the frontal cortex and the hippocampus are two important regions associated with induction and treatment of depressive-like behavior (Caldecott-Hazard et al. 1991; Sheline et al 1996; Mayberg 1994; Drevets et al., 1997; Bremner et al 2002; Schmidt and Duman, 2007). Our finding of cortical NE involvement specifically in alcohol-induced mood dysregulation may implicate the ascending noradrenergic projection from the locus coeruleus to the cortical region (Rajkowska, 2000). In this regard, it would be of relevance to determine the extent to which damage to this trajectory by alcohol may be contributing to the observed behavioral deficits.
Since mood disturbances in general and depression in particular can significantly increase the risk to alcohol relapse, intervention towards mood stabilization or alleviation of depression would be essential in relapse prevention. Thus, it would be of considerable interest to determine whether interventions with antidepressants as suggested by this study would result in relapse prevention. It is also of relevance to note that the level of biogenic amines as determined in this study does not identify the dynamic component (e.g. synthesis, release, metabolism and/or reuptake) that might be affected by alcohol treatment. However, given that the selective NE uptake inhibitors rectify the behavioral abnormalities, it might be suggested that deficiency of synaptic NE, particularly in the frontal cortical region plays a significant role in alcohol-induced mood dysregulation.
The co-morbid condition of alcoholism and depression poses specific treatment challenge as the response to antidepressants may be less than optimal with the presence of alcohol (Goldstein et al., 2009; Howland et al., 2009). Although some of this diminished response could be due to pharmacokinetic interactions between alcohol and the antidepressant (Mattila, 1990; Salvadori et al., 1990), the nature of the antidepressant used and the varied biological substrates contributing to depression may be important determinants of the outcome. In this regard it would be helpful to establish whether depression has preceded alcoholism or vice-versa. Since delineation of such an association might not be readily feasible, it would be important to start the medication with an antidepressant that would have the highest chance of success. Although further verifications are necessary, the results of this study suggest that at least in a subpopulation of alcoholism-depression co-morbid condition administration of a selective NE transport inhibitor might be more effective than other antidepressants.
In summary, the findings confirm a depressogenic effect of relatively high alcohol level in a rodent model and suggest cortical NE as a neurobiological substrate for this association. Moreover, treatments with selective NE uptake inhibitors may normalize the behavioral effects of alcohol and hence may be of particular interest in co-morbid condition of alcoholism and depression.
Research highlights.
Our results implicate cortical NE as a major player in alcohol-induced depression. Moreover, it is suggested that selective NE uptake inhibitors may be of particular therapeutic potential in co-morbid condition of alcoholism and depression.
Acknowledgments
Supported by NIAAA (P20 AA014643) and NIH/NIGMS (2 SO6 GM08016-39)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldessarini . Drug therapy for depression and anxiety disorders. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gillman's the pharmacologic basis of therapeutics. New York: McGraw-Hill; 2006. pp. 429–559. [Google Scholar]
- Ballenger J, Goodwin F, Major L, Brown G. Alcohol and central serotonin metabolism in man. Arch Gen Psychiat. 1979;36:224–7. doi: 10.1001/archpsyc.1979.01780020114013. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49:412–7. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Caldecott-Hazard S, Morgan DG, DeLeon-Jones F, Overstreet DH, Janowsky D. Clinical and biochemical aspects of depressive disorders: II. Transmitter/receptor theories. Synapse. 1991;9:251–301. doi: 10.1002/syn.890090404. [DOI] [PubMed] [Google Scholar]
- Clausius N, Born C, Grunze H. The relevance of dopamine agonists in the treatment of depression. Neuropsychiatr. 2009;23:15–25. [PubMed] [Google Scholar]
- D'Aquila PS, Collu M, Devoto P, Serra G. Chronic Lithium chloride fails to prevent imipramine-induced sensitization to the dopamine D(2)-like receptor agonist quinpirole. Eur J Pharmacol. 2000;395:157–60. doi: 10.1016/s0014-2999(00)00189-8. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Stuckey RF, Gold MS, Pottash AC. Dexamethasone suppression test testing of depressed alcoholics. Alcohol Clin Exp Res. 1986;10:59–60. doi: 10.1111/j.1530-0277.1986.tb05615.x. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dixit AD, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. Am J Psychiatry. 2000;157:751–8. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Letters to Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008;91:97–103. doi: 10.1016/j.pbb.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Spirito A, Emslie G, Clarke G, Wagner KD, et al. Substance Use and the Treatment of Resistant Depression in Adolescents. J Am Acad Child Adolesc Psychiatry. 2010 doi: 10.1097/CHI.0b013e3181bef6e8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology. 1994;113:463–0. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Heinz A, Dufeu P, Kuhn S, Dettling M, Gräf K, Kürten I, et al. Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Arch Gen Psychiatry. 1996;53:1123–8. doi: 10.1001/archpsyc.1996.01830120061011. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol. 1995;63:400–7. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Howland RH, Rush AJ, Wisniewski SR, Trivedi MH, Warden D, Fava M, et al. Concurrent anxiety and substance use disorders among outpatients with major depression: clinical features and effect on treatment outcome. Drug Alcohol Depend. 2009;99:248–60. doi: 10.1016/j.drugalcdep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt SM. Antidepressant drug induced alteration to central dopamine transporter sites in the Wistar Kyoto rat strain. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30:30–41. doi: 10.1016/j.pnpbp.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ. A twin-family study of alcoholism in women. Amer J of Psych. 1994;151:707–15. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Co-morbidity Survey: a family history study. Br J Psych. 1997;170:541–8. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the U.S. National Co-morbidity Survey. Psychol Med. 1997;27:1101–19. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–19. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Lahmame A, del Arco C, Pazos A, Yritia M, Armario A. Are Wistar-Kyoto rats a genetic animal model of depression resistant to antidepressants? Eur J Pharmacol. 1997;337:115–23. doi: 10.1016/s0014-2999(97)01276-4. [DOI] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, et al. Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry. 1999;4:189–91. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000;24:110–2. [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–9. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–32. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Martijena ID, Bustos SG, Bertotto ME, Molina VA. Antidepressants attenuate both the enhanced ethanol intake and ethanol-induced anxiolytic effects in diazepam withdrawn rats. Eur Neuropsychopharmacol. 2005;15:119–30. doi: 10.1016/j.euroneuro.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Mattila MJ. Alcohol and drug interactions. Ann Med. 1990;22:363–9. doi: 10.3109/07853899009147921. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6:428–42. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Gelernter CS. Comorbidity for alcoholism and depression. Psychiatr Clin North Am. 1990;13:613–32. [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Berrettini W. Psychiatric Genetics. London: Chapman & Hall; 1998. [Google Scholar]
- Overstreet DH, Rezvani AH, Janowsky DS. Maudsley reactive and nonreactive rats differ only in some tasks reflecting emotionality. Physiol Behav. 1992;52:149–52. doi: 10.1016/0031-9384(92)90444-7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–59. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Cui CL, Ma YY, Guo CY, Han JS, Lukas SE, et al. Electroacupuncture reduces voluntary alcohol intake in alcohol-preferring rats via an opiate-sensitive mechanism. Neurochem Res. 2008;33:2166–70. doi: 10.1007/s11064-008-9791-9. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–22. [PubMed] [Google Scholar]
- Paré WP, Kluczynski J. Negative Affect and Voluntary Alcohol Consumption in Wistar–Kyoto (WKY) and Sprague–Dawley Rats. Physiology & Behavior. 1999;67:219–25. doi: 10.1016/s0031-9384(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, LePichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–32. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res. 2000;126:397–412. doi: 10.1016/S0079-6123(00)26026-3. [DOI] [PubMed] [Google Scholar]
- Ratsma JE, Van Der Stelt O, Gunning WB. Neurochemical markers of alcoholism vulnerability in humans. Alcohol Alcohol. 2002;37:522–33. doi: 10.1093/alcalc/37.6.522. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Parsian A, Overstreet DH. The Fawn-Hooded (FH/Wjd) rat: a genetic animal model of comorbid depression and alcoholism. Psychiatr Genet. 2002;12:1–16. doi: 10.1097/00041444-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Cleves M, Parsian A. Further genetic characterization of the Fawn-Hooded (FH/Wjd) rat, an animal model of co-morbid depression and alcoholism. Psychiatric Genetics. 2007;17:77–83. doi: 10.1097/YPG.0b013e328012d7c3. [DOI] [PubMed] [Google Scholar]
- Rodgers B, Korten AE, Jorm AF, Jacomb PA, Christensen H, Henderson AS. No-Linear relationships in associations of depression and anxiety with alcohol use. Psychol Med. 2000;30:421–32. doi: 10.1017/s0033291799001865. [DOI] [PubMed] [Google Scholar]
- Rozas I. Improving antidepressant drugs: update on recently patented compounds. Expert Opin Ther Pat. 2009;19:827–45. doi: 10.1517/13543770902932934. [DOI] [PubMed] [Google Scholar]
- Salvadori C, Ward C, Defrance R, Hopkins R. The pharmacokinetics of the antidepressant tianeptine and its main metabolite in healthy humans--influence of alcohol co-administration. Fundam Clin Pharmacol. 1990;4:115–25. doi: 10.1111/j.1472-8206.1990.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psych. 1997;154:948–57. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci. 1995;92:5880–4. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang P, Gado M, Csernansky J, Vannier M. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spak L, Spak A, Allebeck P. Alcoholism and depression in a Swedish female population: Co-morbidity and risk factors. Acta Psychiatr Scand. 2000;102:44–51. doi: 10.1034/j.1600-0447.2000.102001044.x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S, Kluczynski J, Pare WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology. 1999;142:193–9. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Davila-Garcia M, Taylor RE. Alcohol preference: association with reduced striatal nicotinic receptors. Alcohol Alcohol. 2001;36:318–22. doi: 10.1093/alcalc/36.4.318. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–47. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Willner P. The mesolimbic DA system as a target for rapid antidepressant action. Int Clin Psycholpharmacology. 1997;12:7–14. doi: 10.1097/00004850-199707003-00002. [DOI] [PubMed] [Google Scholar]


