Abstract
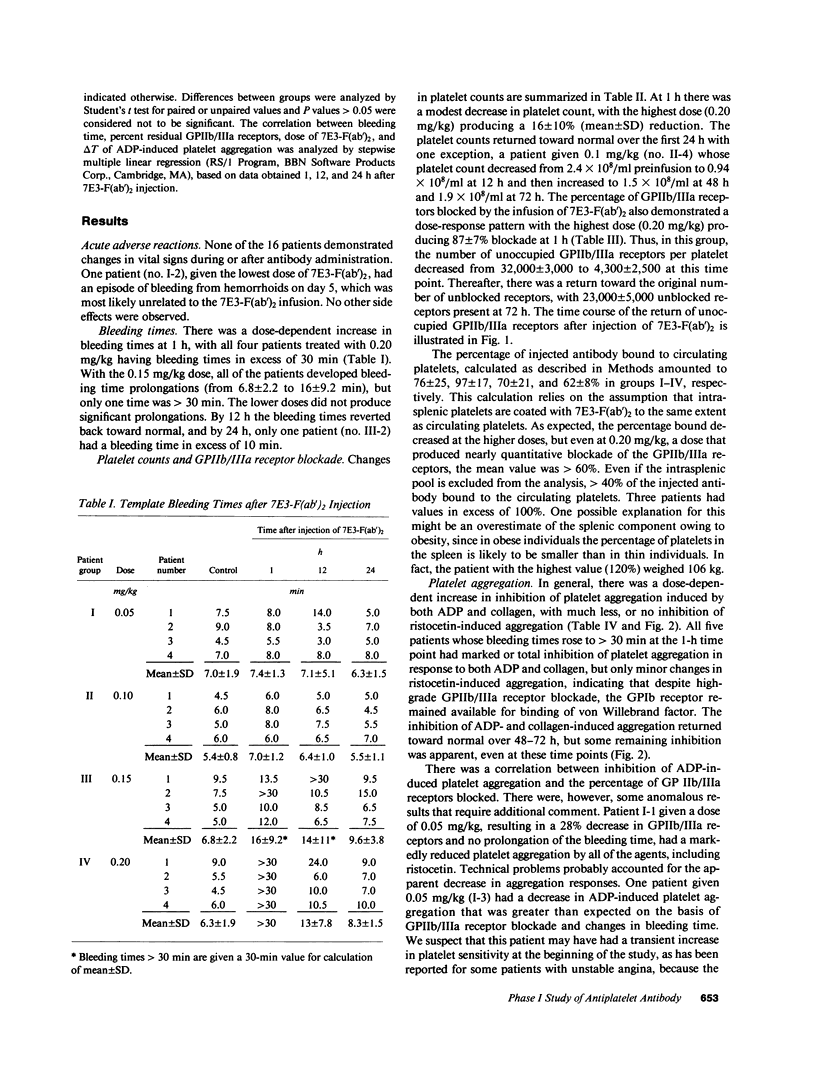
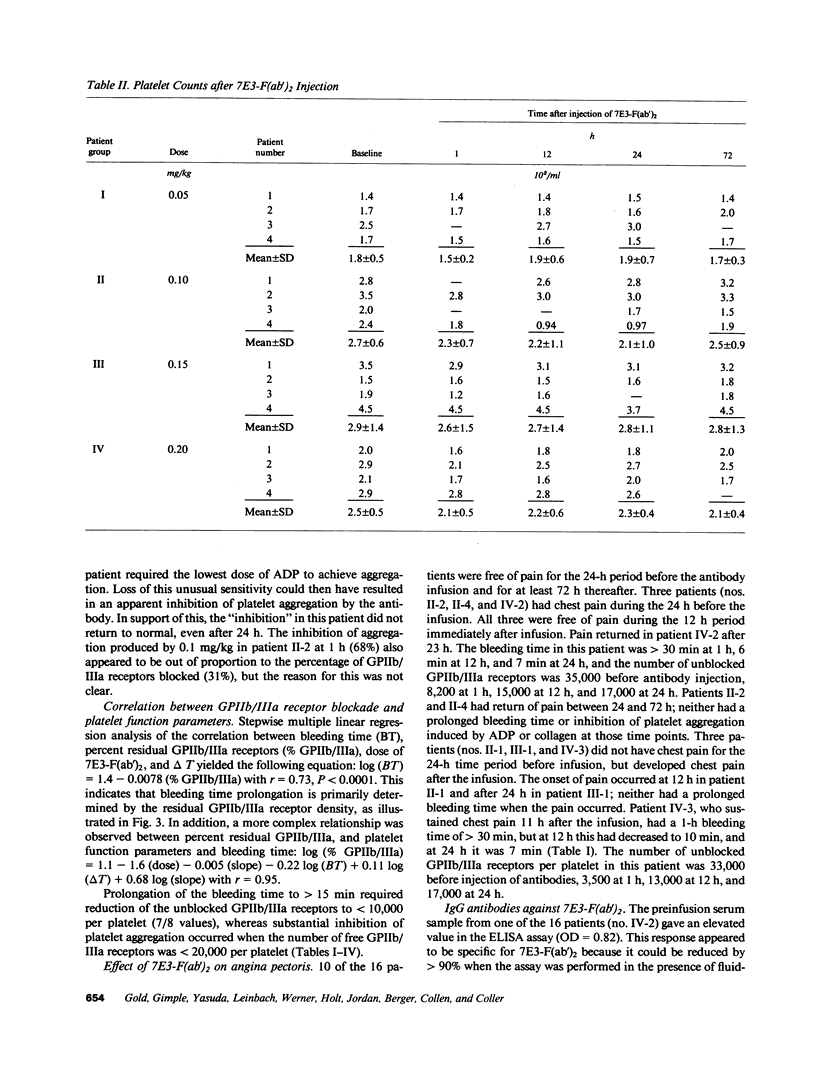
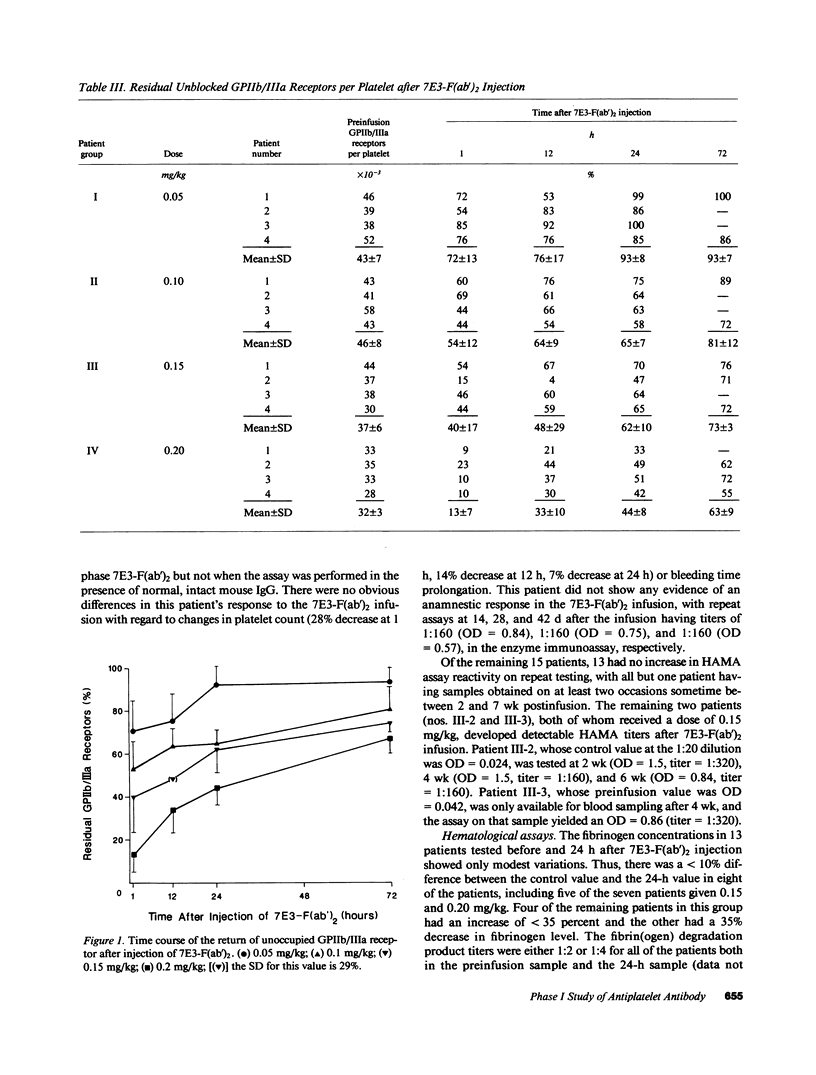
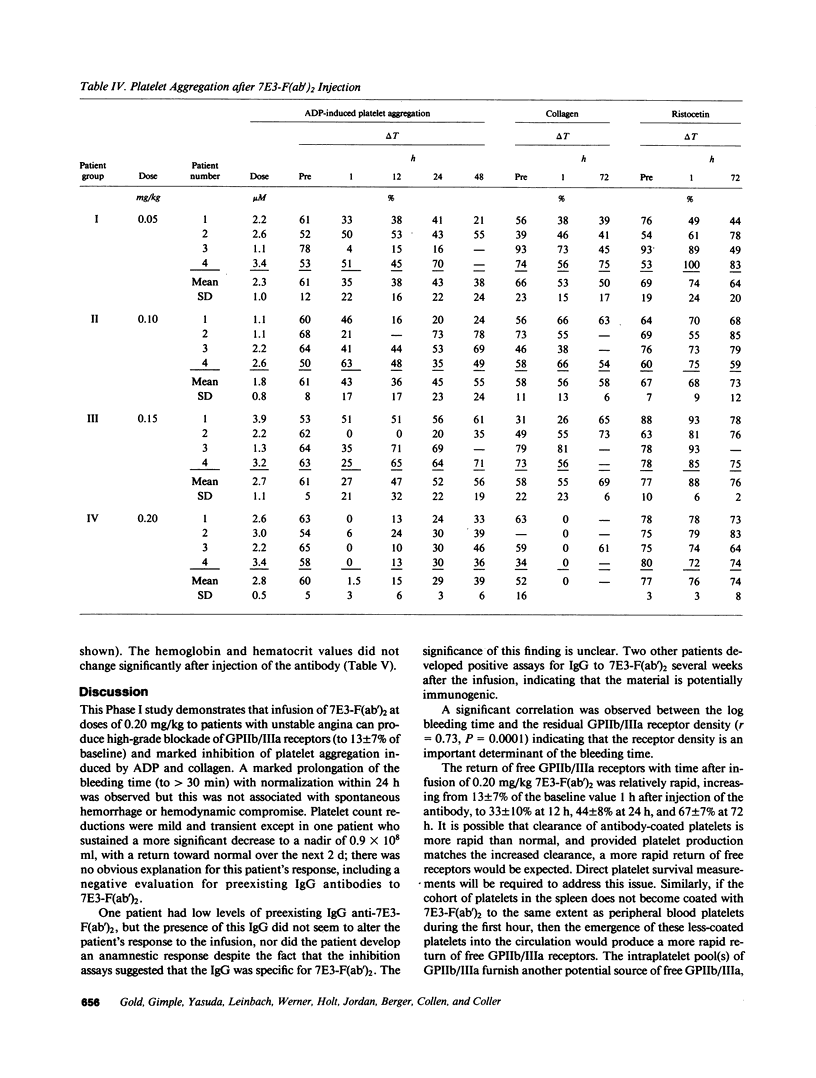
The pharmacodynamics of intravenous bolus injections of 0.05, 0.10, 0.15, and 0.20 mg/kg of F(ab')2 fragments of the murine monoclonal antibody 7E3, 7E3-F(ab')2, directed against the glycoprotein IIb/IIIa (GPIIb/IIIa) receptor of human platelets, were studied in groups of four patients with unstable angina pectoris. With 0.20 mg/kg, the template bleeding time prolonged from 6.3 +/- 1.9 (mean +/- SD) to greater than 30 min; it subsequently decreased to 13 +/- 7.8 min after 12 h and to 8.3 +/- 1.5 min after 24 h. The number of unblocked GPIIb/IIIa receptors (preinfusion value, 32,000 +/- 3,000 per platelet) decreased to 13 +/- 7% of the preinfusion value 1 h after infusion, and then increased to 33 +/- 10% at 12 h, 44 +/- 8% at 24 h and 67 +/- 7% at 72 h. The logarithm of the bleeding time was inversely proportional with the residual GPIIb/IIIa receptors (r = 0.73, P less than 0.0001). ADP-induced platelet aggregation (measured by changes in light transmittance in percent) decreased from 60 +/- 5% before infusion to 1.5 +/- 3% 1 h after infusion; it then increased to 29 +/- 3% after 24 h and 39 +/- 6% after 72 h. Platelet counts decreased by 16% at 1 h and returned to control values within 24 h. Proportionally smaller effects were seen at lower doses of 7E3-F(ab')2. Antibody injection did not induce spontaneous bleeding. Angina was not observed during the first 12 h when the bleeding time was significantly prolonged, but occurred in 6 of the 16 patients within the next 3 d. 2 of the 16 patients developed low titers of IgG antibodies specific for 7E3-F(ab')2. Thus 7E3-F(ab')2 induces dose-related inhibition of platelet function; at a dose of 0.20 mg/kg, it causes profound inhibition of platelet aggregation and prolongation of the bleeding time, but no spontaneous bleeding.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Folts J. D., Scudder L. E., Smith S. R. Antithrombotic effect of a monoclonal antibody to the platelet glycoprotein IIb/IIIa receptor in an experimental animal model. Blood. 1986 Sep;68(3):783–786. [PubMed] [Google Scholar]
- Coller B. S., Folts J. D., Smith S. R., Scudder L. E., Jordan R. Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor. Correlation with bleeding time, platelet aggregation, and blockade of GPIIb/IIIa receptors. Circulation. 1989 Dec;80(6):1766–1774. doi: 10.1161/01.cir.80.6.1766. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Scudder L. E., Berger H. J., Iuliucci J. D. Inhibition of human platelet function in vivo with a monoclonal antibody. With observations on the newly dead as experimental subjects. Ann Intern Med. 1988 Oct 15;109(8):635–638. doi: 10.7326/0003-4819-109-8-635. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Scudder L. E. Inhibition of dog platelet function by in vivo infusion of F(ab')2 fragments of a monoclonal antibody to the platelet glycoprotein IIb/IIIa receptor. Blood. 1985 Dec;66(6):1456–1459. [PubMed] [Google Scholar]
- Coller B. S., Seligsohn U., Zivelin A., Zwang E., Lusky A., Modan M. Immunologic and biochemical characterization of homozygous and heterozygous Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations of Israel: comparison of techniques for carrier detection. Br J Haematol. 1986 Apr;62(4):723–735. doi: 10.1111/j.1365-2141.1986.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Thomas A. C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985 Apr;53(4):363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J., Wright F., FitzGerald G. A. Increased thromboxane biosynthesis during coronary thrombolysis. Evidence that platelet activation and thromboxane A2 modulate the response to tissue-type plasminogen activator in vivo. Circ Res. 1989 Jul;65(1):83–94. doi: 10.1161/01.res.65.1.83. [DOI] [PubMed] [Google Scholar]
- Friedman M. The coronary thrombus: its origin and fate. Hum Pathol. 1971 Mar;2(1):81–128. doi: 10.1016/s0046-8177(71)80022-9. [DOI] [PubMed] [Google Scholar]
- Gold H. K., Coller B. S., Yasuda T., Saito T., Fallon J. T., Guerrero J. L., Leinbach R. C., Ziskind A. A., Collen D. Rapid and sustained coronary artery recanalization with combined bolus injection of recombinant tissue-type plasminogen activator and monoclonal antiplatelet GPIIb/IIIa antibody in a canine preparation. Circulation. 1988 Mar;77(3):670–677. doi: 10.1161/01.cir.77.3.670. [DOI] [PubMed] [Google Scholar]
- Gold H. K., Johns J. A., Leinbach R. C., Yasuda T., Grossbard E., Zusman R., Collen D. A randomized, blinded, placebo-controlled trial of recombinant human tissue-type plasminogen activator in patients with unstable angina pectoris. Circulation. 1987 Jun;75(6):1192–1199. doi: 10.1161/01.cir.75.6.1192. [DOI] [PubMed] [Google Scholar]
- Hanson S. R., Pareti F. I., Ruggeri Z. M., Marzec U. M., Kunicki T. J., Montgomery R. R., Zimmerman T. S., Harker L. A. Effects of monoclonal antibodies against the platelet glycoprotein IIb/IIIa complex on thrombosis and hemostasis in the baboon. J Clin Invest. 1988 Jan;81(1):149–158. doi: 10.1172/JCI113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson J. K., Simpson P. J., Lucchesi B. R. Antiplatelet monoclonal F(ab')2 antibody directed against the platelet GPIIb/IIIa receptor complex prevents coronary artery thrombosis in the canine heart. J Mol Cell Cardiol. 1989 Apr;21(4):393–405. doi: 10.1016/0022-2828(89)90650-0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Cellular adhesion: GPIIb-IIIa as a prototypic adhesion receptor. Prog Hemost Thromb. 1989;9:117–156. [PubMed] [Google Scholar]
- Swahn E., Wallentin L. Platelet reactivity in unstable coronary artery disease. Thromb Haemost. 1987 Jun 3;57(3):302–305. [PubMed] [Google Scholar]
- Torem S., Schneider P. A., Hanson S. R. Monoclonal antibody-induced inhibition of platelet function: effects on hemostasis and vascular graft thrombosis in baboons. J Vasc Surg. 1988 Jan;7(1):172–180. doi: 10.1067/mva.1988.avs0070172. [DOI] [PubMed] [Google Scholar]
- Wencel-Drake J. D. Plasma membrane GPIIb/IIIa. Evidence for a cycling receptor pool. Am J Pathol. 1990 Jan;136(1):61–70. [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Gold H. K., Fallon J. T., Leinbach R. C., Guerrero J. L., Scudder L. E., Kanke M., Shealy D., Ross M. J., Collen D. Monoclonal antibody against the platelet glycoprotein (GP) IIb/IIIa receptor prevents coronary artery reocclusion after reperfusion with recombinant tissue-type plasminogen activator in dogs. J Clin Invest. 1988 Apr;81(4):1284–1291. doi: 10.1172/JCI113446. [DOI] [PMC free article] [PubMed] [Google Scholar]



