Abstract
It is well known that thyroid disease is more frequent in women than in men, however the molecular basis for this gender difference is still poorly understood. PI3K activation, through different mechanisms including loss of the PTEN tumor suppressor, is being increasingly recognized as a major player in the development of thyroid neoplastic lesions. Loss of Pten in the mouse thyroid results in a significant increase in the thyrocyte proliferative index, which is more prominent in the female mice. Here we show that 52% of the Pten−/− female mice, but only 12% of the males, develop follicular adenomas by one year of age. In addition, 50% of female mutants, but only 35% of males older than one year of age develop invasive, and often metastatic, follicular carcinomas. Mutant females have a significantly shorter overall survival compared to male mutants. Hormonal manipulation experiments established a direct role of estrogens in controlling the increased thyrocyte proliferation index in mutant females. Furthermore, while genetic ablation of one Cdkn1b allele accelerated the development of neoplastic lesions, it also abolished the gender differences in survival and reduced the difference in neoplastic lesion development rate, underlining a key role of p27 in mediating estrogen action in the thyroid follicular cells.
These data, based on a clinically relevant model of thyroid follicular carcinoma, provide for the first time in vivo evidence that circulating estrogens are directly responsible for the increased female susceptibility to thyroid disease, at least upon activation of the PI3K pathway, and provide novel insights into the gender differences characterizing thyroid neoplastic disorders.
Keywords: Thyroid cancer, Pten, Estrogen
Introduction
An extensive body of epidemiological data supports the notion that thyroid disorders, including nodular disease and thyroid carcinoma, are more frequent in women than in men (Libutti 2005). Several studies have attempted to link the pro-proliferative action of circulating estrogens on the thyroid epithelial cells to the increased prevalence of thyroid disease in females(Chen et al 2008, Kumar et al 2010, Manole et al 2001, Rajoria et al 2010, Vivacqua et al 2006). However, to date, solid evidence linking sex hormone action to thyroid pathogenesis in vivo is still lacking, also because of the limited number of model systems available to dissect these interactions in a physiologically relevant context.
The central role played by the PI3K signaling cascade in the pathogenesis of thyroid proliferative and neoplastic disorders has been recently corroborated by the development and analysis of a relevant mouse model (Miller et al 2009, Yeager et al 2007, Yeager et al 2008), as well as by solid clinicopathological data (Garcia-Rostan et al 2005, Hou et al 2007, Hou et al 2008, Vasko and Saji 2007, Wang et al 2007).
Thyrocyte-specific deletion of the Pten tumor suppressor constitutively activates the PI3K signaling cascade, leading to hyperplastic thyroid glands at birth, and to the development of thyroid nodules and follicular adenomas by 6–8 months of age (Yeager et al 2007). One striking feature of the Pten mouse model of thyroid disease is the higher proliferative index of female mutant thyrocytes, compared to males, which leads to hypercellular female glands at a young age, and to an increased incidence of thyroid adenomas in the females at 8 months of age (Yeager et al 2007).
While these data support a central role for the PI3K pathway in the regulation of thyroid proliferative homeostasis, they also suggest that its constitutive activation is not sufficient for full neoplastic transformation. In support of this notion, an oncogenic allele of Kras synergizes with Pten loss and leads to the rapid development of thyroid follicular carcinomas (Miller et al 2009).
Here, the analysis of Pten mutant mice for up to two years not only reveals their high susceptibility to developing metastatic follicular carcinomas, but also sheds light on the molecular basis of the different thyrocyte proliferative index and risk of adenoma and carcinoma development between male and female mutant mice.
Results
We followed up a total of 105 mutant mice for two years to determine the long-term consequences of constitutive PI3K activation in the thyroid follicular cells.
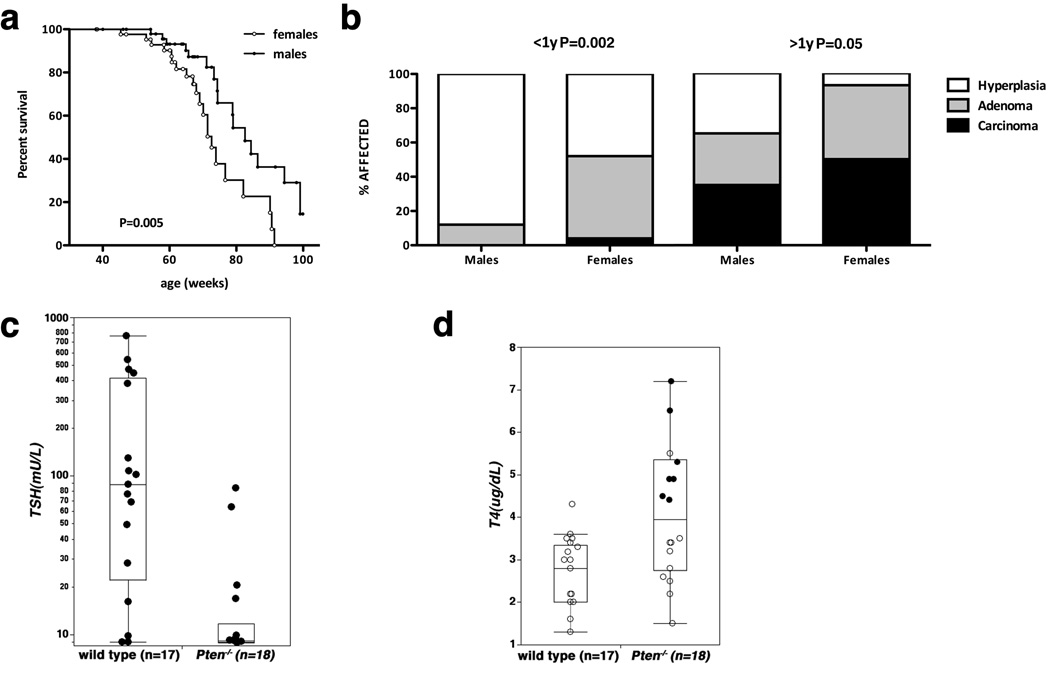
After one year of age, Pten mutant mice started showing signs of illness, and thyroid enlargement became macroscopically visible. At the end of the follow-up, we found that the female mutants had a significantly reduced lifespan(mean survival 73 weeks)compared to males(mean survival 83 weeks)(Figure 1a). We also analyzed the thyroid pathological features of a large group of these mice (n=54) between 8 and 12 months of age in order to further expand and validate our previously published more limited series (Yeager et al 2007). We found that, at this age, 52% of the females had developed thyroid follicular adenomas, compared to only 12% of the males(P=0.002)(Figure 1b). Remarkably, analysis of mice older than one year (n=34) revealed that 50% of the females, but only 35% of the males, had developed invasive and often metastatic thyroid follicular carcinomas (see below). When combined with the incidence of follicular adenomas, 93% of the females older than one year had developed neoplastic features, compared to only 65% of the males (P=0.05)(Figure 1b). These data establish the thyroid-specific Pten−/− strain as a physiologically and clinically relevant mouse model of follicular thyroid carcinoma. In addition, these mice represent a tool to further analyze the mechanisms responsible for the increased incidence of thyroid disorders in females.
Figure 1.
Aging thyroid-specific Pten−/− mice develop thyroid follicular carcinomas. a, Kaplan-Meyer analysis of the gender differences in survival of mutant mice. b, prevalence of thyroid lesions in Pten mutant mice. c, d, serum levels of TSH and T4 in aging mutant mice. Black circles in “d” correspond to mice with thyroid adenomas.
We have previously reported that the levels of TSH and T4 hormones are virtually identical in young (3-month old) wild type and mutant mice (Yeager et al 2007). In contrast, aging mutant mice displayed TSH suppression, in most cases to levels below the detection limit of our assay (Figure 1c). Conversely, T4 levels were slightly but significantly increased in all adenoma-bearing mice (Figure 1D, black dots). These data strongly suggest that the development of thyroid neoplasia in thyroid-specific Pten−/− mice is a cell-autonomous event, independent of TSH stimulation.
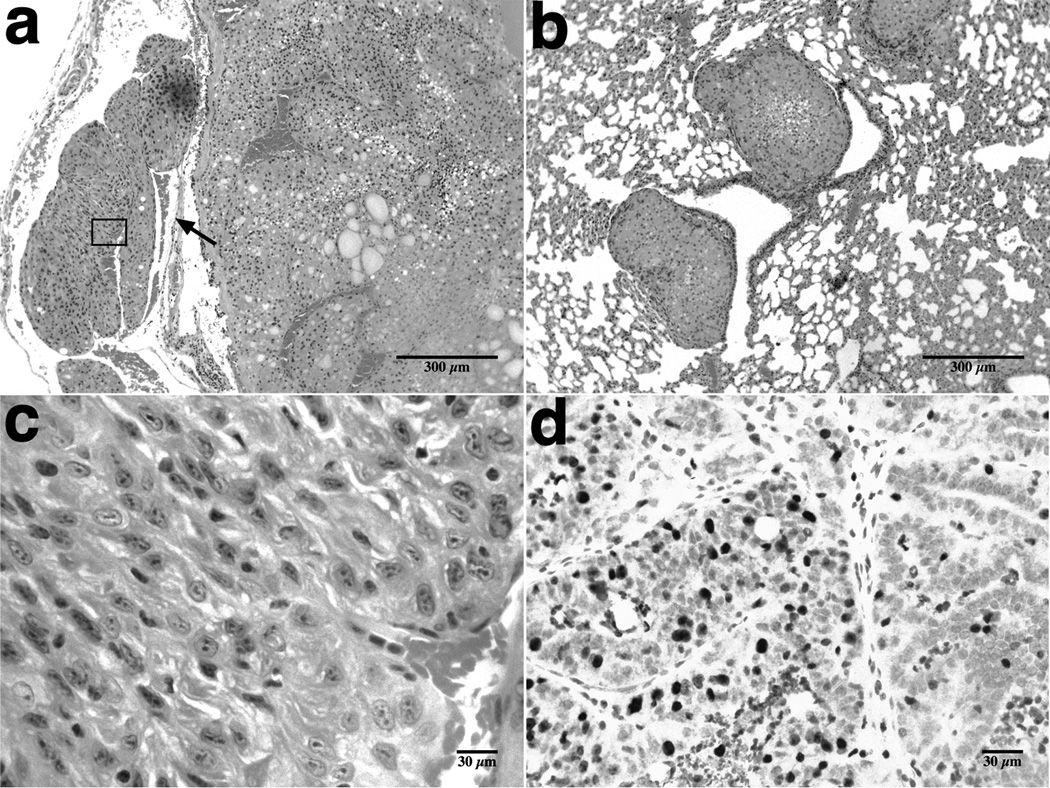
Histopathological analysis of the organs from moribund mutant mice revealed the development of follicular thyroid carcinomas with local invasion into the fibrous capsule and into the adjacent muscles and vessels (Figure 2a), as well as multiple lung metastases (Figure 2b). Neoplastic cells appeared generally well differentiated, although some areas displayed features compatible with poorly differentiated thyroid carcinomas (pleomorphic nuclei, prominent nucleoli, and a higher proliferative index)(Figure 2c,d).
Figure 2.
Histopathological features of thyroid carcinomas in Pten mutant mice. a, Follicular thyroid carcinoma with vascular invasion (arrow). b, multiple metastatic foci in the lungs of a Pten mutant mouse. c, magnification of the poorly differentiated tumor area indicated in “a”; note the enlarged, pleomorphic nuclei and prominent nucleoli. d, Ki-67 staining of the area shown in “c” reveals a high proliferative index.
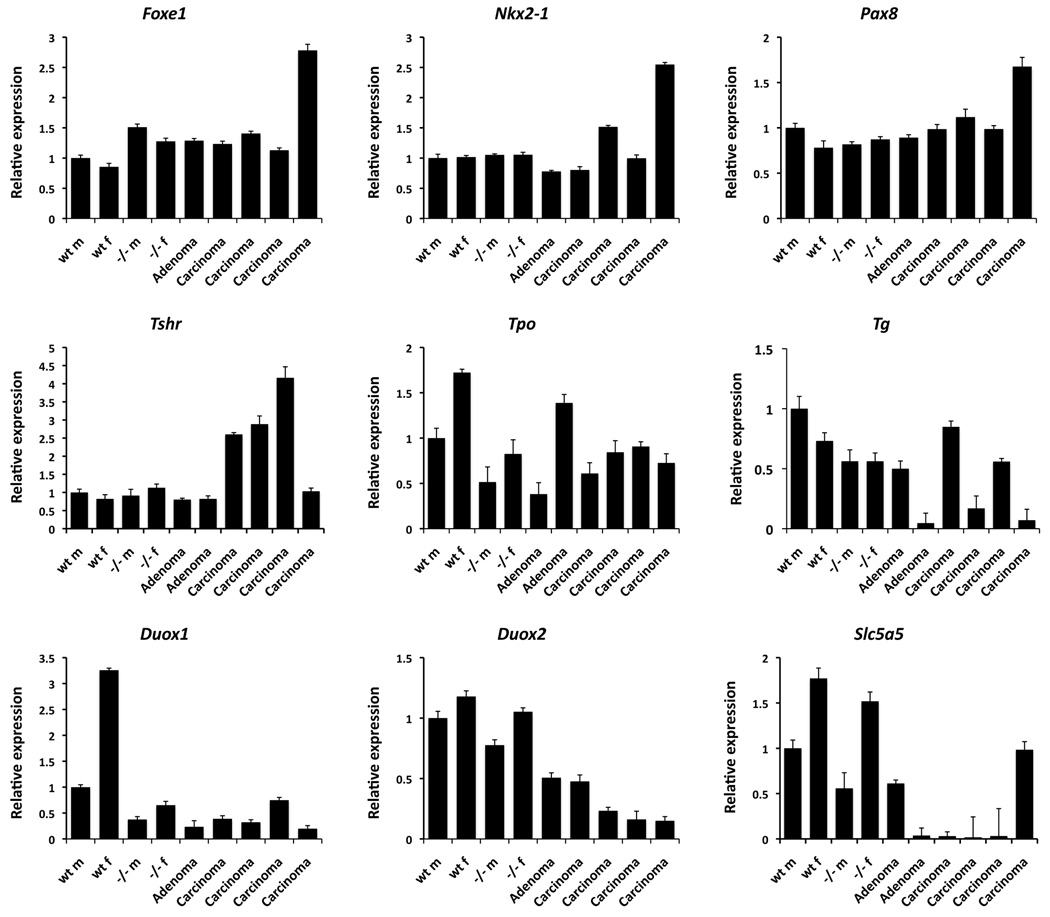
In order to characterize the effects of PI3K constitutive activation on the genes responsible for thyroid specification and function, we determined by quantitative real time PCR the expression levels of a battery of thyroid-relevant genes (Figure 3). Loss of Pten, as well as neoplastic transformation of Pten−/− thyrocytes, did not significantly or consistently change the expression of the three master genes involved in the specification and differentiation of the thyroid gland, namely Foxe1, Nkx2-1, and Pax8. Hyperplastic Pten−/− thyroids had normal mRNA levels of Tshr, Duox2, Slc5a5 (NIS), and slightly reduced expression of Tpo, Tg, and Duox1. Interestingly, we found that the expression of Tpo, Duox1, and Slc5a5 was significantly higher in thyroids from female mice, irrespective of Pten status, suggesting a role for estrogen in the control of the expression of these genes. Carcinomas and, to a lesser extent, adenomas developing in Pten−/− mice showed an increase in the expression of Tshr, as well as a reduction of mRNA levels for Duox1, Duox2 and, notably, Slc5a5.
Figure 3.
Analysis of the expression levels of several thyroid differentiation and functional markers in thyroids from male (m) and female (f) control and young mutant mice, as well as adenoma-and carcinoma-bearing female mutants.
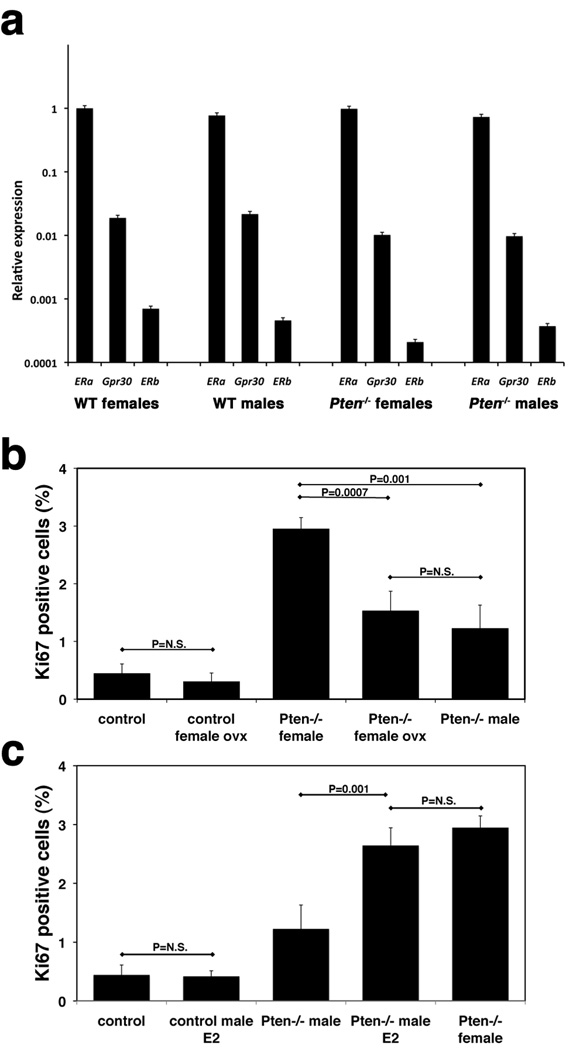
The differences in survival and neoplastic transformation rates between mutant males and females, as well as the differences in expression levels of Tpo, Duox1, and Slc5a5, prompted us to test the hypothesis that estrogen plays a direct causal role in these gender-dependent effects. We first measured the mRNA expression of the three known estrogen receptors, ERα, ERβ, and Gpr30 in the thyroids from control and mutant mice. No significant differences were noted comparing males to females, and controls to mutants (Figure 4a). ERα was expressed at much higher levels than the other two receptors. Furthermore, ERα expression in isolated follicles was comparable to that in whole thyroids (data not shown), suggesting that ERα is the main estrogen receptor in the thyroid and that it is expressed by the follicular cells. Next, we ovariectomized a cohort of 4-week old control and Pten−/− immature mice (n=6) and then measured their thyrocyte proliferative index at 12 weeks of age by Ki-67 immunohistochemistry. Strikingly, complete estrogen ablation reduced the proliferative index of female thyroids to the same levels observed in mutant males (Figure 4b). These data provide for the first time in vivo evidence that circulating estrogens are directly responsible for the increased female susceptibility to thyroid disease, at least upon activation of the PI3K pathway. We then performed the reverse experiment: 4 week-old male mutants (n=5/group) were implanted subcutaneously with pellets for the continuous release of 17β-estradiol (0.5mg/90d) or control vehicle. After 8 weeks, thyrocyte proliferation was determined. Strikingly, estrogen treatment of mutant (but not control) males increased the thyrocyte proliferation index to the same levels observed in mutant females (Figure 4c). These data provide further compelling support to the direct role of estrogen in the gender-specific proliferation differences observed in Pten−/−thyroids.
Figure 4.
Estrogen directly affects thyrocyte proliferation. a, expression analysis of the three estrogen receptors in thyroids from young, tumor-free mice. b, c, thyrocyte proliferation rates in mice subjected to hormonal manipulation. Ovx: ovariectomized, E2: estrogen-treated, N.S.: not significant differences.
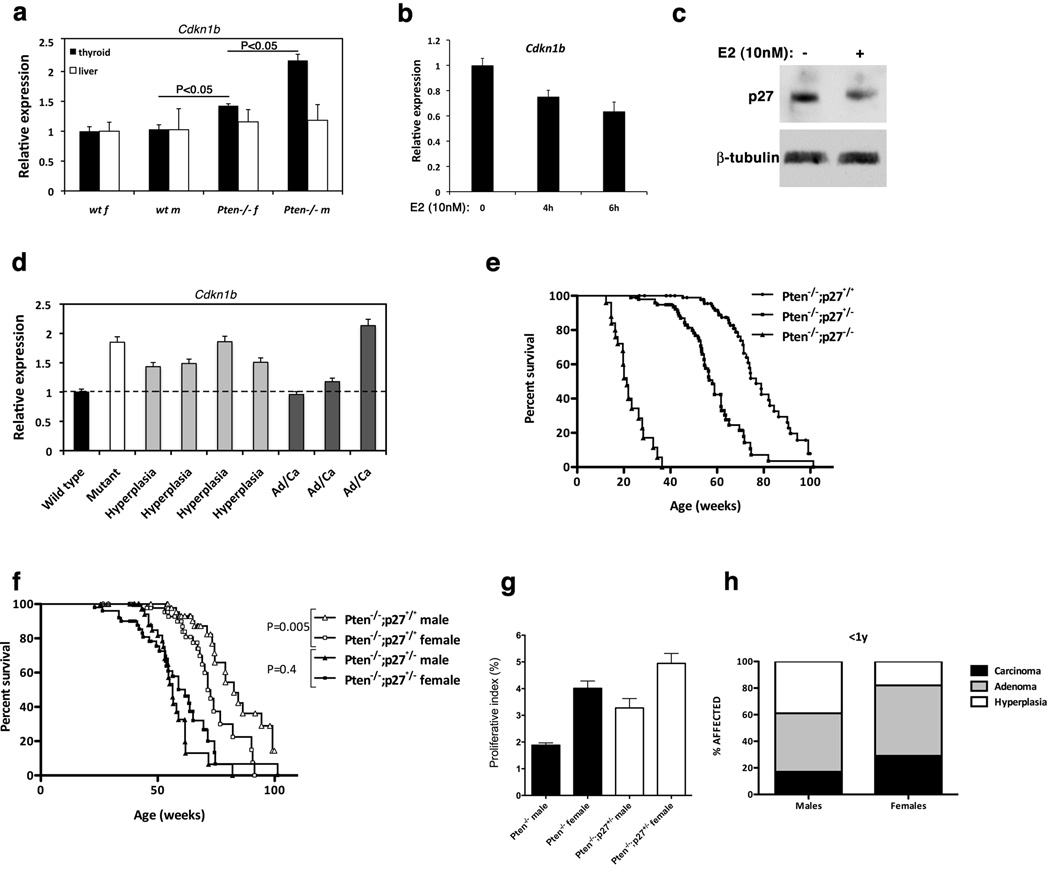
Using a candidate gene approach, we searched for differentially expressed genes that might mediate the estrogen-dependent proliferation increase in female mutants. The expression level of Cdkn1b (p27) was found higher in the thyroid, but not in the liver(used as a Pten wild type control), of Pten−/− male mice, compared to females (Figure 5a). Using Pten−/− mouse thyroid carcinoma cell lines (Miller et al 2009), we found that estrogen could in fact repress the expression of p27 both at the mRNA and protein level (Figure 5b,c), leading to the hypothesis that lower p27 levels in female mutants might be, at least in part, responsible for the increased thyrocyte proliferative index.
Figure 5.
p27 regulation is involved in the growth-promoting effects of estrogen. a, expression analysis of p27 mRNA in the thyroid and liver from control and mutant mice. b, expression analysis of p27 mRNA in a mouse thyroid cancer cell line upon estrogen treatment. c, Western blot analysis of p27 levels in a mouse thyroid cancer cell line 24 hours after estrogen treatment. d, expression analysis of p27 mRNA in the thyroid from control and mutant males, as well as old (>1 year) males that had developed hyperplastic or neoplastic (Ad/Ca: Adenoma, Carcinoma) lesions. e, Kaplan-Meyer analysis showing the effect of progressive reduction of p27 gene dosage on the survival of mutant mice. f, Kaplan-Meyer analysis showing that 50% reduction of p27 gene dosage abolishes the gender-dependent differences in mutant survival. g, proliferative index (Ki-67 positive thyrocytes) of single and Pten−/−;p27+/− compound mutant thyroids. h, prevalence of thyroid lesions in compound mutant mice.
To test whether it is possible that the tumors developed by Pten−/− male mice spontaneously select for lower p27 expression levels, we measured p27 mRNA expression in a small series of aged male mutants (>1 year of age) that had developed hyperplastic only or neoplastic lesions (Figure 5d). Although the small size of the sample does not allow us to draw a final conclusion, the finding that in two out of three tumors developed by male mutants p27 expression was reduced to wild type levels support the notion that reduction of p27 in the context of PI3K activation favors tumor development.
To genetically test this hypothesis, we crossed thyroid-specific Pten−/− mice to mice carrying a p27 null allele(Kiyokawa et al 1996). Not surprisingly, stepwise reduction of p27 gene dosage resulted in a progressive decrease of the survival in compound mutants(Figure 5e). Pten−/−;p27−/− mice had a mean survival of only 21 weeks, and all died by six months of age, with dramatically hyperplastic thyroids that caused dyspnea and prevented feeding. On the other hand, Pten−/−;p27+/− mutants had a mean survival of 58 weeks, a 25% reduction compared to Pten−/−;p27+/+ mice. Strikingly, the gender differences in survival observed in Pten−/−;p27+/+ mice were totally rescued by the reduction of p27 gene dosage (Figure 5f), suggesting that p27 is a relevant mediator of the effects of estrogen on thyroid tumor incidence. In fact, when we compared the proliferative index of thyroids from young, tumor-free Pten−/−;p27+/+ and Pten−/−;p27+/− mice, we found that loss of one p27 allele in the males resulted in a larger proliferation increase than it did in the females (Figure 5g). As a consequence, the gender differences in thyrocyte proliferation were drastically reduced in Pten−/−;p27+/− mice.
The Pten−/−;p27+/− compound mutants survived long enough to develop adenomas and carcinomas: contrary to what we had observed in Pten−/− mice (Figure 1b), the incidence of neoplastic lesions was not significantly different between Pten−/−;p27+/− males and females, although a trend towards higher incidence in the latter was still detectable(Figure 5h). These data strongly support a role for p27 as one important mediator of estrogen action in thyroid hyperproliferation and neoplastic transformation.
Discussion
Mouse models closely recapitulating both the genetic and the clinicopathological features of human carcinomas represent an invaluable tool to dissect the molecular pathways involved in the transformation process, as well as for preclinical studies aimed at validating novel biomarkers and therapeutic approaches. Of the three cancer types originating from thyroid follicular cells, only papillary carcinoma has been effectively modeled in transgenic mice, either by over-expressing the RET-PTC fusion gene (Jhiang et al 1996)or the BRAFV600E oncogenic allele (Knauf et al 2005). No genetically defined model of anaplastic thyroid cancer is available to date. Follicular carcinomas have been obtained in a small percentage of transgenic mice over-expressing the NrasQ61K allele (Vitagliano et al 2006) and in 100% of mice carrying a mutation (PV) in the TRβ receptor associated with thyroid hormone resistance (Suzuki et al 2002). In both cases, and contrary to what is commonly observed in human patients, the genetic alteration increased TSH levels, making it difficult to separate the effects of the oncogenic alterations from those of constitutive Tshr stimulation. In fact, a compound mutant carrying the PV mutation together with a Tshr−/− allele did not develop carcinomas, further underlining the importance of uncoupling these two effects (Lu et al 2010).
Previously reported data from our group (Yeager et al 2007)have provided the first in vivo evidence that constitutive PI3K activation confers thyroid follicular cells a proliferative advantage leading to the development of hyperplasia and nodular lesions. We now show that the vast majority of the hyperplastic lesions developing in thyroid-specific Pten−/− mice progress to aggressive, metastatic thyroid follicular carcinomas.
Two unique and extremely important features characterize this mouse model: first, the levels of TSH in mutant mice carrying pre-neoplastic lesions are within the normal range and then they decrease in older, tumor bearing mice, thus eliminating the confounding effects of constitutive Tshr stimulation. Second, and most strikingly, this model recapitulates the gender differences in disease prevalence observed in human thyroid disorders.
The follicular carcinomas developing in the thyroid-specific Pten−/− model underline the insufficiency of constitutive PI3K signaling for neoplastic transformation: the 10–12 months necessary for invasive tumor development are clearly needed to accumulate additional genetic alterations. In fact, we have shown that an oncogenic Kras allele drastically reduces the time required for thyroid carcinoma development in the context of Pten loss (Miller et al 2009). On the other hand, the extremely high incidence of invasive and metastatic carcinomas developing in aging thyroid-specific Pten−/− mice strongly supports a major role of the PI3K pathway in providing an optimal environment for the development of neoplastic lesions.
These tumors recapitulate several features of human aggressive follicular carcinomas: they invade through the capsule and into the vasculature, metastasize to the lungs, often display solid areas with enlarged, pleomorphic nuclei and a high proliferative index. Furthermore, as previously shown for human thyroid carcinomas, they consistently have reduced expression of Duox1 and −2 (Lacroix et al 2001) and, more importantly, suppression of sodium-iodide symporter (NIS) expression(Sodre et al 2008). The latter feature is particularly clinically relevant, since this mouse model can now be utilized to test approaches aimed at increasing NIS expression in radioiodine-refractory thyroid carcinomas.
A unique and unexpected feature of this model is the increased tumor incidence in female mutants, mirroring the increased susceptibility of women to thyroid disorders. A role for estrogen in thyroid proliferation has been proposed by several groups, based on the analysis of the effects of estradiol on thyroid carcinoma cells in culture (Chen et al 2008, Kumar et al 2010, Manole et al 2001, Rajoria et al 2010, Vivacqua et al 2006). Here, for the first time, our hormone manipulation experiments have provided in vivo evidence that, in the context of PI3K activation, circulating estrogens increase thyroid follicular cells proliferation. It is tempting to suggest that the relatively mild effect of estrogens on thyroid cells is uncovered and amplified by oncogenic events lowering the thyrocyte proliferation threshold. Further studies are needed to validate this hypothesis in the context of different oncogenic mutations. An additional novel finding is the increased expression level of Tpo, Duox1 and Slc5a5 genes in female mice irrespective of their genotype, strongly suggesting that estrogen plays a significant role in their transcriptional regulation.
Finally, we have provided in vivo evidence that the gender differences in thyrocyte proliferation and neoplastic transformation elicited by estrogens are due at least in part to the ability of these hormones to regulate p27 levels through mechanisms that include transcriptional regulation in addition to the known effects of estrogens on p27 protein degradation through Skp2 (Foster et al 2003).
In conclusion, our study validates the thyroid-specific Pten−/− mouse as a clinically relevant model of advanced and aggressive follicular carcinomas, uniquely recapitulating several features of human thyroid cancer. In addition, it provides evidence that estrogen receptor alpha activation contributes to the increased susceptibility of females to thyroid proliferative disorders and neoplastic transformation at least in part through the control of p27 levels.
Materials and Methods
Animals and treatments
The PtenL/L and TPO-Cre strains have been described (Yeager et al 2007). All strains were backcrossed in the 129Sv background for at least eight generations. Ovariectomy was performed on four-week old mice under Isoflurane anesthesia. Four week-old male mutants were implanted subcutaneously with pellets (Innovative Res., Sarasota, FL) for the continuous release of E2 (0.5mg/90d)or control vehicle.
Hormone measurements
Blood was collected by cardiac puncture. Serum thyroid-stimulating hormone (TSH) was measured using a sensitive, heterologous, disequilibrium double-antibody precipitation RIA (Pohlenz et al 1999), and results were expressed in mU/liter. All samples were individually analyzed for each mouse. Total T4 concentrations were measured by a solid-phase RIA (Coat-a-Count; Diagnostic Products Corp., Los Angeles, CA) adapted for mice. Values of the respective limits of assays sensitivities were assigned to samples with undetectable TSH and T4 concentration.
Proliferation analysis
Ki-67-stained thyroid sections were photographed at 400x magnification and analyzed using the ImageJ software. Between 1500 and 3000 cells per slide were analyzed.
Cells and treatments
Several independent cell lines established from primary thyroid tumors developed by Pten mutant mice (Miller et al 2009)were grown in phenol red-free DMEM with 10% charcoal-stripped FBS. Estradiol (Sigma, St. Louis, MO) was added 24h after plating. At the indicated time points, cells were harvested in RIPA buffer for protein analysis and in Trizol (Invitrogen, Carlsbad, CA) for RNA analysis.
Western blot analysis
Thyroids and cells were homogenized on ice in RIPA buffer supplemented with Complete protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN). Western blot analysis was carried out on 20–40µg proteins with a p27-specific antibody from Santa Cruz Biotechnology (Santa Cruz, CA) and a β-tubulin antibody from Sigma-Aldrich.
Real time PCR
Total RNA was extracted with Trizol and reverse transcribed using the Thermoscript kit(Invitrogen). qRT-PCR was performed on a StepOne Plus apparatus using the Absolute Blue qPCR Rox Mix(Thermo Scientific, Waltham, MA) and TaqMan expression assays (Applied Biosystems, Carlsbad, CA). Each sample was run in triplicate and GusB was used to control for input RNA. Data analysis was based on the Ct method, and experiments were repeated at least three times using at least two independent organ pools(at least five mice/pool).
Statistical analysis
Experiments were performed at least three times. Data were analyzed using the JMP 5.1 and Prism software packages. Differences with P- values <0.05 were considered statistically significant.
Acknowledgements
The authors acknowledge the Animal Housing and the Histotechnology and Comparative Pathology Facilities of Albert Einstein College of Medicine. This work was supported by the AECC Core Grant, and by NIH grants to ADC (CA97097 and CA128943)and SR (DK15070 and DK20595).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets. 2008;8:367–377. doi: 10.2174/156800908785133150. [DOI] [PubMed] [Google Scholar]
- Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–41366. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- Garcia-Rostan G, Costa AM, Pereira-Castro I, Salvatore G, Hernandez R, Hermsem MJ, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- Hou P, Ji M, Xing M. Association of PTEN gene methylation with genetic alterations in the phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid tumors. Cancer. 2008;113:2440–2447. doi: 10.1002/cncr.23869. [DOI] [PubMed] [Google Scholar]
- Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, et al. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996;137:375–378. doi: 10.1210/endo.137.1.8536638. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010;36:1067–1080. doi: 10.3892/ijo_00000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix L, Nocera M, Mian C, Caillou B, Virion A, Dupuy C, et al. Expression of nicotinamide adenine dinucleotide phosphate oxidase flavoprotein DUOX genes and proteins in human papillary and follicular thyroid carcinomas. Thyroid. 2001;11:1017–1023. doi: 10.1089/105072501753271699. [DOI] [PubMed] [Google Scholar]
- Libutti SK. Understanding the role of gender in the incidence of thyroid cancer. Cancer J. 2005;11:104–105. doi: 10.1097/00130404-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Lu C, Zhao L, Ying H, Willingham MC, Cheng SY. Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma. Endocrinology. 2010;151:1929–1939. doi: 10.1210/en.2009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen Promotes Growth of Human Thyroid Tumor Cells by Different Molecular Mechanisms. J Clin Endocrinol Metab. 2001;86:1072–1077. doi: 10.1210/jcem.86.3.7283. [DOI] [PubMed] [Google Scholar]
- Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–3694. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9:1265–1271. doi: 10.1089/thy.1999.9.1265. [DOI] [PubMed] [Google Scholar]
- Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41. doi: 10.1089/thy.2009.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodre AK, Rubio IG, Galrao AL, Knobel M, Tomimori EK, Alves VA, et al. Association of low sodium-iodide symporter messenger ribonucleic acid expression in malignant thyroid nodules with increased intracellular protein staining. J Clin Endocrinol Metab. 2008;93:4141–4145. doi: 10.1210/jc.2007-0353. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- Vitagliano D, Portella G, Troncone G, Francione A, Rossi C, Bruno A, et al. Thyroid targeting of the N-ras(Gln61Lys) oncogene in transgenic mice results in follicular tumors that progress to poorly differentiated carcinomas. Oncogene. 2006;25:5467–5474. doi: 10.1038/sj.onc.1209527. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, et al. 17 beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol. 2006;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hou P, Yu H, Wang W, Ji M, Zhao S, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J Clin Endocrinol Metab. 2007;92:2387–2390. doi: 10.1210/jc.2006-2019. [DOI] [PubMed] [Google Scholar]
- Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res. 2007;67:959–966. doi: 10.1158/0008-5472.CAN-06-3524. [DOI] [PubMed] [Google Scholar]
- Yeager N, Brewer C, Cai KQ, Xu XX, Di Cristofano A. mTOR is the key effector of PI3K-initiated proliferative signals in the thyroid follicular epithelium. Cancer Res. 2008;68:444–449. doi: 10.1158/0008-5472.CAN-07-3030. [DOI] [PubMed] [Google Scholar]