Hepatic allografts are more resistant than other solid organs to antibody-mediated rejection, and human hepatic transplants can be successfully performed in the face of donor-specific lymphocytotoxic antibodies (positive crossmatch).1,2 However, we recently reported a significant decrease in patient and graft survival when a liver transplant was performed against a positive IgG lymphocytotoxic crossmatch.3 Grafts that did or did not fail had histopathologic findings mimicking preservation injury, although specific deposits of immunoglobulins and complement were rarely found. 4 These data suggest that lymphocytotoxic antibodies can have a deleterious effect in liver transplantation, even if they do not precipitate hyperacute rejection.
Complement plays a crucial role in the pathophysiology of antibody-mediated rejection. We therefore prospectively analyzed total complement activity and detection of circulating immune complexes in primary liver allograft recipients with positive IgG lymphocytotoxic antibodies. The objective was to determine if in allosensitized patients complement consumption (low peripheral blood levels) was reflective of an antibody-mediated reaction. Because low peripheral complement levels may be a result of consumption or poor production, the crossmatch positive patients were compared with a control group of cross-match negative primary liver allograft recipients with severe hepatocellular damage.
MATERIALS AND METHODS
Between March 1, 1991, and December 31, 1991, 22 consecutive adult patients received a liver from an IgG lymphocytotoxic positive crossmatch donor (more than 50% of donor lymphocytes were killed by dithiothreitol [DTT]-pretreated recipient serum), and were prospectively followed during the first month after transplantation. A group of 10 patients with negative crossmatch and severe hepatocellular damage, assessed by elevation of transaminase (AST) above 2500 U/mL on day 1 posttransplant, served as controls.5
Immunosuppression
Standard postoperative immunosuppression consisted of FK 506 and low-dose steroids. FK 506 was initially given in a continuous IV infusion at 0.1 mg/kg/d, which was converted to an oral dose of 0.15 mg/kg every 12 hours with the return of bowel function. Subsequent dosage adjustments were guided by the quality of the graft, the presence of rejection, toxicity, and the FK 506 plasma trough level (normal value <2 ng/mL). Rejection episodes were treated with either 1 g bolus of methylprednisolone or a recycling of high-dose steroids starting at 200 mg and tapering to 20 mg over 5 days. If rejection persisted, a 3 to 5-day course of 5 to 10 mg/d of OKT3 was given.
Treatment With Prostaglandin E1 (PGE1)
Fourteen patients with positive crossmatch and all the patients with hepatocellular damage received treatment with PGE1 (Prostin VRR) 0.2 to 0.6 μg/kg/h IV during 5 to 7 days after transplantation.
Crossmatch Test
Pretransplant sera were drawn immediately before liver transplantation and used for the crossmatching. All sera were DTT treated to inactivate IgM. The donor T lymphocytes were isolated from lymph nodes using CD3-conjugated dynabeads. The cytotoxicity test was performed according to National Institutes of Health (NIH) standards with one washing. Briefly, 1 μL of 2 × 106/mL T lymphocytes were added to 1 μL of serum, which was twofold diluted up to 1:8 using RPMI 1640 solution, for 1 hour at room temperature. After one washing, 5 μL of rabbit complement was added for an additional 1 hour at room temperature and trypan blue was added to stain dead cells.
Total Complement Activity Test
The method is based on the ability of complement to lyse red blood cells. In summary, serum to be tested is placed in wells and diffuses radially through an agarose gel containing standardized sheep erythrocytes sensitized with hemolysin. An estimate of total complement activity (CH100) is made by comparison of the extent of lysis caused by the serum sample and that caused by reference sera run simultaneously. Results are given in U/mL (normal value >60 U/mL).
Detection of Circulating Immune Complexes
Circulating immune complexes were detected qualitatively using zonal electrophoresis on agarose gels.6
Statistical Analysis
Repeated measures one-way analysis of variance (ANOVA) was used to compare the average complement levels across the time.
RESULTS
Demographics
Table 1 shows the circumstances of 22 patients with positive crossmatch and 10 patients with negative cross-match and hepatocellular damage.
Table 1.
IgG Lymphocytotoxic Crossmatch Positive Cases and Crossmatch Negative Control With Hepatocellular Damage
| Positive Crossmatch | Negative Crossmatch | P | |
|---|---|---|---|
| Number of patients | 22 | 10 | NS |
| Age* | 46.8 ± 13.0 | 53.5 ± 13.5 | NS |
| Male/female | 7/15 | 6/4 | NS |
| Cold ischemic time (h)* | 12.0 ± 4.6 | 13.3 ± 3.9 | NS |
| Panel reactive antibody (%)* | 80.0 ± 29.3 | 3.5 ± 1.7 | <.001 |
| AST on day 1* | 1434.2 ± 1039.6 | 6093.7 ± 3699.5 | <.001 |
| Original disease | |||
| Hepatocellular | 17 | 7 | NS |
| Cholestatic | 7 | 3 | NS |
Mean ± SE.
Crossmatch After Liver Transplantation
In 14 of the 22 patients with a positive pretransplant crossmatch repeat crossmatch testing 1 day after liver transplantation became negative. However, eight patients persistently tested positive for donor-specific lymphocytotoxic antibodies after transplantation. The positive cross-match persisted positive for 4 weeks in five patients and for 3 weeks in two patients. One patient was retransplanted on day 2, and the cross match became negative thereafter.
Survival
Four of eight patients (50%) with persistently positive crossmatches after transplantation died at an interval of 36 to 120 days following transplantation. The cause of death was sepsis in three patients and respiratory arrest in one. Two of 14 patients (14%) whose crossmatch became negative died at days 44 and 45 following transplantation because of sepsis. No patient with a negative crossmatch died. Four of eight grafts (50%) with persistent positive crossmatch failed at an interval of 2 to 43 days following transplantation. No graft failures occurred in patients whose crossmatch became negative after transplantation. Of 10 grafts with a negative pretransplant crossmatch and hepatocellular damage one failed at day 15 after transplantation. All four patients with persistent positive cross-match whose graft did not fail received treatment with PEG1. In contrast only one of these patients whose graft failed received treatment with PEG1. Nine of the 14 patients whose crossmatch became negative received treatment with PEG1.
Total Complement Activity (CH100)
In patients with persistent positive crossmatch after transplantation the median CH100 levels at weeks 1, 2, 3, and 4 were 24 U/mL (range 19 to 39), 23 U/mL (range 19 to 27), 32 U/mL (range 23 to 45), and 25 U/mL (range 19 to 76), respectively (NS). In patients whose crossmatch became negative after transplantation the median CH100 levels were 42 U/mL (range 26 to 77), 65 U/mL (range 53 to 108), 93 U/mL (range 66 to 150), and 100 U/mL (range 72 to 110) at weeks 1, 2, 3, and 4, respectively (P <.001). In patients with negative crossmatch and hepatocellular damage the median CH100 levels were 67 U/mL (range 28 to 90), 109 U/mL (range 49 to 165), 106 U/mL (range 72 to 146), and 125 U/mL (range 79 to 165) (P < .001) (Fig 1).
Fig 1.
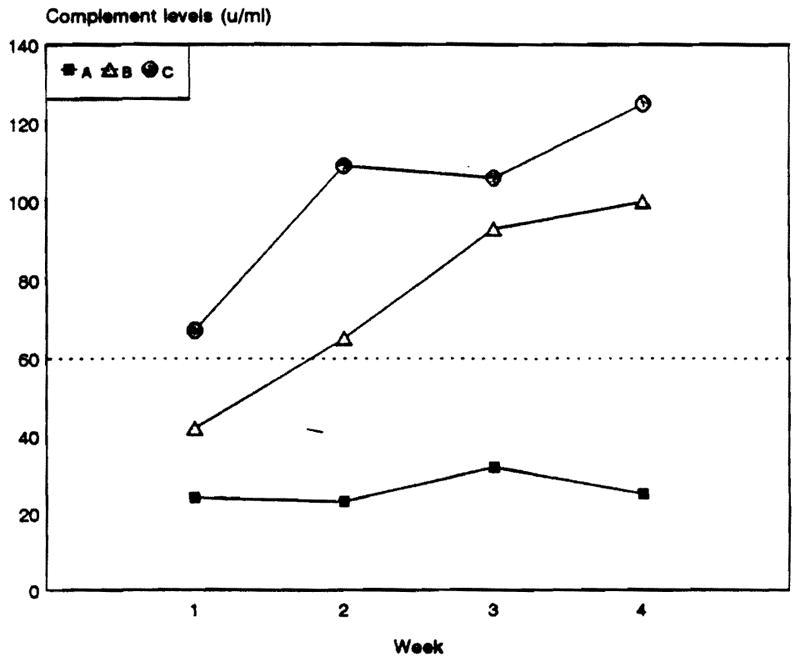
Median complement levels in liver transplant recipients with positive IgG lymphocytotoxic crossmatch pre- and posttransplantation (A), positive pretransplant that became negative post-transplant (B). and crossmatch negative controls with severe hepatocellular damage (C).
Immune Complex Detection
As Fig 2 shows, circulating immune complexes were detected on weeks 1, 2, and 3 in all patients with persistently positive crossmatches. In contrast, circulating immune complexes were detected on weeks 1, 2, and 3 in 50%, 33%, and 30%, respectively, of patients whose crossmatch became negative (P < .05). In those patients with hepatocellular damage immune complexes were detected in 20%, 20%, and 33% on weeks 1, 2, and 3, respectively (P < .01). On week 4, circulating immune complexes were detected in 67% of patients with persistent positive crossmatch, 33% of patients whose crossmatch became negative, and 20% of patients with hepatocellular damage.
Fig 2.
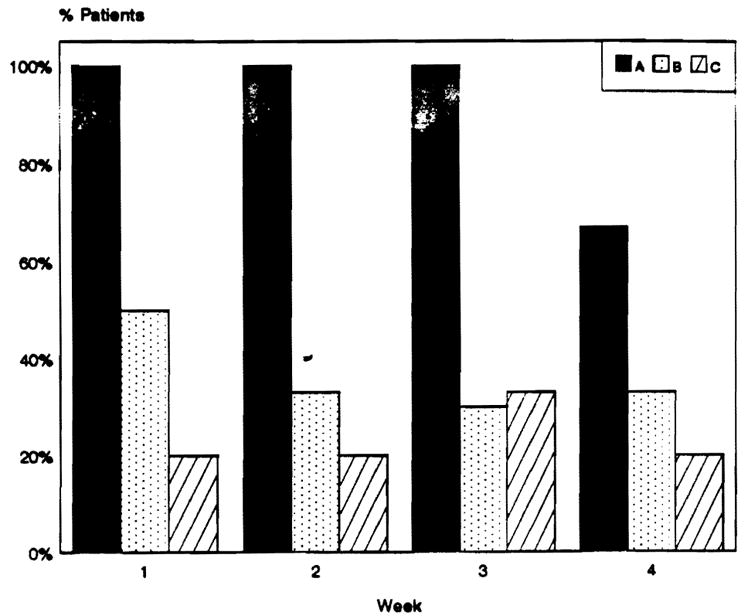
Immune complexes detection in liver transplant recipients with positive IgG lymphocytotoxic crossmatch pre and posttransplantation (A), positive crossmatch pretransplant that became negative posttransplant (B), and crossmatch negative controls with severe hepatocellular damage (C).
DISCUSSION
Two different patterns were seen in patients with specific antidonor IgG lymphocytotoxic antibodies receiving a hepatic allograft. In about 65% of cases, antibodies disappeared immediately after transplantation. The outcome of these patients did not differ from those with negative crossmatch. However, in about 35% of cases antibodies persisted after transplantation for a variable interval, usually longer than 1 month. The outcome of these patients was poor, with a 50% graft loss and 50% mortality. The use of PGE1 was associated with an improvement in the outcome of these patients.
A decrease in total complement activity and detection of circulating immune complexes were observed during weeks 1, 2, and 3 following transplantation in patients with persistently positive crossmatches in contrast to patients whose crossmatch became negative and patients with hepatocellular damage. This suggests that a humoral anti-body-mediated reaction occurred in those patients. However, graft failure, if it occurs, is usually delayed in contrast with kidney or heart allografts.
We postulate that after graft unclamping the humoral reaction develops in patients with preformed lymphocytotoxic antibodies. In several cases the new liver graft may neutralize the antibodies by various mechanisms. In other cases the protective mechanisms are overwhelmed and antibodies persist, and humoral reaction continues after transplantation. In both cases the dual blood supply is an important advantage and likely the most important factor to avoid graft failure. The microvascular thrombosis and intense vasoconstriction associated with humoral events only occur in the arterial tree. Monitoring complement activity and circulating immune complex may be useful to distinguish patients with persistent antibodies after transplantation. This would permit an adjustment of therapy in this high-risk population, particularly the use of PGE1 which seems to improve the outcome.
References
- 1.Starzl TE, Ishikawa M, Putnam CW, et al. Transplant Proc. 1974;6:129. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Iwaki Y, Kano T, et al. Transplant Proc. 1981;13:286. [PMC free article] [PubMed] [Google Scholar]
- 3.Takaya S, Bronsther O, Iwaki Y, et al. Transplantation. 1992;53:400. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetris AJ, Nakamura K, Yagihashi A, et al. Hepatology. (in press) [Google Scholar]
- 5.Mor E, Klintrnalm GB, Gonwa TA, et al. Transplantation. 1992;53:383. doi: 10.1097/00007890-199202010-00022. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RH, Scholl MA, Harvey VS, et al. Clin Chern. 1980;26:396. [PubMed] [Google Scholar]


