Abstract
2-Fluoro-1,3-thiazoles were rapidly and efficiently labeled with no-carrier-added fluorine-18 (t1/2 = 109.7 min) by treatment of readily prepared 2-halo precursors with cyclotron-produced [18F]fluoride ion. The [18F]2-fluoro-1,3-thiazolyl moiety constitutes a new and easily-labeled structural motif for prospective molecular imaging radiotracers.
Keywords: Radiofluorination; 1,3-thiazole; microwave-assisted synthesis
Interest in the efficient synthesis of new fluoro compounds as potential drugs and imaging agents is growing dramatically.1 Fluorinated drugs include antibiotics, sedatives, antidepressants and anti-tumor agents.1,2 Moreover, methods for introducing fluorine-18 (t1/2 = 109.7 min) into organic molecules have become increasingly important for the development of radiotracers for positron emission tomography (PET)3, a sensitive and powerful technique for molecular imaging in animal and human subjects in vivo, and hence for clinical research and diagnosis.4 For the development of PET radiotracers, the preferred source of fluorine-18 is [18F]fluoride ion, obtained from the 18O(p,n)18F reaction on 18O-enriched water.5 This production method is very high yielding and importantly gives the [18F]fluoride ion at a very high ‘no-carrier-added’ (NCA) specific radioactivity. High specific radioactivity (typically > 1 Ci/μmol) is often mandatory for the safety and efficacy of PET radiotracers.6
The introduction of fluoride ion or [18F]fluoride ion at an alkyl carbon is usually straightforward.6 Fluoroalkanes are however generally more susceptible to metabolic defluorination in vivo than fluoroarenes.7 Therefore, the latter are often more desirable targets in the development of drugs and of 18F-labeled imaging agents. [18F]Fluoride ion may be incorporated readily into electron-deficient benzenes6 and also into pyridines6,8 at their 2- and 4-positions by aromatic nucleophilic substitution reactions, and at all positions in electron-rich or electron-poor benzenes and pyridines by reaction with diaryliodonium salts.9 Fast efficient methods for utilizing [18F]fluoride ion in the fluorination of other arenes, especially other heteroarenes, have however been little explored.
1,3-Thiazole can be regarded as isosteric with benzene and pyridine.10 Replacement of a phenyl or pyridinyl group with a 1,3-thiazolyl group may therefore lead to an improved drug or receptor ligand. A striking example occurs among diarylacetylene-type metabotropic glutamate sub-type receptor 5 (mGluR5) ligands. Here pairing of a substituted 1,3-thiazolyl group with a substituted phenyl group in the diarylacetylene provides ligands with similar or higher potency than the pairing of other aryl groups.11 Such 1,3-thiazoles appear susceptible to metabolism by oxidative ring opening.12 However, one report suggests that fluorination of a 1,3-thiazolyl group might lead to better resistance to metabolism in vivo.13 We hypothesized that the 2-position in 1,3-thiazoles would be an attractive site for facile incorporation of fluorine-18 from [18F]fluoride ion and moreover might deliver a relatively metabolically stable [18F]2-fluoro-1,3-thiazolyl structural motif.
In order to establish reaction conditions for the preparation of [18F]2-fluorothiazoles, we first sought a simple method to prepare reference 2-fluorothiazoles. Among the few methods reported for preparing such fluoro compounds, including fluoro-dediazoniation14 or nucleophilic substitution15,16, nucleophilic substitution with fluoride ion in 2-halo-1,3-thiazoles16 appeared attractive since the precursor halo compounds were quite accessible.
2-Bromo-1,3-thiazole (1) was submitted to fluorination with potassium fluoride and an equimolar amount of the cryptand, Kryptofix® 2.2.2 (K 2.2.2) or the crown ether 18-crown-6, in various solvents (DMSO, MeCN or DMF) under microwave irradiation. We found that fluorination was almost quantitative after irradiation for 10 minutes at 150 °C (35 W) in DMSO. The conversion of 2-bromo-1,3-benzothiazole (2) into the corresponding 2-fluoro derivative (8) also proceeded in very high yield under these conditions (Table 1).
Table 1.
Yields of 2-fluorothiazoles produced under microwave irradiation.
 | ||||
|---|---|---|---|---|
| Precursor |
Fluoro-product | Yield (%)a | ||
| R | X | |||
| 1 | H | Br | 7 | 95 |
| 2 |
|
Br | 8 | 89 |
| 3 |
|
Cl | 9 | 78 |
| 4 |
|
Br | 9 | 72 |
| 5 |
|
I | 9 | 9 |
| 6 |
|
Br | 10 | 55 |
Yield for isolated and analytically pure compound.
We decided to test the generality of these microwave conditions for the rapid high-yield preparation of other 2-fluoro-thiazole derivatives. First we prepared the 2-chloro-, 2-bromo- and 2-iodo-1,3-thiazolyl compounds, 3–5,17 as simple analogs of a potent mGluR5 ligand, namely 3-[(2-methyl-1,3-thiazol-4-yl)-ethynyl]-pyridine.18 These halo compounds were subjected to fluorination under the most effective conditions found for 2. The target fluoro derivative 9 was successfully isolated in high yield from the chloro (3) and bromo (4) precursors (Table 1). The yield of 9 from the iodo precursor 5 was however low (Table 1).
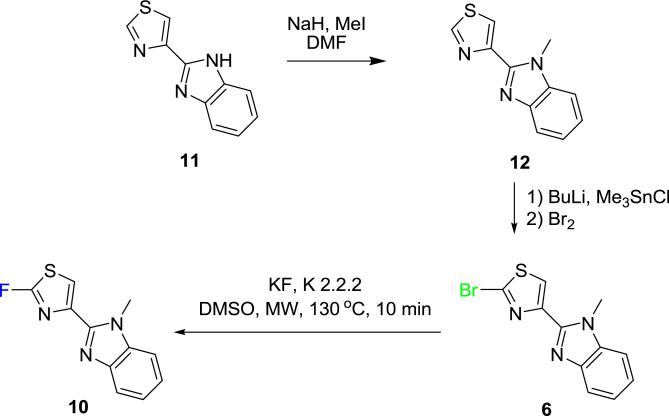
We also prepared the 2-bromo and N-Me derivative (6) of the powerful antibiotic, thiabendazole, as a precursor for fluorination (Scheme 1). Previous work has shown that thiabendazole retains high fungicidal and bactericidal activity after 2-halogenation19 (halo = Cl or Br) of the thiazolyl group or N-methylation20 of the imidazolyl ring. Fluorination of 6 in DMSO for 10 min with microwave heating at 130 °C gave N-methyl-2-fluorothiabendazole (10) in good yield (Scheme 1; Table 1).
Scheme 1.
Synthesis of N-methyl-2-fluoro-thiabendazole (10).
The apparent rapidity and effectiveness of the rapid fluorination of 2-halo-1,3-thiazoles with potassium fluoride under microwave irradiation encouraged us to explore the preparation of [18F]2-fluoro-1,3-thiazoles with cyclotron-produced NCA [18F]fluoride ion under thermal or microwave heating conditions (Table 2). We found that treatment of a low amount of the 2-fluoro-thiazole 7 (1.1 mg; 1.0 mmol) with [18F]fluoride ion in the presence of K+-K 2.2.2 in acetonitrile (500 μL) at 110 °C produced carrier-added [18F]7 in 26% decay-corrected radiochemical yield (RCY) within 35 min (Scheme 2).
Table 2.
RCYs of [18F]2-fluoro-thiazoles from NCA [18F]fluoride ion.
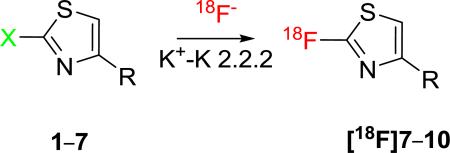 | |||||
|---|---|---|---|---|---|
| Precursor |
Reaction conditions | [18F]Fluoro- product | RCYa (%) | ||
| R | X | ||||
| 1 | H | Br | DMSO, 150 °C, 30 min | [18F]7 | 29 |
| 1 | H | Br | DMSO, MW (130 °C, 10 min) | [18F]7 | 81 |
| 2 |
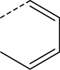
|
Br | MeCN, 80 °C, 30 min | [18F]8 | 19 |
| 2 |
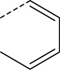
|
Br | MeCN, 150 °C, 30 min | [18F]8 | 19 |
| 2 |
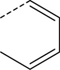
|
Br | DMF, 80 °C, 30 min | [18F]8 | 21 |
| 2 |
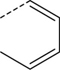
|
Br | DMF, 150 °C, 30 min | [18F]8 | 24 |
| 2 |
|
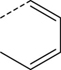
Br | DMSO, 80 °C, 30 min | [18F]8 | 26 |
| 2 |
|
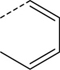
Br | DMSO, 150 °C, 30 min | [18F]8 | 35 |
| 2 |
|
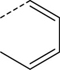
Br | DMSO, MW (150 °C, 10 min) | [18F]8 | 47 |
| 3 |
|
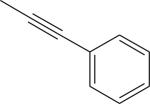
Cl | DMSO, MW (130 °C, 8 min) | [18F]9 | 14.5 |
| 4 |
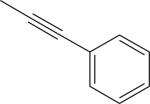
|
Br | DMSO, MW (130 °C, 8 min) | [18F]9 | 14 |
| 5 |
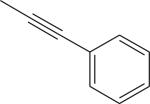
|
I | DMSO, MW (130 °C, 8 min) | [18F]9 | 2.0 |
| 4 |
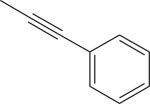
|
Br | DMSO, MW (130 °C, 10 min) | [18F]9 | 45 |
| 6 |
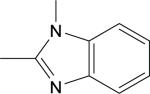
|
Br | DMSO, MW (130 °C, 10 min) | [18F]10 | 23 |
| 7 | H | F | MeCN, 110 °C, 35 min | [18F]7 | 26 |
Isolated decay-corrected radiochemical yield from starting [18F]fluoride ion.
Similar treatment of the 2-bromo compound 1 in DMSO with NCA [18F]fluoride ion at 150 °C for 30 min gave [18F]7 in 29% RCY (Scheme 2). Use of microwave heating (10 min at 40–90 W; 130 °C) dramatically increased the RCY of [18F]7 to 81%. Similarly, under thermal heating conditions, the RCY of [18F]8 increased with temperature between 80 and 150 °C, and with solvent polarity, among acetonitrile, DMF or DMSO. Thus, at 80 °C in DMSO, [18F]8 was obtained in only 26% RCY. A higher RCY of [18F]8 (35%) was obtained in DMSO at 150 °C for 30 min. Under inert atmosphere in DMSO with brief microwave heating (90 W, 150 °C, 10 min), [18F]8 was obtained in 47% RCY, and with a measured specific activity of 2.5 Ci/μmol. Increase in temperature had less effect when using acetonitrile or DMF as solvent. In acetonitrile at 80 or 150 °C for 30 min, [18F]8 was obtained in 19% RCY, respectively. RCYs were similar in DMF under the same conditions. Thus, the 2-bromo-1,3-thiazoles 1 and 2 show reactivity towards radiofluoridation that is similar to that of 2-bromo-pyridine.21
The effect of halo leaving group on radiofluorination yield was examined with the 2-halo-thiazole precursors 3–5. In one set of experiments, using a single batch of dry [18F]F--K+-K 2.2.2 under identical reaction conditions (DMSO, 130 °C, 8 min, MW), [18F]9 was obtained from 3, 4 and 5 in 14.5, 14 and 2.0% RCY, respectively. This shows that chloro and bromo are the preferred halo leaving groups, as for halo-benzenes.6
The production of [18F]9 from bromo precursor 4 was performed in DMSO under argon with microwave heating at 130 °C for 10 min and an improved 45% RCY was obtained. Similarly, the bromo precursor 6 was radiofluorinated under microwave condition to give [18F]10 in 23% RCY. Conditions for this later reaction were not optimized.
In conclusion, we developed a mild and operationally simple procedure for preparing 2-fluoro-1,3-thiazoles in high yield by nucleophilic substitution with fluoride ion from readily prepared halo precursors under microwave irradiation. This procedure was successfully adapted to the rapid labeling of potential new radioligands with NCA [18F]fluoride ion under thermal or microwave heating conditions for potential application in PET imaging. The generated 2-fluoro compounds are expected to show desirably high resistance to defluorination in vivo. Thus 2-fluoro-1,3-thiazolyl and [18F]2-fluorothiazolyl moieties may now prove to be usefully new structural motifs for drugs and radioligands, respectively.
Supplementary Material
Acknowledgement
This work was supported by the Intramural Research Program of the National Institutes of Health (NIMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data: Experimental procedures, characterization data for new compounds 9−10, and representative radio-HPLC chromatograms for compounds [18F]7–[18F]10.
REFERENCES
- 1.Hagmann WK. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 2.Müller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 3.Phelps ME. Proc. Natl. Acad. Sci. USA. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Watson DA, Su M, Teverovskiy G, Zhang Y, García FJ, Kinzel T, Buchwald SL. Science. 2009;325:1661–1664. doi: 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Furuya T, Kaiser HM, Ritter T. Angew. Chem., Int. Ed. 2008;47:5993–5996. doi: 10.1002/anie.200802164. [DOI] [PubMed] [Google Scholar]
- 5.a Ruth TJ, Wolf AP. Radiochim. Acta. 1979;26:21–24. [Google Scholar]; b Guillaume M, Luxen A, Nebeling B, Argentini M, Clark JC, Pike VW. Appl. Radiat. Isot. 1991;42:749–762. [Google Scholar]
- 6.Cai L, Lu S, Pike VW. Eur. J. Org. Chem. 2008;17:2853–2873. [Google Scholar]
- 7.Pike VW. TIPS. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Dolci L, Dollé F, Jubeau S, Vaufrey F, Crouzel C. J. Label. Compd. Radiopharm. 1999;42:975–985. [Google Scholar]; b Dollé F. In: PET Chemistry – the Driving Force in Molecular Imaging. Schubiger PA, Lehman L, Friebe M, editors. Springer; 2007. pp. 114–157. Ch. 5. [Google Scholar]
- 9.a Pike VW, Aigbirhio FI. J. Chem. Soc., Chem. Commun. 1995:2215–2216. [Google Scholar]; b Shah A, Pike VW, Widdowson DA. J. Chem. Soc., Perkin Trans I. 1998:2043–2046. [Google Scholar]; c Ross TL, Ermert J, Hocke C, Coenen HH. J. Am. Chem. Soc. 2007;129:8018–8025. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]; d Carroll MA, Nairne J, Woodcraft JL. J. Labelled Compd. Radiopharm. 2007;50:452–454. [Google Scholar]; e Chun J-H, Lu S, Lee Y-S, Pike VW. J. Org. Chem. 2010;75:3332–3338. doi: 10.1021/jo100361d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wermuth CG, editor. The Practice of Medicinal Chemistry. 2nd Ed. Academic Press; 2003. [Google Scholar]
- 11.a Slasssi A, Isaac M, Edwards L, Minindis A, Wensbo D, Mattson J, Nilsson K, Raboisson P, McLeod D, Stormann TM, Hammerland LG, Johnson E. Curr. Topics Med. Chem. 2005;5:897–911. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]; b Siméon FG, Brown AK, Zoghbi SS, Patterson VM, Innis RB, Pike VW. J. Med. Chem. 2007;50:3256–3266. doi: 10.1021/jm0701268. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Chen W. Xenobiotica. 2005;35:797–809. doi: 10.1080/00498250500230412. [DOI] [PubMed] [Google Scholar]
- 13.Briner PH, Fyfe MCT, Martin P, Murray PJ, Naud F, Procter MJ. Org. Process Res. Dev. 2006;10:346–348. [Google Scholar]
- 14.a Grünert C, Wiechert K. Zeitsch. Chem. 1970;10:188–189. [Google Scholar]; b Lowe G, Potter BVL. J. Chem. Soc., Perkin Trans 1. 1980:2026–2028. [Google Scholar]; c Kim YH, Lee CH, Ki YC. Tetrahedron Lett. 1990;31:3019–3022. [Google Scholar]; d Yoneda N, Fukuhara T. Tetrahedron. 1996;52:23–26. [Google Scholar]; e Berger R, Chang L, Edmondson SD, Globe SD, Ha SN, Kar NF, Kopka IE, Li B, Morriello GJ, Moyes CR, Shen D-M, Wang L, Zhu C. 2009 US Patent 253705 A1.
- 15.Bartoli G, Latrofa A, Naso F, Todesco PE. J. Chem. Soc., Perkin Trans. 1. 1972;21:2671. [Google Scholar]
- 16.a Gallagher PT, Iddon B, Suschitzky H. J. Chem. Soc., Perkin Trans. 1. 1980:2358–2361. [Google Scholar]; b Beck G, Schubert R. 1988 US Patent 4,788,208.; c Benoit M, Demoute J-P, Wehrey C. 1993 Eur. Patent 0556123 A1.; d Kim Y-H. Phosphorus, Sulfur, Silicon and the Related Elements. 1993;74:249–260. [Google Scholar]
- 17.Siméon FG, Wendahl MT, Pike VW. J. Org. Chem. 2009;74:2578–2581. doi: 10.1021/jo802799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosford NDP, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. J. Med. Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 19.a Guczoghy L, Puklics M, Toth G, Szabo G, Palfi D. 1973 Fr. Patent 7230738.; b Tashika Y, Ito T, Takanashi K, Ono M. 1967 Jpn. Tokkyo Koho JP 19650309.
- 20.Schiffmann R, Neugebauer A, Klein CD. J. Med. Chem. 2006;49:511–222. doi: 10.1021/jm050476z. [DOI] [PubMed] [Google Scholar]
- 21.a Greguric I, Taylor SR, Denoyer D, Ballantyne P, Berghofer P, Roselt P, Pham TQ, Mattner F, Bourdier T, Neels OC, Dorow DS, Loc'h C, Hicks RJ, Katsifis A. J. Med. Chem. 2009;52:5299–5302. doi: 10.1021/jm9008423. [DOI] [PubMed] [Google Scholar]; b Dolci L, Valette H, Vaufrey F, Fuseau C, Bottlaender M, Crouzel C. Bioorg. Med. Chem. 1999;7:467–479. doi: 10.1016/s0968-0896(98)00261-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.