Abstract
Background
Hepatic resection for metastatic colorectal cancer provides excellent longterm results in a substantial proportion of patients. Although various prognostic risk factors have been identified, there has been no dependable staging or prognostic scoring system for metastatic hepatic tumors.
Study Design
Various clinical and pathologic risk factors were examined in 305 consecutive patients who underwent primary hepatic resections for metastatic colorectal cancer. Survival rates were estimated by the Cox proportional hazards model using the equation: S(t) = [So(t)]exp(R−Ro), where So(t) is the survival rate of patients with none of the identified risk factors and Ro=0.
Results
Preliminary multivariate analysis revealed that independently significant negative prognosticators were: (1) positive surgical margins, (2) extrahepatic tumor involvement including the lymph node(s), (3) tumor number of three or more, (4) bilobar tumors, and (5) time from treatment of the primary tumor to hepatic recurrence of 30 months or less. Because the survival rates of the 62 patients with positive margins or extra-hepatic tumor were uniformly very poor, multivariate analysis was repeated in the remaining 243 patients who did not have these lethal risk factors. The reanalysis revealed that independently significant poor prognosticators were: (1) tumor number of three or more, (2) tumor size greater than 8cm, (3) time to hepatic recurrence of 30 months or less, and (4) bilobar tumors. Risk scores (R) for tumor recurrence of the culled cohort (n = 243) were calculated by summation of coefficients from the multivariate analysis and were divided into five groups: grade 1, no risk factors (R = 0); grade 2, one risk factor (R = 0.3 to 0.7); grade 3, two risk factors (R = 0.7 to 1.1); grade 4, three risk factors (R = 1.2 to 1.6); and grade 5, four risk factors (R > 1.6). Grade 6 consisted of the 62 culled patients with positive margins or extrahepatic tumor. Kaplan-Meier and Cox proportional hazards estimated 5-year survival rates of grade 1 to 6 patients were 48.3% and 48.3%, 36.6% and 33.7%, 19.9% and 17.9%, 11.9% and 6.4%, 0% and 1.1 %, and 0% and 0%, respectively (p < 0.0001).
Conclusions
The proposed risk-score grading predicted the survival differences extremely well. Estimated survival as determined by the Cox proportional hazards model was similar to that determined by the Kaplan-Meier method. Verification and further improvements of the proposed system are awaited by other centers or international collaborative studies. (J Am Coll Surg 1999;189:291–299.
Hepatic resection for metastases from colorectal carcinoma can be performed quite safely and provides excellent longterm results in a substantial proportion of patients. 1–18 Now that various clinical and pathologic risk factors have been identified, 1–18 the efforts of investigation should be shifted to establishing an accurate staging system for metastatic hepatic tumors or a dependable prognostic scoring method to predict the results after curative operations.
We examined our 305 consecutive patients with colorectal metastases who underwent hepatic resection with curative intent to identify clinical and pathologic prognosticators. We propose here a new prognostic scoring method and associated proportional hazards model for survival.
METHODS
Patients and tumors
During the 15-year period between 1981 and 1996, 305 consecutive patients were treated with primary hepatic resection for hepatic metastases from adenocarcinoma of colorectal origin at the University of Pittsburgh Medical Center. All hepatic resections were carried out with curative intent. There were 178 men and 127 women. Their ages ranged from 26 to 82 years (mean ± SE 60 ± 0.6 years).
The primary tumor was located in the right colon of 71 patients (23.3%), the left colon of 156 (51.1 %), and the rectum of 78 (25.6%). Five patients (1.6%) had Dukes A (stage I) primary tumors and 70 (23.0%) had Dukes B (stage II). Dukes C (stage III) tumors represented the largest group with 141 patients (46.2%); 89 patients (29.2%) had synchronous hepatic metastases (Dukes D; stage IV). 19, 20 Most patients with Dukes D tumors underwent hepatic resection within the first 3 months after their colorectal resection. Twenty-three patients were not referred or evaluated until after this interval. Metastases to the mesenteric lymph nodes were present at the time of colorectal operation in 154 patients (50.5%) and were absent in 148 (48.5%). The status of lymph node involvement was not available in three patients.
The interval between the primary colorectal resection and hepatic resection ranged from −6 months (primary not discovered until after resection) to 228 months, with a median of 16 months.
One hundred thirty-seven patients (44.9%) had solitary lesions, 75 (24.6%) had 2 lesions, 31 (10.2%) had 3 lesions, and 62 (21.0%) had 4 or more lesions (as many as 11). The size of the hepatic metastasis ranged from 1.2 to 18 cm with a median of 5 cm; the size exceeded 8 cm in 48 (15.7%) of the 305 patients. The hepatic metastases were unilobar in 200 patients (65.6%) and bilobar in the other 105 (34.4%).
At the time of hepatic resection, abdominal lymph node metastases were present in 9 patients (3.0%) and absent in 296 (97.0%). Because of direct tumor invasion, the diaphragm, the right adrenal gland, the greater omentum, or localized peritoneal seeding were removed in continuity with the resected liver in 32 patients (10.5%).
The metastatic tumors were histologically well differentiated (grade 1) in 59 patients (19.3%), moderately differentiated (grade 2) in 239 (78.4%), and poorly differentiated (grade 3) in 7 (2.3%).18,19
Right or left hepatic lobectomy was performed in 158 patients, more than lobectomy (trisegmentectomy, extended lobectomy, and lobectomy plus wedge resection) in 85 patients, multiple bilateral wedge resections in 20 patients, and less than lobectomy (left lateral segmentectomy and nonanatomic resection) in 42 patients. Of note, 243 (79.7%) of the 305 patients were treated by lobectomy or greater hepatic resection. Despite the curative intent of resection, 28 (9.2%) of the 305 patients had microscopically positive margins at postoperative pathologic examinations, although all gross tumors were removed.
After hepatic resection with curative intent, 202 (66.2%) of the 305 patients received adjuvant chemotherapy. Although no single chemotherapeutic protocol was applied, the usual regimen contained 5-FU with levamisole or leucovorin for 6 months. Recurrent tumors after hepatic resection were surgically removed in 32 patients, including 12 thoracoscopic pulmonary resections, 11 hepatic re-resections, 3 bone resections (2 sacrum, 1 sternum), 3 abdominal-wall resections, 1 adrenalectomy, and 2 colectomies.
Data analysis
We retrospectively reviewed all available inpatient and outpatient records, including operative and pathologic reports. Patient followup was performed prospectively every 6 months after hepatic resection, and the results were summarized as of June 30, 1998. The median followup period was 32 months.
The 16 clinical and pathologic risk factors listed in Table 1 were examined for prognostic influence. Patient survival time was calculated from the date of hepatic resection until death, and tumor-free survival was determined from the date of resection until the time of tumor recurrence. Survival curves were generated with the method of Kaplan and Meier and were compared using the log-rank test. A multivariate stepwise Cox regression analysis (backward elimination method) was performed to identify the factors that were independently associated with mortality and tumor recurrence. A two-sided p value of < 0.05 was considered statistically significant.
Table 1.
Influences of Various Clinical and Pathologic Risk Factors on Overall Patient and Tumor-Free Survival
| Characteristic | n | Overall patient survival |
Tumor-free survival |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 y (%) | 5 y (%) | 10 y (%) | P Value | 3 y (%) | 5 y (%) | 10 y (%) | P Value | ||
| Patient | |||||||||
| Gender | |||||||||
| Male | 178 | 49.3 | 33.5 | 24.7 | NS | 34.9 | 28.2 | 24.7 | <0.03 |
| Female | 127 | 47.1 | 30.5 | 23.3 | 26.4 | 15.7 | 13.5 | ||
| Age (y) | |||||||||
| ≤60 | 143 | 51.5 | 36.5 | 28.6 | NS | 32.1 | 23.8 | 21.0 | NS |
| > 60 | 162 | 46.6 | 28.2 | 19.0 | 30.5 | 22.3 | 18.8 | ||
| Primary tumor | |||||||||
| Site | |||||||||
| Rectum | 78 | 50.1 | 40.5 | 31.1 | NS | 37.6 | 26.0 | 21.4 | NS |
| Left colon | 156 | 50.8 | 30.9 | 20.0 | 31.5 | 22.0 | 18.6 | ||
| Right colon | 71 | 41.2 | 26.4 | 24.2 | 23.6 | 21.6 | 21.6 | ||
| Dukes classification | |||||||||
| A + B | 75 | 60.3 | 44.9 | 31.5 | NS | 39.9 | 28.8 | 28.8 | < 0.06 |
| C | 141 | 42.8 | 27.4 | 21.5 | 27.4 | 19.9 | 18.8 | ||
| D (synchronous) | 89 | 47.1 | 28.6 | 20.9 | 30.4 | 22.8 | 14.7 | ||
| Dukes classification | |||||||||
| A + B | 75 | 60.3 | 44.9 | 31.5 | < 0.053 | 39.9 | 28.8 | 28.8 | < 0.002 |
| C + D | 230 | 44.6 | 28.1 | 21.8 | 28.4 | 20.9 | 16.8 | ||
| Lymph node | |||||||||
| Negative | 148 | 56.9 | 36.8 | 27.3 | < 0.03 | 35.9 | 25.9 | 24.7 | < 0.02 |
| Positive | 154 | 39.9 | 27.7 | 21.3 | 26.7 | 20.5 | 16.6 | ||
| Hepatic metastases | |||||||||
| Interval (mo) | |||||||||
| ≤ 12 | 132 | 48.8 | 28.7 | 25.1 | NS | 28.8 | 24.0 | 21.9 | NS |
| 12–24 | 77 | 38.8 | 30.9 | 20.7 | 25.1 | 19.7 | 17.5 | ||
| 25–36 | 42 | 48.1 | 40.5 | 21.6 | 34.2 | 15.4 | 7.7 | ||
| 36–48 | 22 | 65.7 | 23.6 | 23.6 | 40.0 | 21.0 | 21.0 | ||
| > 48 | 32 | 58.2 | 45.8 | 32.8 | 47.0 | 37.6 | 32.3 | ||
| Interval (mo) | |||||||||
| ≤ 30 | 235 | 44.6 | 29.9 | 22.5 | < 0.04 | 27.6 | 20.9 | 18.2 | < 0.01 |
| > 30 | 70 | 61.4 | 40.1 | 29.6 | 43.3 | 29.3 | 26.4 | ||
| No. of tumors | |||||||||
| 1 | 137 | 57.3 | 41.4 | 32.3 | < 0.001 | 40.9 | 33.1 | 31.0 | < 0.0001 |
| 2 | 75 | 50.6 | 37.6 | 24.9 | 35.0 | 22.8 | 17.8 | ||
| 3 | 31 | 38.4 | 11.7 | – | 19.4 | 14.5 | – | ||
| ≥ 4 | 62 | 31.4 | 16.1 | 10.7 | 11.9 | 5.0 | – | ||
| No. of tumors | |||||||||
| 1–2 | 212 | 55.0 | 40.2 | 29.8 | < 0.0001 | 38.8 | 29.4 | 26.3 | < 0.0001 |
| ≥ 3 | 93 | 33.8 | 14.6 | 11.2 | 14.4 | 8.1 | – | ||
| Size (cm) | |||||||||
| ≤ 2 | 15 | 58.2 | 43.6 | 29.1 | NS | 46.7 | 46.7 | 46.7 | NS |
| 2–5 | 152 | 51.0 | 32.0 | 23.4 | 31.8 | 25.1 | 21.7 | ||
| 5–10 | 102 | 45.8 | 34.4 | 26.3 | 29.6 | 17.7 | 15.8 | ||
| > 10 | 31 | 39.9 | 20.7 | 15.4 | 27.8 | 17.4 | 11.6 | ||
| Size (cm) | |||||||||
| ≤ 8 | 257 | 50.9 | 34.6 | 25.5 | < 0.03 | 33.5 | 24.9 | 22.0 | < 0.02 |
| > 8 | 48 | 34.7 | 19.3 | 16.1 | 18.8 | 11.7 | 7.8 | ||
| Distribution | |||||||||
| Unilobar | 200 | 56.0 | 39.2 | 29.2 | < 0.0001 | 38.0 | 28.7 | 26.0 | <0.0001 |
| Bilobar | 105 | 33.8 | 18.9 | 13.9 | 18.2 | 11.7 | 8.9 | ||
| Node metastasis | |||||||||
| No | 296 | 49.5 | 32.9 | 24.5 | < 0.007 | 31.9 | 23.4 | 20.4 | < 0.05 |
| Yes | 9 | 11.1 | – | – | 11.1 | – | – | ||
| Metastasis | |||||||||
| No | 273 | 51.1 | 34.6 | 26.2 | < 0.0001 | 34.5 | 25.3 | 22.9 | < 0.0001 |
| Yes | 32 | 20.8 | 5.5 | 0 | 0 | 0 | 0 | ||
| Differentiation | |||||||||
| Grade 1 | 59 | 50.4 | 39.6 | 32.1 | NS | 39.4 | 28.1 | 28.1 | NS |
| Grade 2 | 239 | 48.1 | 30.2 | 21.8 | 29.2 | 22.0 | 17.4 | ||
| Grade 3 | 7 | 42.9 | 28.6 | – | 28.6 | 14.3 | 0 | ||
| Surgery | |||||||||
| Margin | |||||||||
| > 1 cm | 130 | 54.2 | 38.4 | 26.7 | < 0.003 | 38.5 | 28.9 | 27.0 | < 0.0008 |
| ≤ 1 cm | 147 | 48.4 | 31.4 | 25.8 | 30.2 | 22.2 | 18.1 | ||
| Involved | 28 | 20.9 | 8.4 | 0 | 4.8 | 0 | 0 | ||
| Resection | |||||||||
| Multiple wedges | 20 | 38.9 | 13.0 | 0 | < 0.03 | 16.0 | 0 | 0 | < 0.006 |
| More than lobe | 85 | 33.3 | 21.1 | 19.3 | 17.1 | 15.4 | 13.7 | ||
| Lobectomy | 158 | 54.7 | 37.2 | 25.3 | 36.6 | 24.8 | 22.9 | ||
| Less than lobe | 42 | 57.5 | 40.6 | 34.8 | 41.1 | 37.6 | 26.9 | ||
| Adjuvant therapy | |||||||||
| No | 103 | 44.4 | 33.6 | 23.0 | NS | 33.4 | 24.2 | 24.2 | NS |
| Yes | 202 | 50.4 | 31.3 | 24.5 | 30.3 | 22.4 | 18.2 | ||
| Surgery for recurrence | |||||||||
| No | 197 | 27.0 | 7.7 | 0.9 | < 0.0001 | 8.6 | 2.5 | 0 | < 0.002 |
| Yes | 32 | 87.5 | 77.2 | 52.2 | 30.2 | 6.7 | 3.4 | ||
RESULTS
Early mortality and morbidity
There were no deaths within the first 30 days after hepatic resection, although three patients died within 90 days (perioperative mortality of less than 1%). One death was in a 71-year-old man after right trisegmentectomy who developed liver failure and stress-ulcer bleeding. Two other deaths from liver failure and septicemia after extended right lobectomy were in 62- and 67-year-old men.
In addition, there were 16 cases of severe hyper-bilirubnemia (serum total bilirubin greater than 8 mg/l00 mL), 7 subphrenic abscesses, 5 cases of prolonged ascites or pleural effusion, 2 deep vein thromboses, 2 prolonged bile leaks, 2 cardiac arrhythmias, and 1 stress ulcer with bleeding. These complications occurred in 25 (8.2%) of the 305 patients (one patient had multiple complications) and were resolved without permanent consequences.
Survival
As of June 30, 1998, 198 (64.9%) of the 305 patients were known to be dead with tumor recurrence, 12 (3.9%) were dead without tumor recurrence, 67 (22.0%) were alive and free of tumor recurrence, and 28 (9.2%) were alive with tumor recurrence. None of the patients were lost to followup. Ten-year overall and tumor-free Kaplan-Meier survival curves for the 305 patients after hepatic resection are depicted in Figure 1; 5-year overall survival was 32.3%. The tumor-free survival at this milestone was 23.0%.
Figure 1.
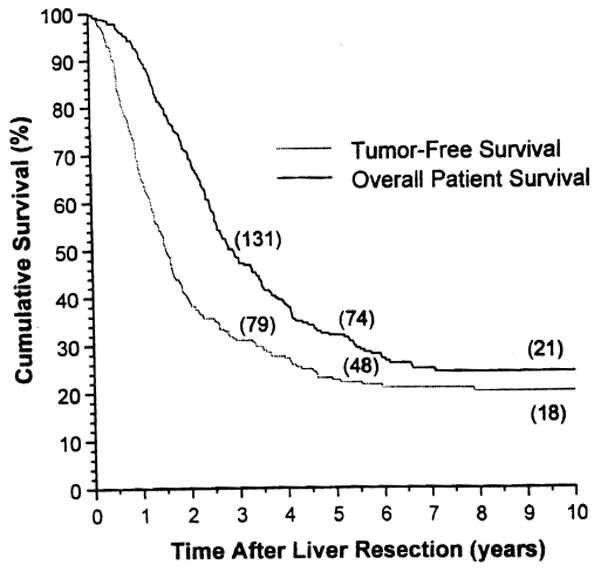
Ten-year overall patient and tumor-free Kaplan-Meier survival.
Examinations of clinical and pathologic risk factors
The influences of 16 clinical and pathologic risk factors on overall patient and tumor-free survival rates were examined (Table 1). For both end points, a significantly better prognosis was associated with the following: (1) primary colorectal cancer of Dukes A and B, (2) no metastasis to the mesenteric lymph nodes at the time of colorectal operation, (3) interval between colorectal operation and hepatic resection of longer than 30 months, (4) two or fewer hepatic metastases, (5) greatest tumor diameter of 8 cm or less, (6) unilobar distribution of hepatic metastases, (7) no nodal metastasis at the time of hepatic resection, (8) no distant metastasis at the time of hepatic resection, (9) microscopically negative surgical margins, and (10) lobectomy or smaller hepatic resection.
The patients whose recurrent hepatic metastases could be resected surgically lived longer than those whose recurrent tumors were not resected. Although overall patient survival was similar for men and women, tumor-free survival for men was significantly better than that for women (Table 1).
Multivariate analysis
Multivariate analysis based on the 305 patients identified the following significant poor prognosticators for overall and tumor-free survival: (1) positive surgical margins, (2) extrahepatic metastasis including lymph nodes, (3) tumor number of three or more, (4) bilobar distribution of hepatic metastases, and (5) interval between colorectal resection and hepatic resection of 30 months or less.
Because the survival of the 62 patients who had positive surgical margins and extrahepatic metastasis (including lymph nodes) was uniformly poor, univariate and multivariate analyses were repeated after excluding these 62 patients to identify the independent factors that could be used to calculate a risk score. The repeat univariate analysis on the remaining 243 patients confirmed the significant effect of all previously discovered risk factors except for the status of the mesenteric lymph nodes at the time of colorectal operation (p > 0.17). The lymph node status at the time of colorectal operation was better represented by Dukes classification (positive mesenteric lymph nodes are limited to Dukes C and D). The remaining six risk factors (size, number, lobar distribution, time to recurrence, Dukes classification, and extent of resection) met the assumption of proportionality of hazards by assessment of log-minus-log survival plot.
A stepwise Cox regression analysis with backward selection was used to determine independent predictors of mortality and tumor recurrence. The likelihood ratio test based on maximum partial likelihood estimates was used for elimination of confounding variables from the model. Variables were considered eligible for removal if the likelihood ratio test significance level was ≥ 0.1. Four variables (tumor number greater than two, tumor size greater than 8 cm, interval of 30 months or less, and bilobar metastases) were found to be independent predictors of tumor recurrence (Table 2). The results of the multivariate analysis for overall patient survival are shown in Table 3. The exclusion of Dukes classification can be explained by the strong inverse relation (p < 0.0001) between the time to recurrence (interval) and Dukes stages (the shorter the interval, the more advanced the Dukes stage). The extent of hepatic resection is an immediate consequence of the size, number, and distribution of metastases, which explains its exclusion from a set of independent predictors.
Table 2.
Significant Prognostic Risk Factors for Tumor Recurrence (Tumor-Free Survival) Identified by Multivariate Analysis*
| Risk factor | Coefficient (B) | Relative risk | 95% Confidence interval |
|---|---|---|---|
| Tumor number > 2 | 0.6286 | 1.87 | 1.33–2.64 |
| Tumor size > 8 cm | 0.4724 | 1.60 | 1.06–2.43 |
| Interval ≤ 30 mo | 0.3894 | 1.48 | 1.00–2.18 |
| Bilobar tumors | 0.3308 | 1.39 | 0.98–1.97 |
Excludes the 62 patients with positive surgical margins, lymph node invasion, or distant metastasis.
Table 3.
Significant Prognostic Risk Factors for Mortality (Overall Patient Survival) Determined by Multivariate Analysis*
| Risk factor | Coefficient (B) | Relative risk | 95% Confidence interval |
|---|---|---|---|
| Tumor number > 2 | 0.5514 | 1.74 | 1.21–2.50 |
| Tumor size > 8 cm | 0.4006 | 1.49 | 0.96–2.32 |
| Interval ≤ 30 mo | 0.2585 | 1.29 | 0.87–1.93 |
| Bilobar tumors | 0.2256 | 1.25 | 0.86–1.82 |
Excludes the 62 patients with positive surgical margins, lymph node invasion, or distant metastasis.
Calculation of risk score and prediction of survival
Based on the results of the multivariate analysis, the risk score can be calculated for each patient by the following formula: Risk score (R) = B1X1 + B2X2 + B3X3 + B4X4, where B = coefficient from the Cox model (Tables 2, 3) and Xi = 0 when the risk factor is absent or Xi = 1 when the risk factor is present.
Correspondingly, the probability of which patient with risk score R will be recurrence-free t years after hepatic resection (S(t)) can be calculated by the following21: S(t) = [So(t)]exp(R − Ro), where Ro is the risk score corresponding to the baseline survival function So(t). Because all of the four risk factors are presented as binary variables, So(t) was calculated for a patient with no risk factors. The cumulative tumor-free survival (Kaplan-Meier) of the 243 patients was then compared with the predicted probability of cohort tumor-free survival after hepatic resection (S(t)). The fit of the developed model was assessed heuristically by comparison of overall patient and tumor-free survival rates estimated by the Kaplan-Meier method versus the predicted survival by the Cox model of probability for various patient risk groups. As shown in Table 4, S(t) agrees reasonably well with tumor-free survival as determined by the Kaplan-Meier method.
Table 4.
Tumor-Free Survival Prediction*
| Prediction | 1 y | 2 y | 3 y | 4 y | 5 y | 6 y | 7 y | 8 y | 9 y | 10 y |
|---|---|---|---|---|---|---|---|---|---|---|
| Actuarial (Kaplan-Meier) | 72.3 | 46.9 | 38.4 | 33.6 | 28.5 | 26.7 | 26.7 | 25.8 | 24.9 | 24.9 |
| So(t) (estimated) | 87.3 | 47.2 | 39.3 | 30.6 | 26.6 | 26.6 | 26.6 | 26.6 | 26.6 | 26.6 |
Tumor-free survival prediction based on patients with no risk factors (So(t)) correlated well with actuarial tumor-free survival (Kaplan-Meier).
Data are presented as percentages.
Practical application of risk score
Risk scores for tumor-free survival were grouped into the following strata: grade 1, none of the four risk factors present (risk score = 0); grade 2, one of the four risk factors present (risk score = 0.3308 to 0.6286); grade 3, two of the four risk factors present (risk score = 0.7202 to 1.101); grade 4, three of the four risk factors present (risk score = 1.1926 to 1.4904); grade 5, all of the four risk factors present (risk score = 1.8212); and grade 6, positive surgical margins and lymph node or distant metastasis.
Tumor-free survival rates (Kaplan-Meier) for the above-defined six grades of patients are depicted in Figure 2, and the tumor-free survival rates calculated by S(t) (Cox model) are shown in Figure 3 for comparison. Note that the survival curves were similar.
Figure 2.
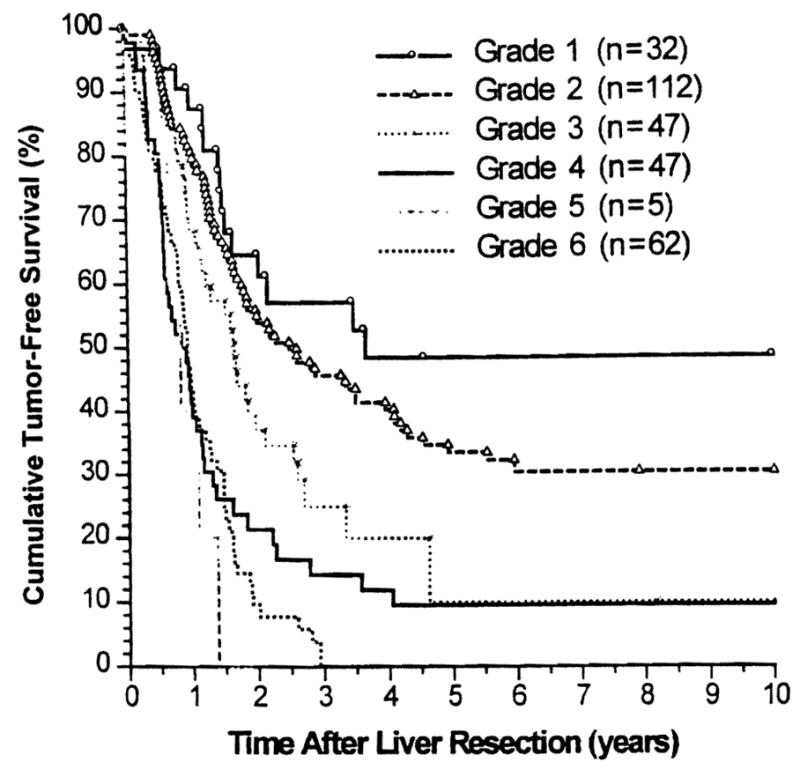
Kaplan–Meier tumor-free survival stratified by six grades.
Figure 3.
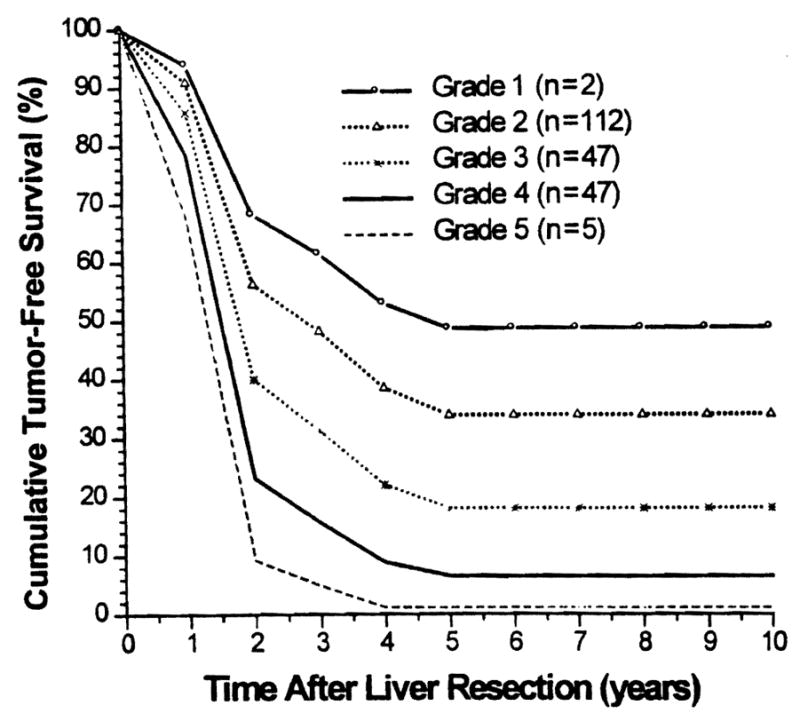
Estimated (Cox regression model) tumor-free survival stratified by five grades (grade 6 cannot be estimated with this model).
Overall patient survival rates (Kaplan-Meier) of the six grades of patients and those calculated by S(t) are shown in Figures 4 and 5, respectively.
Figure 4.
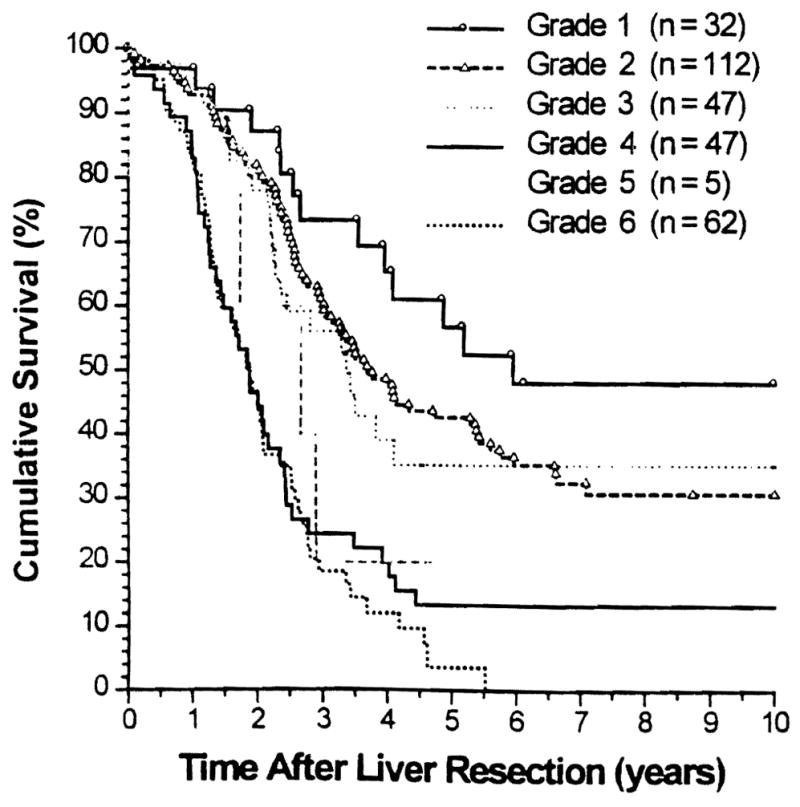
Kaplan-Meier overall patient survival stratified by six grades.
Figure 5.
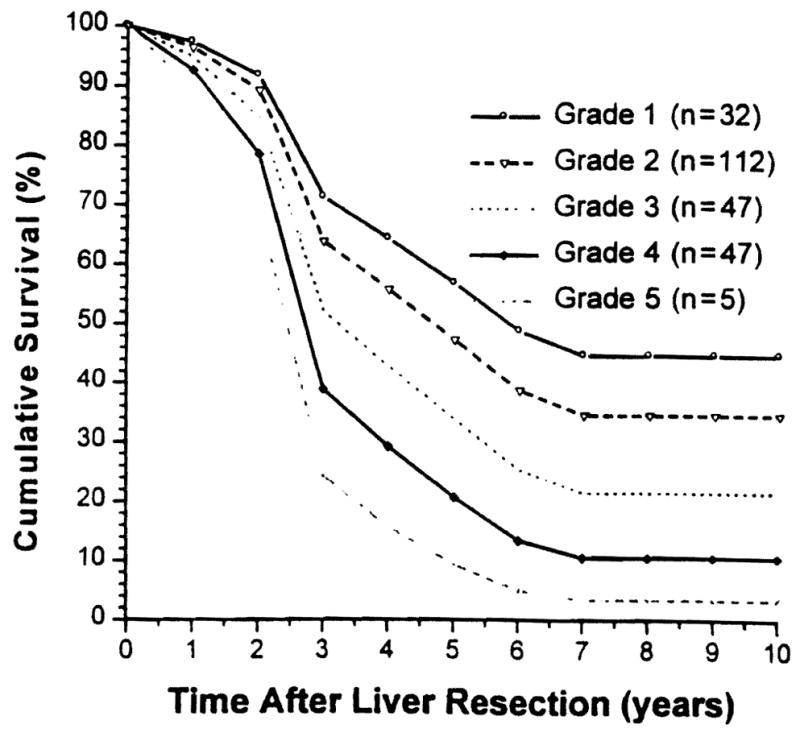
Estimated (Cox regression model) overall patient survival stratified by five grades (grade 6 cannot be estimated with this model).
DISCUSSION
Hepatic resection for metastatic colorectal cancer can now be performed with minimal surgical risks. With this treatment, an overall 5-year survival rate of 25% to 40% has been commonly achieved.1–18 Various factors influencing outcomes have been reported in the literature.1–18 Positive surgical margins, lymph node invasion, and distant metastasis have proved to be prognosticators for failure in all studies. The stages of primary colorectal cancer (TNM stage, Dukes classification, or status of mesenteric lymph nodes at the time of colorectal resection); the size, number, and lobar distribution of hepatic metastases; and the time from colorectal resection to hepatic metastasis (synchronous versus metachronous) have been identified as significant prognostic determinants. In some studies, blood transfusion during hepatectomy, type of hepatic resection, histologic grades of primary and metastatic tumors, serum CEA levels, and gender have been found to be significant. Repeated hepatic resection for recurrent metastases has been reported to prolong overall survival.22,23
In our univariate analyses, 10 of the 16 variables studied were significantly associated with overall patient and tumor-free survival (Table 1). Although our findings agree in general with others,1–18 some of the individual variables that were significant in our investigation were not in several other studies. Except for gender,8 none of the prognosticators noted in the univariate analysis have been reported in other studies to have an opposite association from the ones that we saw. Differences between our study and others are mostly due to differences in the number of patients, length of followup, and grouping of continuous variables.
The proposed formula derived from our current study (S(t) = So(t)]exp(R − Ro)) appears to be simple and practical. It reflected reasonably well both tumor-free survival (Figs. 2, 3) and overall patient survival (Figs. 4, 5).
Two other prognostic scoring systems for patients with hepatic metastases from colorectal carcinoma have been reported in the literature. The one proposed by Cady and Stone7 in 1991 included surgical margins, the time to hepatic recurrence, the number of metastases, and serum CEA levels. Because this scoring system was not based on statistical analyses, it could not be compared with ours. The second scoring system, advanced in 1996 by Nordinger and associates13 on behalf of a French surgical consortium, was based on the study of 1,568 patients collected from 85 institutions over the last 3 decades. The following seven factors were found to be significant by multivariate analysis: (1) age (60 or older versus less than 60 years), (2) serosal involvement of primary tumor, (3) peritumoral lymph node invasion by the primary, (4) time to hepatic recurrence (more than 2 years versus 2 years or less), (5) tumor size (greater than 5 cm versus 5 cm or less), (6) number of tumors (four or more versus fewer than four), and (7) surgical margins (1 cm or more versus less than 1 cm). The patients were classified into three categories, each with significantly poorer survival: grade 1, zero to two risk factors; grade 2, three to four risk factors; and grade 3, five to seven risk factors. This system was applied to our 144 patients with the best prognosis (ie, those with our Pittsburgh grades 1 and 2). Our results with patients in grades 1 and 2 were essentially identical to those of the French13 grade 1 patients. Only 55 (38.2%) of these patients qualified for a French grade 1, however. The French grading system13 failed to identify more than 60% of the patients with the most hopeful prognosis according to our Pittsburgh system.
The failure of the French grading system to accurately predict the prognosis of our patients, and especially those with Pittsburgh grades 1 and 2, may be related to several factors identifiable in the report by Nordinger and associates13: (1) The two closely linked factors of serosal involvement and peritumoral lymph node invasion were designated as independent predictors in the French study by stepwise multivariate analysis; (2) a positive surgical margin was assumed if the tumor-free margin was less than 1 cm, inevitably excluding from the French grade 1 patients with negative and positive margins; (3) accrual of patients in the French study took place over 3 decades but was not analyzed by era; (4) the institutional factor (85 centers) was not examined24; (5) more than 200 patients (including those with operative death) were excluded from the study; (6) age was not found to be a significant factor (p> 0.05); and (7) death without recurrence was not censored in the calculation of tumor-free survival but was considered as death with recurrence.
When the French group reanalyzed the factors influencing 5-year survival, using the same database as in their previous report,15 they found only three factors that influenced the 5-year survival: serosa infiltration, peritumoral lymph nodes, and surgical margin of less than 1 cm. The factors of age, time to hepatic recurrence, tumor size, and the number of metastases did not significantly influence survival at 5 years.
In view of these disparities between our scoring system and the French system,13 our Pittsburgh survival-prediction formula will have to be validated by other large series of patients. It is possible that refinements will be needed before it can be accepted universally. An international collaborative study by major centers could quickly accomplish this objective. Until then, our results indicate the following. First, excellent survival or even cure can be expected in more than one third of the patients with hepatic metastases if none or only one of the four risk factors is present (tumor number of three or more, bilobar tumors, tumor size greater than 8 cm, and time to hepatic recurrence of 30 months or less). Second, the prognosis is extremely poor when all of the four risk factors are present, when extrahepatic metastasis includes the lymph nodes, or when the surgical margins are positive after hepatic resection.
Footnotes
No competing interests declared.
References
- 1.Fortner JG, Silva JS, Golby RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. Ann Surg. 1984;199:306–316. doi: 10.1097/00000658-198403000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekberg M, Tranberg KG, Anderson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 3.Adson MA. Resection of liver metastases—when is it worthwhile? World J Surg. 1987;11:511–520. doi: 10.1007/BF01655817. [DOI] [PubMed] [Google Scholar]
- 4.Nordinger B, Parc R, Belva E, et al. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987;205:256–269. doi: 10.1097/00000658-198703000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson KR, Steinberg SM, Hughes KS, et al. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resections of colorectal liver metastases. Ann Surg. 1988;208:679–687. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes KS, Simon RM, Songhoraboodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resections. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 7.Cady B, Stone MD. The role of surgical resection of liver metastases in colorectal carcinoma. Semin Oncol. 1991;28:399–406. [PubMed] [Google Scholar]
- 8.Doci R, Gennari L, Bignami P, et al. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78:797–801. doi: 10.1002/bjs.1800780711. [DOI] [PubMed] [Google Scholar]
- 9.Scheele J, Stangl R, Altendorf-Hofman A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 10.Rose CB, Nagarney DM, Taswell HF, et al. Perioperative transfusion and determinants of survival after hepatic resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–505. doi: 10.1097/00000658-199210000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–711. [PMC free article] [PubMed] [Google Scholar]
- 12.Scheele J, Stangl R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 13.Nordinger B, Guiguet M, Vaillant J-C, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 14.Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg. 1997;84:1136–1140. [PubMed] [Google Scholar]
- 15.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Br J Surg. 1997;84:977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 16.Bakalakos EA, Kim JA, Young DC, Martin EW., Jr Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg. 1998;22:399–405. doi: 10.1007/s002689900404. [DOI] [PubMed] [Google Scholar]
- 17.Cady B, Jenkins R, Steele GD, Jr, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improveable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohlsson BB, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998;22:268–277. doi: 10.1007/s002689900381. [DOI] [PubMed] [Google Scholar]
- 19.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 5. Philadelphia: Lippincott-Raven; 1997. pp. 83–90. [Google Scholar]
- 20.International Union Against Cancer. TNM Classification of Malignant Tumors. 5. New York: Wiley-Liss; 1997. pp. 66–69. [Google Scholar]
- 21.Fisher LD, van Belle G. A Methodology for the Health Sciences. New York: John Wiley & Sons Inc; 1993. Biostatistics; pp. 811–822. [Google Scholar]
- 22.Nordinger B, Vaillant J-C, Guiguet M, et al. Survival benefit of repeat liver resection for recurrent colorectal metastases: 143 cases. J Clin Oncol. 1944;12:1491–1496. doi: 10.1200/JCO.1994.12.7.1491. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, Bismuth H, Gastaing D, et al. Repeat hepatectomy for colorectal liver metastasis. Ann Surg. 1997;225:51–62. doi: 10.1097/00000658-199701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:178–179. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]


