Abstract
Bacterial chromosomes often carry integrated genetic elements (e.g., plasmids, transposons, prophages, and islands) whose precise function and contribution to the evolutionary fitness of the host bacterium are unknown. The CTXϕ prophage, which encodes cholera toxin in Vibrio cholerae1, is known to be adjacent to a chromosomally integrated element of unknown function termed the toxin-linked cryptic (TLC)2. Here we report characterization of a TLC-related element that corresponds to the genome of a satellite filamentous phage (TLC-Knϕ1) which uses the morphogenesis genes of another filamentous phage (fs2ϕ) to form infectious TLC-Knϕ1 phage particles. The TLC-Knϕ1 phage genome carries a sequence similar to the dif recombination sequence which functions in chromosome dimer resolution using XerC and XerD recombinases3. The dif sequence is also exploited by lysogenic filamentous phages (e.g., CTXϕ) for chromosomal integration of their genomes. Bacterial cells defective in the dimer resolution often show an aberrant filamentous cell morphology3,4. We found that acquisition and chromosomal integration of the TLC-Knϕ1 genome restored a perfect dif site and normal morphology to V. cholerae wild type and mutant strains that displayed dif- filamentation phenotypes. Furthermore, lysogeny of a dif- nontoxigenic V. cholerae with TLC-Knϕ1 promoted its subsequent toxigenic conversion through integration of CTXϕ into the restored dif site. These results reveal a remarkable level of cooperative interactions between multiple filamentous phages in the emergence of the bacterial pathogen that causes cholera.
The TLC element of V. cholerae encodes the Cri replicase with homology to filamentous phage replication proteins and TlcR, a protein that displays sequence similarity to RstR, the repressor controlling lysogeny of the filamentous CTXϕ and the target for anti-repression by the RstC product of satellite filamentous phage RS1ϕ1,2,5-9. For these reasons we hypothesized that the TLC element corresponds to the genome of a satellite filamentous phage that depended on another filamentous phage for its morphogenesis. As a prelude to the described study, we devised a screen for the postulated “TLC helper phage” and this effort identified filamentous phage fs2ϕ as such a helper10. In brief, our evidence (see supplementary information) that fs2ϕ is a TLC helper phage includes the following: 1) Strains encoding genetically marked versions of the TLC element (e.g., TLC-Kn1) inserted in their chromosome produce infectious TLC-Knϕ1 phage particles only if also infected with fs2ϕ; 2) Such TLC-related phage particles carry ssDNA corresponding to a circularized variant of the TLC element; 3) TLC-Knϕ1 phage infect only cells expressing mannose sensitive hemagglutinin (MSHA) pili, the known receptor of fs2ϕ11. Upon infection of MSHA+ vibrios, the TLC-Knϕ1 ssDNA present in phage particles is converted to the double stranded replicative form (RF) that is detectable in infected cells as a plasmid or as a chromosomally integrated copy; 4) The RF form of the TLC-Knϕ1 (designated pTLC-Kn1) was also shown to be sufficient for formation of TLC-Knϕ1 phage in recipient cells provided that the cells are also infected with fs2ϕ. Thus fs2ϕ is a helper phage that provides essential gene products required for TLC-Knϕ1 phage particle morphogenesis.
In order to better understand the biology of TLCϕ, we sequenced pTLC-Knϕ1 and its chromosomally integrated form in strain AL33457-TLC-Kn1. Strain AL33457 was found to carry two copies of the TLC element that flank a unique ORF (VC1471)(Fig. 1). Each of the two copies of chromosomally integrated TLC elements in AL33457 is comprised of 5 ORFs, spanning from VC1466 to VC1470 and VC1472 to VC1476, respectively. In strain AL33457-TLC-Kn1, the KnR determinant was located in VC1470 and thus, like ORF VC1471 was located between the duplicated copies of TLC. Nucleotide sequence analysis of pTLC-Kn1 indicated that this plasmid likely formed as a result of recombination between two directly repeated 25 base pair (bp) sequences (ACATAATGCGCACTAGGAACATTTT), which are located in the 3’end of VC1465 and within VC1471 (Fig. 1). Remarkably, this 25 bp sequence within VC1471 overlaps 18 bp (bold nucleotides) with the 28 base pair dif1 sequence ATTTAACATAACATACATAATGCGCACT14,15. Dif1 is a site on the large chromosome of V. cholerae which is required for XerC/XerD-mediated resolution of chromosome dimers and similar sites are also exploited by various filamentous phages for integration of their genomes into the host chromosome using XerC/XerD-mediated recombination3,4,12-15. The dif1 sequence is utilized by CTXϕ and RS1ϕ for their chromosomal integration though XerC/XerD-mediated recombination with the corresponding dif/attP site formed by annealing of ssDNA derived from phage genomes14,15. The recombination event that formed pTLC-Kn1 looped out the entire region between the 25bp duplicated sequence in VC1465 and VC1471 including the 18bp identical to a part of the dif1 sequence together with most part of the ORF defined as VC1471. Thus, TLC-Knϕ1 and pTLC-Kn1 encode a part of the dif1 sequence (Fig. 1).
Figure 1.

Schematic diagram showing the formation of plasmid pTLC-Kn1. A conservative recombination event (dotted line and arrow) occurring between two identical 25 bp sequences (boxed nucleotides) located near the 3’ end of VC1465 and within VC1471 excised the plasmid from the chromosome of the V. cholerae AL33457-TLC-Kn1 parental strain. This strain carries two chromosomal copies of the TLC element with a kanamycin resistance (Kn) insert located in VC1470 of the first TLC element (TLC1). Thus pTLC-Kn1 represents a circularized TLC-related element that carries VC1471 (a gene that was located between the 25 bp sequences. Within VC1471 there also exists an 18-bp region (red font) which is identical to a part of the known 28 bp dif1 sequence. TLC1 is composed of genes VC1466, VC1467, VC1468, VC1469, and VC1470 while TCL2 is composed of genes VC1472, VC1473, VC1474, VC1475, and VC1476. Only a subset of these genes are shown for simplicity in this diagram.
These observations suggest that naturally occurring TLC-related phages might be capable of reconstituting a functional chromosomal dif sequence by recombining its partial dif site with a defective dif-like sequence during lysogenic integration of its genome into the chromosome. In order to test this hypothesis, we screened a collection of 97 clinical or environmental V. cholerae of both O1 or non-O1 serogroups and identified 18 strains which were negative for one or more chromosomal regions including TLC, VC1471 or the dif sequence3,14,15 These included 12 non-O1, non-O139 strains and 6 nontoxigenic O1 strains. Sequencing of the relevant region in five such TLC negative CTX negative O1 strains (AO12682, AO7543, AV2684248, AN19908, and AN25049, see Table S1) revealed a gap between the rtxA gene (VC1451) and the gene designated VC1479 in all the five strains analyzed. In toxigenic strains of 7th pandemic El Tor biotype, such as N16961 the CTX prophage and the TLC genes as well as the recombined dif-like sites formed by integration of CTXϕ are located in this space16. Notably in the intergenic region between rtxA and VC1479 in the TLC negative strains, a possible defective dif-like sequence, differing in two nucleotide pairs (G to A, and C to T) from the bona fide dif sequence, was identified (Fig. 2).
Figure 2.
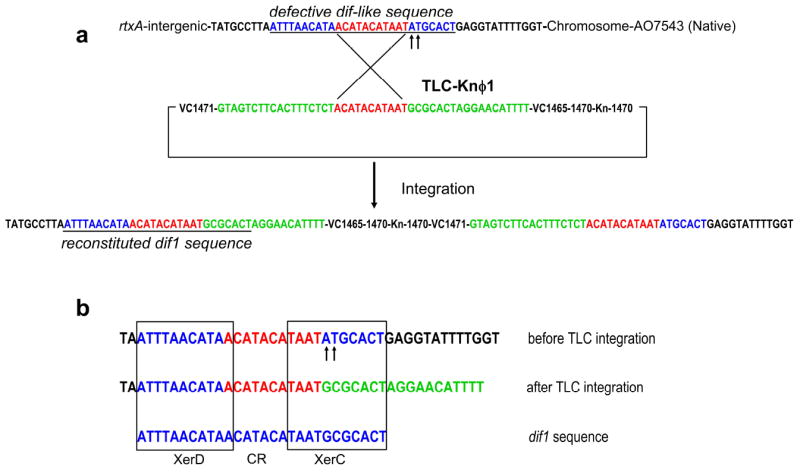
Part a, Schematic diagram showing site specific integration of TLC-Knϕ1 genome into the chromosome of strain AO7543. The region in the vicinity of the phage attachment site is shown in green and the region in the vicinity of the chromosome attachment site is shown in blue. The chromosomal attachment site corresponds to a defective dif-like sequence altered in an AT dinucleotide (arrows). Sequence analysis indicates that the recombination event that integrated the phage genome occurred in the central region of sequence identity (red) which also corresponds in part to the central region (CR) of a dif1 site (see Fig 2, Part b). This recombination event is likely generated at least in part by action of the XerC and XerD on chromosomal and phage nucleic acid substrates in that the TLC-Kn1 genome did not chromosomally integrate in XerC-defective and XerD-defective strains (see text and supplement). Formation of the TLC prophage by integration resulted in formation of a functional dif1 sequence (underlined) on its left border. The central sequence of identity is duplicated on the right border but the dif1 sequence is not duplicated. Part b, Base pair alignments between the defective dif-like sequence on the chromosome of strain AO7543 that is the target for TLC-Knϕ1 integration, the resultant hybrid sequence found after integration of TLC-Knϕ1 (left end) and the authentic V. cholerae dif1 sequence. Colors of nucleotides correspond to those highlighted in Part A of this figure. The binding sequences for XerC and XerD recombinases are indicated with filled boxes.
Cultures of V. cholerae strains with deletions in the dif recombination site are known to contain a subpopulation of cells that display a filamentous morphology3. These filaments reflect aberrant cell division resulting from a defect in XerC/XerD-mediated chromosome dimer resolution3. We examined whether naturally occurring V. cholerae O1 strains that lack TLC, or the bona fide dif sequence, display filamentous morphology. As shown in Fig 3 and Table S3, these strains indeed display filamentous morphology for a noticeable sub-population of their cells. We next tested whether transduction with TLC-Knϕ1 phage could correct this morphology defect. In each case, cell filamentation in these V. cholerae was found to be eliminated following transduction with TLC-Knϕ1 (Fig. 3, Table S3). These results suggest that these filamentous strains are indeed dif-deficient and that lysogeny with TLC-Knϕ1 apparently corrected this defect.
Figure 3.

Transduction with TLC-Knϕ1 phage cures cell filamentation of Vibrio cholerae O1 strains which have likely defects in resolution of chromosome dimers. Panels a through c (a,strain AO12682, b, strain AO7543, and c, strain AV2684248, see Table S1 and Table S4 for details) show the morphology of three different strains before infection with TLC-Knϕ1 whereas panels d through f show the same strains respectively after infection and chromosomal integration of TLC-Knϕ1.
To further verify the natural formation of TLC-related phages and the role of the dif-like sequence encoded by VC1471 in correction of dif-deficient phenotypes, we used a set of chromosomal transposon insertion mutants of C670617. We selected five strains carrying TnFGL3 insertions in the different ORF’s of TLC (VC1466, VC1467, VC1468, VC1469, VC1470 and VC1471). With the exception of VC1469, for each strain, we were able to recover a plasmid corresponding to the RF of TLCϕ-related genome with a TnFGL3 insertion in the corresponding ORF. No plasmid recovery was actually expected for the insertion mutant in VC1469 because this gene encoded Cri, the protein required for replication of TLC-related plasmids2. When these TLC-related plasmids were introduced into each of the strains SA317, AO7543 and AO12682 (naturally occurring strains that showed filamentous morphology and were negative for VC1471), normal cellular morphology was restored except in one case. The RF plasmid derived from the mutant carrying a TnFGL3 insertion in VC1471 failed to complement the morphology defect in these strains (Fig. S4). Notably, the TnFGL3 insertion in VC1471 is located within the dif-like sequence (bold) encoded by VC1471 (insertion indicated by the * in the sequence: ACATACA*TAATGCGCACTAGGAACA). We conclude that the dif-like sequence present in VC1471 is required to correct morphological defects upon introduction of TLC-related plasmids into naturally occurring dif-/TLC- strains of V. cholerae.
We studied the integration of TLC-Knϕ1 genome into the chromosome of naturally occurring TLC negative strains AO7543 and AO12682 after infecting these strains with TLC-Knϕ1 phage particles. As expected, Southern blot hybridization (Fig S5) and PCR analysis indicated that the TLC-Knϕ1 genome had inserted into the bacterial genome in the intergenic region between rtxA and VC1479. This was confirmed by PCR assays using two primers one of which was complementary to this chromosomal region and the other corresponding to pTLC-Knϕ1. Notably, the correction of the cell filamentation phenotype of dif-deficient strains was observed only in strains in which the TLC-Knϕ1 genome had integrated into the chromosome. In contrast, introduction of a pUC18 clone of VC1471 designated pVC1471 into the defective cells did not cure the cell filamentation phenotype despite the fact that it carried the dif-like sequence. This finding indicated that the dif-like sequence present in VC1471 functions only in cis and not in trans. This is what one would expect if the dif-like sequence in VC1471 could indeed function in recombination with XerC/XerD to resolve chromosomal dimers only if it recombined into the chromosome. To verify this assumption, we utilized mutants of dif- strains with transposon insertions in the XerC or XerD genes. As expected, transduction of TLC-Knϕ1 into these XerC or XerD defective strains did not cure their cell filamentation phenotype (Table S4). Furthermore, PCR based analyses as described above confirmed that the TLC-Knϕ1 DNA did not integrate into the chromosome of the XerC or XerD mutant strains.
To further examine the mechanism associated with the elimination of the dif- defect through chromosomal integration of TLC-Knϕ1 DNA, we sequenced the junction of several independent TLC-Knϕ1 integration events. The sequence analysis showed that TLC-Knϕ1 DNA had integrated into the intergenic region between rtxA and VC1479 in strains AO7543, AV2684248 and AO12682 using its defective dif-like site as a recombinational substrate (Fig 2A). Remarkably, the recombination event leading to the integration of the TLC-Knϕ1 genome resulted in formation of a functional dif sequence identical to the bona fide dif1 sequence reported for V. cholerae3 (Fig. 2B). This result also suggests that TLC-deficient V. cholerae strains contain alternative dif-like sequences that can still function in recombination with TLC encoded dif-like sequence but are not fully functional in chromosome dimer resolution. We conclude that the dif-like site in VC1471 recombines with the defective chromosomal dif-like sequence in these TLC negative strains during the process of XerC/D-mediated TLC genome insertion and that the product of this integration event generates a dif sequence that is functional in chromosomal dimer resolution.
In toxigenic V. cholerae, the CTXϕ genome exists as a prophage inserted into the bacterial chromosome at the dif recombination site13-15. Because transduction with TLC-Knϕ1 reconstructs a functional dif sequence in the recipient bacterium (Fig. 2), we tested whether TLC-Knϕ1 transductants could be stably lysogenized by CTXϕ. We chose test strains that were positive for the TCP locus (which encodes the receptor for CTXϕ1), and utilized a CTXϕ that was marked with a chloramphenicol resistance (CTX-Cmϕ). As expected we found that TLC-Knϕ1 transductants were readily superinfected with CTX-Cmϕ and in these cases CTX-Cmϕ was found integrated into the dif site generated through previous integration of TLC-Knϕ1. In contrast, although natural TLC negative strains could also be infected with CTX-Cmϕ, (Table S6) the CTX-Cmϕ genome did not integrate, and the un-integrated CTX-Cmϕ genome was rapidly lost when inoculated in the intestinal loops of adult rabbits. Because the integrated form of the CTXϕ genome is known to be more stably retained in V. cholerae compared to the un-integrated plasmid form18 these data strongly argue that TLCϕ plays a critical role in the natural, stable acquisition of CTXϕ.
Although the TLC element was known to be invariably present in all CTX positive strains and notably absent in CTX negative strains2, the role of this element in the evolution of toxigenic V. cholerae was not clear until the present study. Here we show that the TLC element can give rise to infectious phage particles (TLCϕ) when its morphogenesis is supported by another filamentous phage, previously designated as fs2ϕ10. Furthermore, infectious forms of TLCϕ which encode a dif-like sequence can be easily isolated. These specialized TLCϕ-related transducing phages can, upon chromosomal integration, generate a functional dif sequence and correct aberrant filamentous morphology present in TLC-negative cells that apparently exhibit defective dif/XerC/XerD-mediated chromosome dimer resolution. Lysogeny by such TLC phages leads to the restoration of a functional dif site which also is essential for stable integration of CTXϕ and conversion of V. cholerae to a toxigenic form.
The most common strains of V. cholerae causing cholera in the world today are all highly related to the 7th pandemic clone of V. cholerae that emerged as a human pathogen in 1971 in the Celebes Islands19,20. The arrangement of TLC prophage, and the dif site utilized by CTXϕ in these highly successful pandemic strains is virtually the same as the one we experimentally produced in this study by lysogeny of dif- strains such as AO7543 sequentially with TLC-Knϕ1 followed by CTX-Cmϕ. Thus, it seems highly likely that the precursor of the 7th pandemic clone was a dif-strain which emerged as a pandemic pathogen after sequential lysogeny by three filamentous phages TLCϕ, CTXϕ, and RS1ϕ (Fig 4). Because dif- defects are deleterious to growth, it is possible that the precursor of the 7th pandemic clone may be rare in the environment or that the dif- genotype confers a yet-to-be-determined advantage for nontoxigenic O1 strains in the context of its environmental nitch. Nonetheless, our data suggest that the evolutionary emergence of the toxigenic 7th pandemic clone of V. cholerae likely involved molecular interactions between two satellite filamentous phages (TLCϕ, and RS1ϕ), three helper filamentous phages (fs2ϕ, CTXϕ and KSFϕ21), and two type IV pilus-based phage receptors (MSHA and TCP) (Fig. S6). Accordingly, our results provide a paradigm for understanding the cooperative interactions of multiple genetic elements in the evolution of pathogenic bacteria from nonpathogenic environmental progenitors.
Figure 4.

Schematic diagram of phage-bacterial interactions that likely led to the emergence of the 7th pandemic clone of Vibrio cholerae. Boxes indicate the integrated prophages and dif sequence in the 7th pandemic strain, whereas the solid lines indicate the corresponding empty sites where these elements are absent in the precursor strains. Sequential lysogeny by three different filamentous phages TLCϕ, CTXϕ, and RS1ϕ, and the role of two helper phages fs2 and KSF-1 are shown. In order to generate the observed organization of RS1 prophages found in most 7th pandemic strains, it is postulated that two rounds of RS1 prophage integration would be needed if this prophage integrates into only a functional dif site and then reconstitutes only one dif site after integration. For a more complete explanation of these hypothetical steps see Fig. S6 in the supplementary information.
Methods Summary
A genetic marker encoding kanamycin resistance (KnR) was introduced into the TLC element carried by multiple V. cholerae strains followed by screening of the marked strains for production of TLC-related KnR transducing particles in the culture supernatants. Mung bean-nuclease digestion as well as hybridization analysis with strand-specific oligonucleotide probes corresponding to the (+) and (-) strand of TLC element was conducted to test whether the DNA carried by putative TLC-related phage particles present in filter-sterilized culture supernatants was single stranded. The role of fs2ϕ as a helper of TLC satellite phage was established by demonstrating the formation of TLC-Knϕ1 phage in recipient cells which contained pTLC-Kn1 provided that the cells were also infected with fs2ϕ.
For transduction assays, recipient V. cholerae strains were mixed with genetically marked phage preparations5, and transductants were selected using Luria-Bertini agar medium containing appropriate antibiotics. Integration of TLC-Knϕ1 genome was detected by Southern blot hybridization, and PCR assays using two primers one of which was complementary to the chromosomal region and the other corresponding to pTLC-Knϕ1. DNA sequencing was conducted to further confirm the integration event and detect the generation of the dif sequence. Subsequently, a chloramphenicol resistance (CmR)-marked CTX phage was used to study the susceptibility and chromosomal integration5,18 of CTX phage into the restored dif site. The CmR-marked CTX phage genome (pCTX-Cm) was constructed by replacing the KnR-marker in pCTX-Km1 derived from strain SM44 with a CmR cassette.
The full list of strains and plasmids is available as Table S1. Full methods and associated references are available in the supplementary information linked to the paper at www.nature.com/nature.
Supplementary Material
Acknowledgments
This research was funded in part by the National Institutes of Health grants RO1-GM068851, and RO1-AI070963 under different sub-agreements between the Harvard Medical School, and the ICDDR,B. The ICDDR,B is supported by countries and agencies which share its concern for the health problems of developing countries. We thank T.M. Zaved Waise, and S.M. Nashir Udden for technical assistance.
Footnotes
Author contributions. F.H., M.K., and S.M.F. conducted the experiments, and performed analyses of bacterial strains and phages. S.M.F. and J.J.M. designed the studies, analyzed data and wrote the manuscript.
Relevant nucleotide sequence data described in the article have been deposited at GenBank under accession numbers HM134797, HM134798, HM134799, and HM134800.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous bacteriophage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 2.Rubin EJ, Lin W, Mekalanos JJ, Waldor MK. Replication and integration of a Vibrio cholerae cryptic plasmid linked to the CTX prophage. Molecular Microbiology. 1998;28:1247–1254. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 3.Huber KE, Waldor MK. Filamentous phage integration requires the host recombinases XerC and XerD. Nature. 2002;417:656–659. doi: 10.1038/nature00782. [DOI] [PubMed] [Google Scholar]
- 4.Blakely G, et al. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell. 1993;75:351–361. doi: 10.1016/0092-8674(93)80076-q. [DOI] [PubMed] [Google Scholar]
- 5.Faruque SM, Asadulghani, Kamruzzaman M, Nandi RK, Ghosh AN, Nair GB, Mekalanos JJ, Sack DA. RS1 element of Vibrio cholerae can propagate horizontally as a filamentous phage exploiting the morphogenesis genes of CTXphi. Infect Immun. 2002;70:163–70. doi: 10.1128/IAI.70.1.163-170.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis BM, Kimsey HH, Kane AV, Waldor MK. A satellite phage-encoded antirepressor induces repressor aggregation and cholera toxin gene transfer. EMBO J. 2002;21:4240–9. doi: 10.1093/emboj/cdf427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimsey HH, Waldor MK. The CTXphi repressor RstR binds DNA cooperatively to form tetrameric repressor-operator complexes. J Biol Chem. 2004;279:2640–7. doi: 10.1074/jbc.M311109200. [DOI] [PubMed] [Google Scholar]
- 8.Waldor MK, Rubin EJ, Pearson GD, Kimsey H, Mekalanos JJ. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis BM, Waldor MK. CTXΦ contains a hybrid genome derived from tandemly integrated elements. Proc Natl Acad Sci USA. 2000;97:8572–8577. doi: 10.1073/pnas.140109997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikema M, Honma Y. A novel filamentous phage, fs-2, of Vibrio cholerae O139. Microbiology. 1998;144:1901–1906. doi: 10.1099/00221287-144-7-1901. [DOI] [PubMed] [Google Scholar]
- 11.Ehara M, Shimodori S, Kojima F, Ichinose Y, Hirayama T, Albert MJ, Supawat K, Honma Y, Iwanaga M, Amako K. Characterization of filamentous phages of Vibrio cholerae O139 and O1. FEMS Microbiol Lett. 1997;154:293–301. doi: 10.1111/j.1574-6968.1997.tb12659.x. [DOI] [PubMed] [Google Scholar]
- 12.Iida T, Makino K, Nasu H, Yokoyama K, Tagomori K, Hattori A, Okuno T, Shinagwa H, Honda T. Filamentous bacteriophage of Vibrios are integrated into the dif-like site of the host chromosome. J Bacteriol. 2002;184:4933–4935. doi: 10.1128/JB.184.17.4933-4935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeod SM, Waldor MK. Characterization of XerC and XerD-dependent CTX phage integration in Vibrio cholerae. Mol Microbiol. 2004;54:935–947. doi: 10.1111/j.1365-2958.2004.04309.x. [DOI] [PubMed] [Google Scholar]
- 14.Val ME, Bouvier M, Campos J, Sherratt D, Cornet F, Mazel D, Barre FX. The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Mol Cell. 2005;19:559–566. doi: 10.1016/j.molcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Das B, Bischerour J, Val ME, Barre FX. Molecular keys of the tropism of integration of the cholera toxin phage. Proc Natl Acad Sci U S A. 2010;107:4377–4382. doi: 10.1073/pnas.0910212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidelberg JF, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci USA. 2008;105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faruque SM, Rahman MM, Hasan AK, Nair GB, Mekalanos JJ, Sack DA. Diminished diarrheal response to Vibrio cholerae strains carrying the replicative form of the CTXΦ genome instead of CTXΦ lysogens in adult rabbits. Infect Immun. 2001;69:6084–90. doi: 10.1128/IAI.69.10.6084-6090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaper JB, Morris JG, Levine MM. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faruque SM, Kamruzzaman M, Asadulghani, Sack DA, Mekalanos JJ, Nair GB. CTX phage-Independent Production of RS1 Satellite Phage by Vibrio cholerae. Proc Natl Acad Sci USA. 2003;100:1280–1285. doi: 10.1073/pnas.0237385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


