Abstract
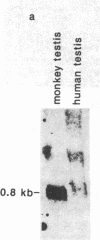
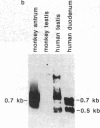
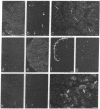
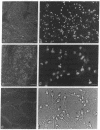
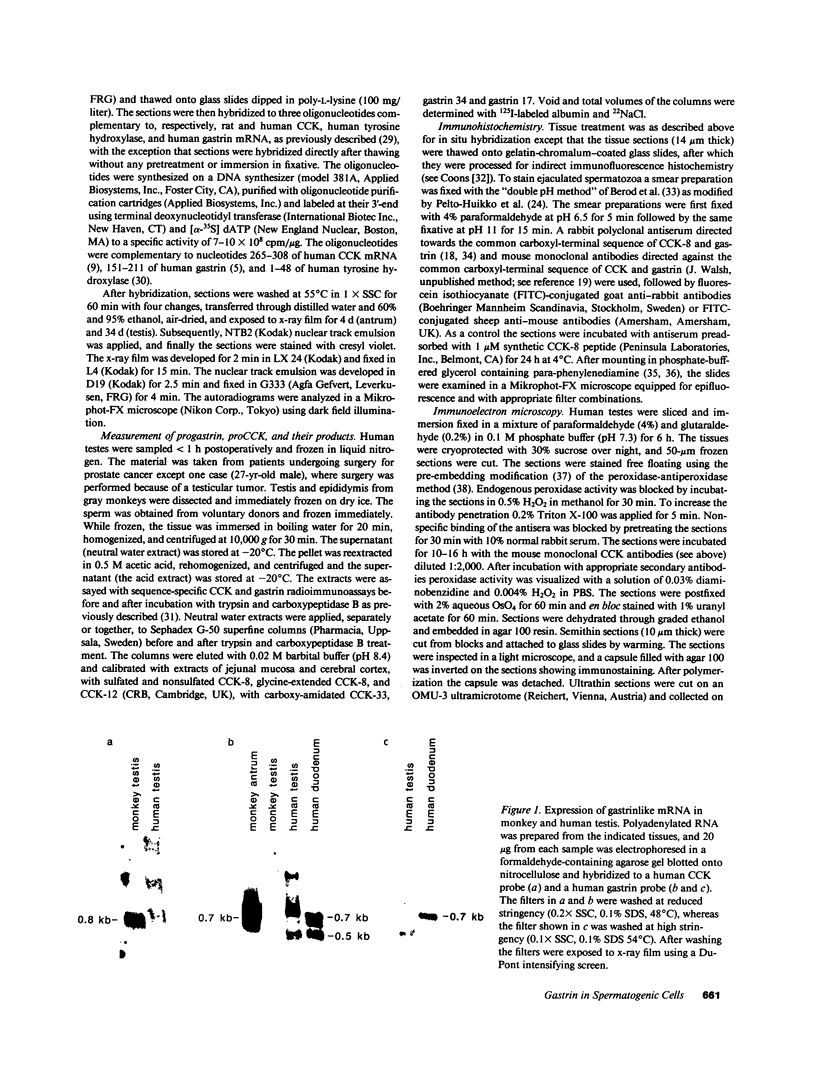
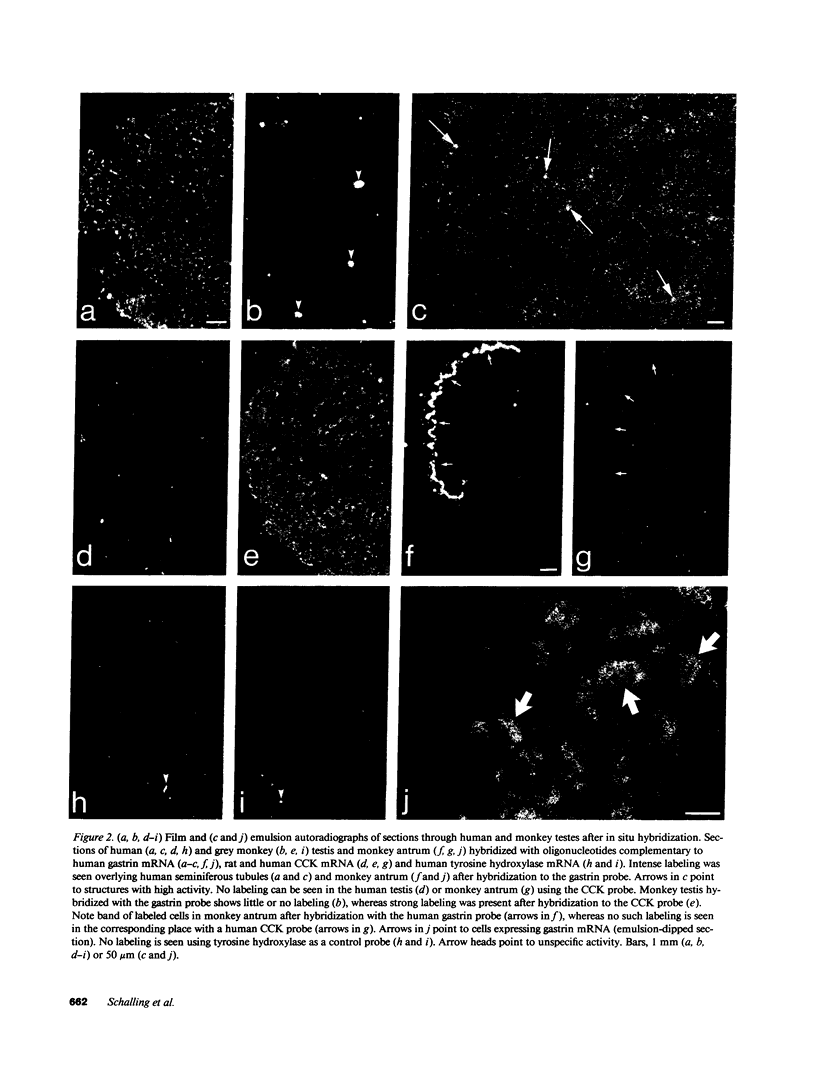
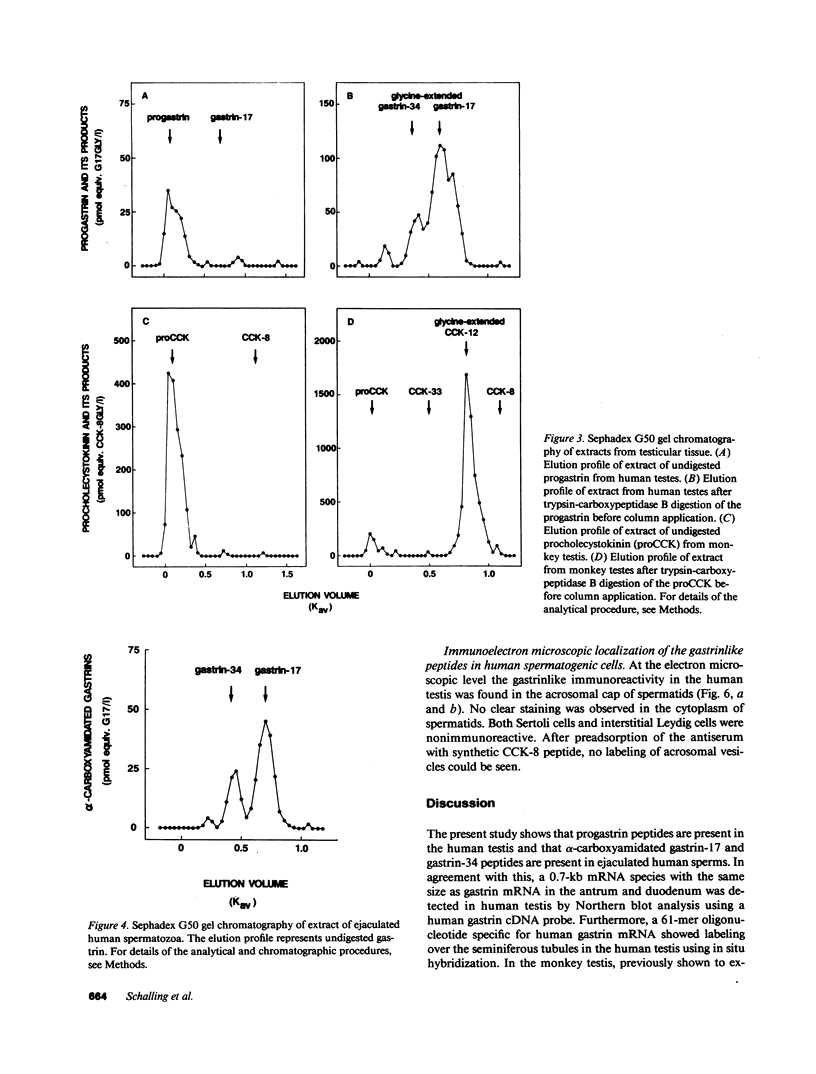
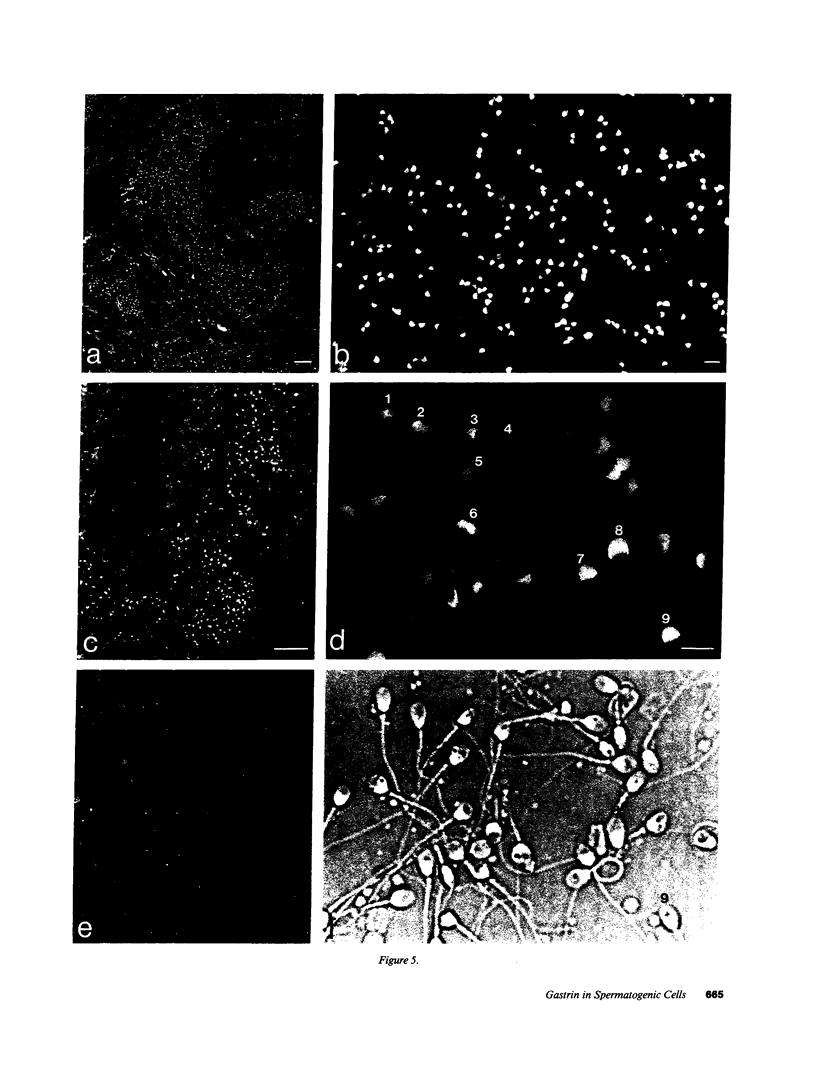
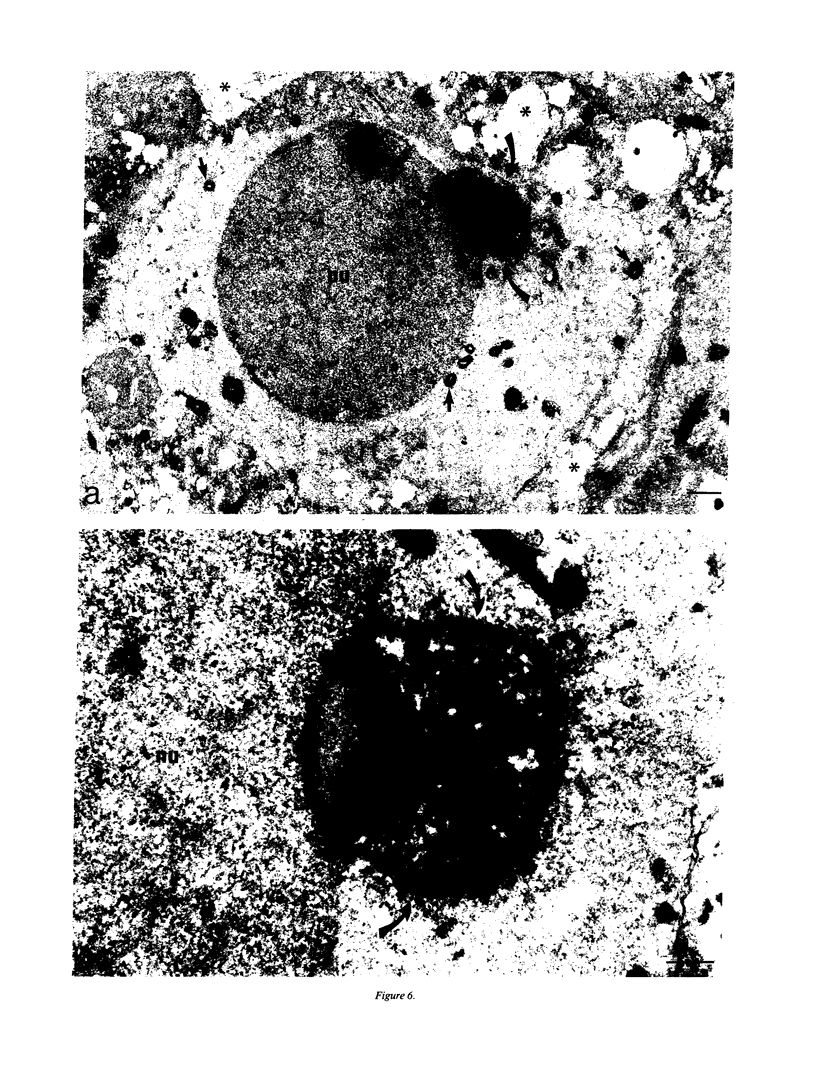
In previous studies we have shown that the gene encoding cholecystokinin (CCK) is expressed in spermatogenic cells of several mammalian species. In the present study we show that a gene homologous to the CCK-related hormone, gastrin, is expressed in the human testis. The mRNA hybridizing to a human gastrin cDNA probe in the human testis was of the same size (0.7 kb) as gastrin mRNA in the human antrum. By in situ hybridization the gastrinlike mRNA was localized to seminiferous tubules. Immunocytochemical staining of human testis revealed gastrinlike peptides in the seminiferous tubules primarily at a position corresponding to spermatids and spermatozoa. In ejaculated spermatozoa gastrinlike immunoreactivity was localized to the acrosome. Acrosomal localization could also be shown in spermatids with electron microscopy. Extracts of the human testis contained significant amounts of progastrin, but no bioactive amidated gastrins. In contrast, ejaculated sperm contained mature carboxyamidated gastrin 34 and gastrin 17. The concentration of gastrin in ejaculated human spermatozoa varied considerably between individuals. We suggest that amidated gastrin (in humans) and CCK (in other mammals) are released during the acrosome reaction and that they may be important for fertilization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A., Hartman B. K., Pujol J. F. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981 Jul;29(7):844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Boel E., Vuust J., Norris F., Norris K., Wind A., Rehfeld J. F., Marcker K. A. Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication. Proc Natl Acad Sci U S A. 1983 May;80(10):2866–2869. doi: 10.1073/pnas.80.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Lorenz L. J., Haun R. S., Roos B. A., Collier K. J., Dixon J. E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Narayana S. V., Argos P., Dixon J. E. Primary structural comparison of the preprohormones cholecystokinin and gastrin. FEBS Lett. 1985 Mar 11;182(1):135–138. doi: 10.1016/0014-5793(85)81170-4. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Hallbök F., Ebendal T., Shooter E. M., Radeke M. J., Misko T. P., Persson H. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1988 Dec;1(10):983–996. doi: 10.1016/0896-6273(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Fuller P. J., Stone D. L., Brand S. J. Molecular cloning and sequencing of a rat preprogastrin complementary deoxyribonucleic acid. Mol Endocrinol. 1987 Apr;1(4):306–311. doi: 10.1210/mend-1-4-306. [DOI] [PubMed] [Google Scholar]
- GREGORY H., HARDY P. M., JONES D. S., KENNER G. W., SHEPPARD R. C. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Boni C., Julien J. F., Javoy-Agid F., Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987 Apr 16;326(6114):707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Measurement of precursors for alpha-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal Biochem. 1986 Jan;152(1):119–126. doi: 10.1016/0003-2697(86)90129-6. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Herrera-Marschitz M., Seroogy K., Ju G., Staines W. A., Holets V., Schalling M., Ungerstedt U., Post C., Rehfeld J. F. Immunohistochemical studies on cholecystokinin (CCK)-immunoreactive neurons in the rat using sequence specific antisera and with special reference to the caudate nucleus and primary sensory neurons. J Chem Neuroanat. 1988 Jan-Feb;1(1):11–51. [PubMed] [Google Scholar]
- Johnsen A. H., Rehfeld J. F. Cionin: a disulfotyrosyl hybrid of cholecystokinin and gastrin from the neural ganglion of the protochordate Ciona intestinalis. J Biol Chem. 1990 Feb 25;265(6):3054–3058. [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jorpes E., Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand. 1966 Jan-Feb;66(1):196–202. doi: 10.1111/j.1748-1716.1966.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Kline D., Simoncini L., Mandel G., Maue R. A., Kado R. T., Jaffe L. A. Fertilization events induced by neurotransmitters after injection of mRNA in Xenopus eggs. Science. 1988 Jul 22;241(4864):464–467. doi: 10.1126/science.3134693. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Evidence for a common evolutionary origin of gastrin and cholecystokinin. Nature. 1977 Sep 22;269(5626):335–338. doi: 10.1038/269335a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979 Apr 13;165(2):201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F., Sundler F., Håkanson R. Pancreatic gastrin in foetal and neonatal rats. Nature. 1976 Aug 12;262(5569):609–610. doi: 10.1038/262609a0. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Gillo B., Sealfon S., Landau E. M. Activation of ionic currents in Xenopus oocytes by corticotropin-releasing peptides. Brain Res. 1988 Nov;464(3):201–205. doi: 10.1016/0169-328x(88)90026-5. [DOI] [PubMed] [Google Scholar]
- Pelto-Huikko M., Persson H., Schalling M., Rehfeld J. F., Hökfelt T. Immunocytochemical demonstration of cholecystokinin-like immunoreactivity in spermatozoa in monkey testis and epididymis. Acta Physiol Scand. 1989 Nov;137(3):465–466. doi: 10.1111/j.1748-1716.1989.tb08781.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Ericsson A., Schalling M., Rehfeld J. F., Hökfelt T. Detection of cholecystokinin in spermatogenic cells. Acta Physiol Scand. 1988 Dec;134(4):565–566. doi: 10.1111/j.1748-1716.1998.tb08534.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Rehfeld J. F., Ericsson A., Schalling M., Pelto-Huikko M., Hökfelt T. Transient expression of the cholecystokinin gene in male germ cells and accumulation of the peptide in the acrosomal granule: possible role of cholecystokinin in fertilization. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6166–6170. doi: 10.1073/pnas.86.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Bardram L., Hilsted L. Gastrin in human bronchogenic carcinomas: constant expression but variable processing of progastrin. Cancer Res. 1989 Jun 1;49(11):2840–2843. [PubMed] [Google Scholar]
- Rehfeld J. F. Four basic characteristics of the gastrin-cholecystokinin system. Am J Physiol. 1981 Apr;240(4):G255–G266. doi: 10.1152/ajpgi.1981.240.4.G255. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F. Localisation of gastrins to neuro- and adenohypophysis. Nature. 1978 Feb 23;271(5647):771–773. doi: 10.1038/271771a0. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Preprocholecystokinin processing in the normal human anterior pituitary. Proc Natl Acad Sci U S A. 1987 May;84(9):3019–3023. doi: 10.1073/pnas.84.9.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Brené S., Hallman H., Djurfeldt M., Persson H., Terenius L., Goldstein M., Schlesinger D., Hökfelt T. Coexistence and gene expression of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and neuropeptide tyrosine in the rat and bovine adrenal gland: effects of reserpine. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Wallensten K., Rehfeld J. F., Larsson L. I., Uvnäs B. Heptadecapeptide gastrin in the vagal nerve. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5707–5710. doi: 10.1073/pnas.74.12.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo O. J., Powell C. T., Agarwal K. L. Molecular cloning and nucleotide sequence of full-length of cDNA coding for porcine gastrin. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1049–1053. doi: 10.1073/pnas.79.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]