Summary
Objective
The Cox-Maze procedure for the surgical treatment of atrial fibrillation traditionally has required a median sternotomy and cardiopulmonary bypass. This study describes a method using ablation technologies to create the full Cox-Maze lesion set through a 5–6 cm right mini-thoracotomy.
Methods
Twenty-two consecutive patients underwent a Cox-Maze procedure via a right mini-thoracotomy and cardiopulmonary bypass. All patients were followed prospectively with ECG and 24 hour Holter monitoring at 3, 6, and 12 months. The Cox-Maze procedure lesion set was created using bipolar radiofrequency energy and cryothermy.
Results
There was no operative mortality or major complications. Two patients required a permanent pacemaker. Five patients (23%) had early atrial tachyarrhythmias. At last follow-up (mean 18 ± 12 months), all of the patients (n=22) were free from atrial dysrhythmias. At 3 months (n=19), 84% of patients were off antiarrhythmic drugs. By 6 months (n=18), 94% of patients were free from AF and off antiarrhythmic medications. At 12 months (n=16), 81% of patients were free from AF and off antiarrhythmic drugs and three patients remained on warfarin for a mechanical mitral valve.
Conclusions
A full Cox-Maze procedure can be performed through a right mini-thoracotomy with outstanding short term results. This less invasive procedure can be offered to patients without compromising efficacy.
Keywords: atrial fibrillation, Cox-Maze procedure, minimally invasive
CLINICAL PERSPECTIVE.
The small surgical series presented here is an excellent example of an outstanding surgical option to perform a full Cox-Maze procedure using minimally invasive approach and a combination of energy sources. The procedure described is being supported by cardiopulmonary bypass and cardioplegic arrest with a documented very low morbidity that seems superior to many of the reports were beating heart and limited ablation protocols were applied.
The great news is that the minimally invasive approach was not associated with compromise of the success rate which is comparable to the cut and sew Cox Maze procedure. This comes to say that when a minimally invasive surgical ablation is being performed appropriately, a far superior result may be achieved when compared to multiple minimally invasive reports where modifications where applied without full comprehension of the pathology, the type of atrial fibrillation and the atrial remodeling process.
The increased demand from patients and cardiologists to perform different types of cardiac procedures using minimally invasive approached by utilizing small incisions mandated the familiarity of surgeons with these approaches. Patient selection and very low morbidity is the key for the penetration of such approaches. Since femoral cannulation and perfusion seems to be associated with higher neurological complications it is imperative to screen the thoracic and abdominal aorta for atheromatous changes that will necessitate different approaches such as axillary arterial cannulation.
In this study the only monitoring techniques in use were ECG and 24 hour Holter monitoring. It is clear that longer monitoring will show higher recurrence rate when the HRS guidelines definition of recurrence is being used, however with many of these events being clinically insignificant. As cardiac surgeons we are being asked to report our results using more extensive monitoring techniques and therefore should modify our programs to establish good and a reliable follow up system such as the one at Washington University. Our ability to perform surgical ablation successfully is well documented; however, the penetration of these procedures is going to happen only if we have a much more aggressive agenda related to acceptable outcome and follow up standards.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia affecting patients in the United States with a prevalence of over 2 million people with projections1. It is associated with significant morbidity, estimated as the cause of 15% of all strokes1. The Cox Maze procedure (CMIII) has been the gold standard for the surgical treatment of AF since its’ introduction in 1987. The strategy of this procedure was to make surgical incisions on the atria to disrupt the re-entrant circuits thought to be the cause of AF2. The Cox-Maze III (CMIII) procedure has been very effective in curing symptomatic atrial fibrillation with an approximately 90% success rate3–6. Unfortunately, it was not widely adopted largely because of its complexity and invasiveness. The procedure was traditionally performed through a median sternotomy and required a long period of cardiopulmonary bypass and cardiac arrest.
In an effort to make the Cox-Maze procedure less invasive and quicker to perform, several groups, including our own, have used ablation technologies to replace the various incisions. A number of different energy sources have been used for ablation, including unipolar and bipolar radiofrequency (RF), cryosurgery, microwave, laser, and ultrasound7–16. These new technologies have simplified the CMP. At this institution, the average cross-clamp time dropped from 93 minutes in the CMIII to 35 minutes for the RF ablation-based Cox-Maze procedure (CMIV)8, 17 while still maintaining equivalent efficacy18.
Despite these successes, the RF ablation-assisted Cox-Maze procedure was initially done through a sternotomy. The purpose of this study was to describe a surgical technique to perform a full CMIV lesion set through a 5–6 cm right mini-thoracotomy approach.
Methods
Study Population
Twenty-two consecutive patients undergoing a CMIV through a right mini-thoracotomy between May 2005 and February 2009 were included in this study. All patients evaluated for CMIV at this institution were considered for a thoracotomy. Relative contraindications included prior thoracotomy, a left atrial thrombus, and large body habitus based on surgeon discretion. In patients with a left atrial thrombus, a median sternotomy has been the preferred approach in order to lower the risk of an embolic event and allow for complete excision of the left atrial appendage, which is not possible via a right thoracotomy. Patients who also required surgical coronary revascularization or an aortic valve replacement were not offered a thoracotomy.
The characteristics of the study population are summarized in Table 1. The mean age was 55± 9 years and approximately half of the study cohort was male. The mean duration of preoperative AF was 6 ± 7 years. The mean left atrial diameter was 4.4 ± 0.9 cm. Over one-third of patients (36%) had concomitant mitral and/or tricuspid procedures (Table 2).
Table 1.
Patient characteristics
| Variables | n = 22 |
|---|---|
| Mean age (years) | 56.1 ± 9.1 |
| Male gender (%) | 10 (45) |
| AF duration (years) | 6.2 ± 7.0 |
| Paroxysmal AF (%) | 14 (63.6) |
| Persistent AF (%) | 1 (4.5) |
| Longstanding persistent AF (%) | 7 (31.8) |
| NYHA Class 3 or 4 (%) | 8 (36.4) |
| Mean LVEF (%) | 52 ± 11 |
| Failed catheter ablation (%) | 4 (18.2) |
| Neurologic indication (%) | 3 (5) |
| LA diameter (cm) | 4.4 ± 0.9 |
AF = atrial fibrillation; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; LA = left atrium.
Table 2.
Operative procedures
| Procedure Types | Total patients (%) |
|---|---|
| Stand alone Cox-Maze IV (CMIV) | 14 (64) |
| CMIV + mitral valve procedure | 6 (27) |
| CMIV + mitral & tricuspid valve procedures | 2 (9) |
Follow-Up
All patients were followed prospectively and no patient was lost to follow-up. At each follow-up visit, the patients received an ECG. Additionally, at 3, 6, and 12 months, each patient had a 24-hour Holter monitor. Mean total follow-up was 18 ± 12 months.
Surgical Technique
The patient was intubated with a double-lumen endotracheal tube after induction of anesthesia and positioned with the right chest elevated approximately 30 to 40 degrees. After prepping and draping, the right femoral artery and vein were exposed through a small right inguinal incision, parallel and just inferior to the inguinal ligament. After heparinization, these vessels were cannulated for cardiopulmonary bypass (CPB). A right lateral thoracotomy was performed for access to the atrium. A 5–6 cm incision was used to access the chest through the fourth intercostal space (Figure 1). The right lung was deflated and the pericardium was opened to expose the heart, and CPB was established. The interatrial groove was dissected. The superior vena cava (SVC) and inferior vena cava (IVC) were mobilized. For the administration of antegrade cardioplegia, a catheter was placed in the proximal ascending aorta. A separate stab incision was made in the right chest for placement of a Blake drain to infuse carbon dioxide into the chest to prevent air embolism.
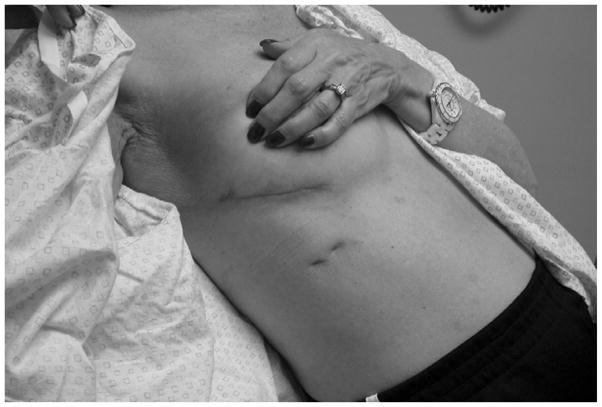
Figure 1.
The incisional scar from the right mini-thoracotomy for a patient undergoing concomitant double-valve surgery with a Cox-Maze procedure.
Once this exposure was complete, the patient was cardioverted if needed into normal sinus rhythm. To create the Cox-Maze lesion set, a bipolar RF clamp and multiple cryoprobes were used to create the lines of conduction block as shown in Figure 2. The right pulmonary veins were bluntly dissected and encircled with umbilical tape. A bipolar RF clamp was used to isolate a cuff of left atrial tissue surrounding the right pulmonary veins. Pulmonary vein isolation was documented by pacing from both the pulmonary veins in order to confirm exit block.
Figure 2.
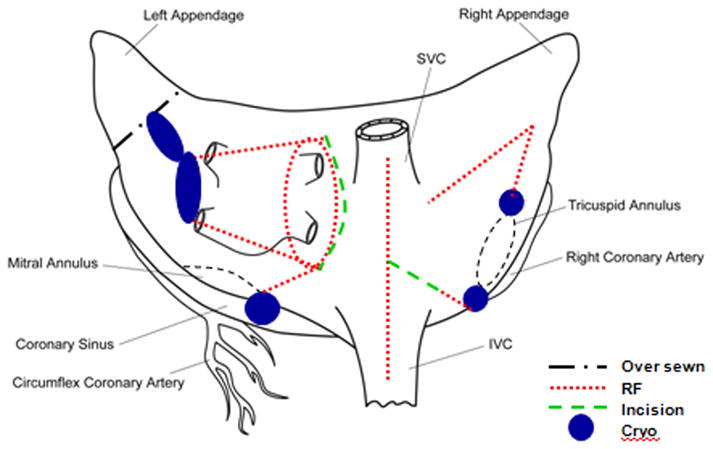
Schematic of the Cox-Maze lesion set performed in this study.
The patient was cooled to 34°C and the right sided lesions of the Cox maze were created on CPB before cross-clamping. A purse string suture was placed in the base of the right atrial appendage, and the bipolar clamp was used to make a right atrial free wall ablation, inserting one of the jaws through a stab incision in the middle of this purse string. A right vertical atriotomy was performed approximately two centimeters inferior to the previous line of ablation, extending from the interatrial septum up toward the atrioventricular groove. The superior aspect of the atriotomy was connected to the tricuspid annulus using a combination of bipolar RF and cryoablation. A linear cryoprobe (Figure 3) cooled to −60°C for two minutes was used to complete the connection to the tricuspid annulus at the 2 o’clock position. From the inferior aspect of the atriotomy, the bipolar clamp was used to create ablation lines up to the SVC and down to the IVC. A final ablation line was placed across the right atrial endocardial surface from the base of the right atrial appendage down toward the tricuspid annulus at the 10 o’clock position using the linear cryoprobe. The right atrium was then closed with a running 4-0 Prolene suture. If a mitral valve procedure was planned, the retrograde cardioplegia catheter was placed into the coronary sinus under direct vision before closing the right atrium.
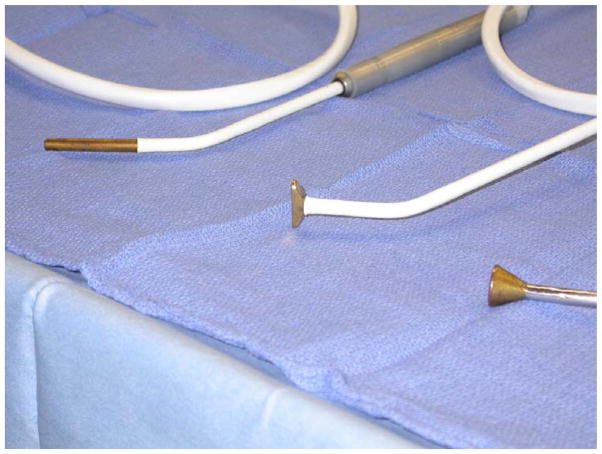
Figure 3.
The linear cryoprobe is used to create an endocardial lesion to the tricuspid annulus in the 10 and 2 o’clock position. The T-shaped cryoprobe is used to complete the isolation of the posterior left atrium with the bell-shaped cryoprobe used to connect the bipolar radiofrequency lesion to the mitral annulus (pictured left to right).
A transthoracic cross-clamp was then positioned through a separate stab incision, and the left-sided lesions of the Cox-Maze procedure were created. After cross-clamping, cold blood cardioplegia was infused in an antegrade fashion into the aortic root. The left atrium was opened with a standard left atriotomy to expose the posterior left atrium. The bipolar clamp was used to create lesions into the left superior pulmonary vein and then the left inferior pulmonary vein from the superior and inferior aspects of this atriotomy, respectively. A final application of the bipolar device was used to create a lesion down towards the mitral valve annulus, approximately at the midpoint of the P2 cusp of the posterior leaflet. The clamp was placed across the coronary sinus. Cryothermy was used to finish this line of conduction block with a 15 mm bell-shaped cryoprobe cooled to −60°C for two minutes. This cryolesion connected the bipolar ablation line to the mitral annulus. An additional cryolesion was occasionally placed across the coronary sinus with a linear cryoprobe if there was doubt about the adequacy of the bipolar ablation. Isolation of the posterior left atrium was completed with a T-shaped cryoprobe (Figure 3). A cryolesion was placed behind the left pulmonary veins to connect the two bipolar RF ablation lines on the posterior left atrium. The base of the left atrial appendage was over sewn and this suture line was connected to the prior ablation line with a final application of the T-shaped cryoprobe. A left ventricular vent was placed through the right superior pulmonary vein and the left atriotomy was closed with a running 4-0 Prolene suture. The aorta was unclamped. After rewarming, the patient was weaned from cardiopulmonary bypass.
Results
There were no operative mortalities or major complications. Cross clamp time for patients undergoing a stand-alone Cox maze procedures averaged 48 ± 7 minutes. Perioperative complications were similar to prior experience with the CMIV at this institution19 and are summarized in Table 3. Two patients required a permanent pacemaker, and five patients had early atrial tachyarrhythmias.
Table 3.
Perioperative parameters
| Variables | Total patients (%) |
|---|---|
| Operative mortality | 0 (0) |
| Mean CCT (minutes) | 66 ± 26 |
| Mean CPB (minutes) | 185 ± 42 |
| Early ATA | 5 (22.7) |
| Permanent PM | 2 (9.1) |
| Myocardial infarction | 0 |
| Stroke | 0 |
| Reoperation for bleeding | 0 |
| Renal failure requiring dialysis | 0 |
| Median ICU LOS in days (range) | 1 (1–15) |
| Median hospital LOS in days (range) | 7 (4–23) |
Early ATA included atrial fibrillation and atrial flutter. CCT = cross clamp time; CPB = cardiopulmonary bypass time; PM = pacemaker; ATA = atrial tachyarrhythmias; ICU = intensive care unit; LOS = length of stay.
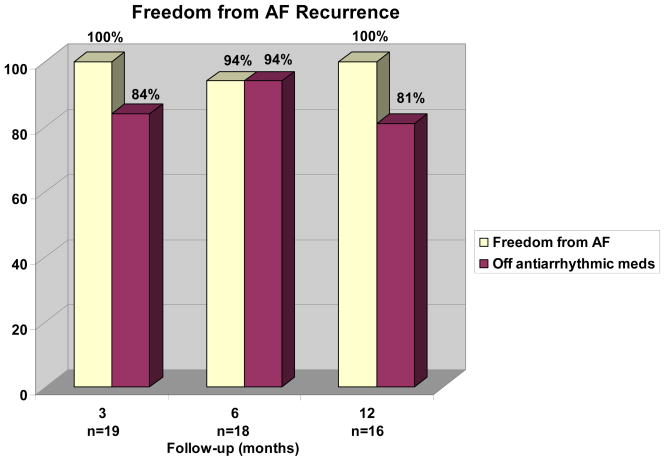
Freedom from atrial fibrillation, as assessed by Holter monitoring, was excellent as shown in Figure 5. By six months follow-up, 94% patients were free from AF. Antiarrhythmic medication usage steadily declined over the first year. (Figure 4) By 12 months, 81% of patients were off antiarrhythmic drugs. All patients on antiarrhythmic drugs at last follow-up were in sinus rhythm. Warfarin use steadily declined as well. At 12 months, two patients (15%) were still on warfarin and did not have a mechanical valve replacement. At our center, anticoagulation is stopped usually at 3 months once the patient is documented to have no episode of AF on prolonged monitoring one month after discontinuation of all antiarrhythmic drugs. Echocardiography is also performed in all patients prior to discontinuation of warfarin to rule out left atrial stasis or left atrial thrombus. There were no late strokes.
Figure 4.
Percent freedom from atrial fibrillation is plotted against follow-up time. The shaded area represents the percent of patients also off antiarrhythmic drugs.
Discussion
This study demonstrated that the full CMP can be performed through a mini-thoracotomy with minimal morbidity and mortality while still maintaining excellent results. In this short term follow-up, 100% of patients were free from AF at 12 months and with over 80% off antiarrhythmic drugs. The entire experience at our institution with the RF based CMIV, including those performed through a sternotomy was similar with 94% freedom from AF at 12 months and 78% off antiarrhythmic medications.
In order to make the CMP technically less demanding and less time consuming, we utilized bipolar radiofrequency and cryoablation to develop the Cox-Maze IV at this institution8. Bipolar radiofrequency clamps are quick, averaging only 11.1 ± 4.3 seconds of ablation per lesion20. More importantly, they reliably create transmural lesions, a prerequisite when surgically treating atrial fibrillation17, 21–23. This technology allowed refinement of the technique into a less invasive approach. Cryoablation was substituted for bipolar RF in places not amenable to clamping. This included the tissue near the tricuspid and mitral annuli and occasionally the coronary sinus. This is a safe and reliable technology that has been used for decades in arrhythmia surgery21–23. Transmurality is assessed by observing the ice ball go through the atrial myocardium. It has the advantage over heat-based ablation in that it preserves tissue collagen and the structural integrity of the heart27. Our group has preferred this technology near the cardiac valves for this reason.
Some minimally invasive surgical treatments for atrial fibrillation have removed many of the original CMP lesions and instead concentrated on pulmonary vein isolation and ganglionated plexus ablation to reduce complexity. Generally, these limited procedures have performed worse than the full CMP lesion set, particularly in patients with persistent and long-standing AF and in patients with organic heart disease28–32. Since patients are reluctant to undergo a second redo surgical procedure for their AF, it has been our policy to perform the full CMP lesion set in all patients undergoing valve surgery and in patients with long-standing lone AF. However, in patients with small left atria (≤ 5cm) and lone paroxysmal AF, our center and others have had good results with pulmonary vein isolation, and this is a reasonable procedure that can be performed off CPB. In patients undergoing valve surgery, we have preferred a full CMP lesion set since the results of pulmonary vein isolation in these patients have generally been poor29, 33–36. Moreover, surgically converting AF into sinus rhythm has been associated with better early and late survival and less thromboembolic events during follow-up for patients with mitral valve disease37–40. Thus, this less invasive full Cox-Maze procedure is an excellent option, particularly in patients with long-standing or persistent lone AF, and patients with AF and concomitant valvular heart disease.
Limitations
This study has some limitations. The small patient population from this study does not represent the population of all AF patients. The majority of the patients in this study had paroxysmal AF, and further study is needed to determine the effectiveness of this procedure in persistent AF, as the percent of paroxysmal AF in other studies examining the efficacy of the Cox-Maze procedure have had a paroxysmal rate closer to 50% 19, 41, 42. Finally, follow-up was limited to one year. Larger series with a more diverse patient population and long-term follow-up are needed to fully assess the efficacy of this approach.
Acknowledgments
The research was supported in part by National Institutes of Health grants 5R01HL32257, RO1 HL085113, T32HL07776.
Footnotes
Presented at the Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothoracic Surgery, Boston, MA USA, June 11–14, 2008.
Disclosure: Ralph J. Damiano, Jr., MD, is a consultant for AtriCure, Inc., West Chester, OH USA, Medtronic, Inc., Minneapolis, MN USA, and ATS Medical, Minneapolis MN USA. The Division of Cardiothoracic Surgery at Washington University School of Medicine has a research grant with AtriCure, Inc.
References
- 1.Callans DJ. In the clinic. Atrial fibrillation. Ann Intern Med. 2008;149 doi: 10.7326/0003-4819-149-9-200811040-01005. ITC5-1-15; quiz ITC5-16. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 3.Ad N, Henry L, Hunt S, Barnett S, Stone L. The Cox-Maze III procedure success rate: comparison by electrocardiogram, 24-hour holter monitoring and long-term monitoring. Ann Thorac Surg. 2009;88:101–5. doi: 10.1016/j.athoracsur.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Isobe F, Kawashima Y. The outcome and indications of the Cox maze III procedure for chronic atrial fibrillation with mitral valve disease. J Thorac Cardiovasc Surg. 1998;116:220–7. doi: 10.1016/s0022-5223(98)70120-5. [DOI] [PubMed] [Google Scholar]
- 5.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 6.Stulak JM, Dearani JA, Sundt TM, 3rd, et al. Superiority of cut-and-sew technique for the Cox maze procedure: comparison with radiofrequency ablation. J Thorac Cardiovasc Surg. 2007;133:1022–7. doi: 10.1016/j.jtcvs.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee A, Singh S, Tempe DK. Intraoperative endocardial ablation of chronic atrial fibrillation along with mitral valve surgery using high frequency ultrasound with a ball-tipped harmonic scalpel probe. Indian Heart J. 2004;56:178–80. [PubMed] [Google Scholar]
- 8.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Gillinov AM, Smedira NG, Cosgrove DM., 3rd Microwave ablation of atrial fibrillation during mitral valve operations. Ann Thorac Surg. 2002;74:1259–61. doi: 10.1016/s0003-4975(02)03760-8. [DOI] [PubMed] [Google Scholar]
- 10.Knaut M, Spitzer SG, Karolyi L, et al. Intraoperative microwave ablation for curative treatment of atrial fibrillation in open heart surgery--the MICRO-STAF and MICRO-PASS pilot trial. MICROwave Application in Surgical treatment of Atrial Fibrillation. MICROwave Application for the Treatment of Atrial Fibrillation in Bypass-Surgery. Thorac Cardiovasc Surg. 1999;47 (Suppl 3):379–84. doi: 10.1055/s-2007-1013205. [DOI] [PubMed] [Google Scholar]
- 11.Kottkamp H, Hindricks G, Autschbach R, et al. Specific linear left atrial lesions in atrial fibrillation: intraoperative radiofrequency ablation using minimally invasive surgical techniques. J Am Coll Cardiol. 2002;40:475–80. doi: 10.1016/s0735-1097(02)01993-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Choo SJ, Kim KI, et al. Atrial fibrillation surgery simplified with cryoablation to improve left atrial function. Ann Thorac Surg. 2001;72:1479–83. doi: 10.1016/s0003-4975(01)03176-9. [DOI] [PubMed] [Google Scholar]
- 13.Mohr FW, Fabricius AM, Falk V, et al. Curative treatment of atrial fibrillation with intraoperative radiofrequency ablation: short-term and midterm results. J Thorac Cardiovasc Surg. 2002;123:919–27. doi: 10.1067/mtc.2002.120730. [DOI] [PubMed] [Google Scholar]
- 14.Mokadam NA, McCarthy PM, Gillinov AM, et al. A prospective multicenter trial of bipolar radiofrequency ablation for atrial fibrillation: early results. Ann Thorac Surg. 2004;78:1665–70. doi: 10.1016/j.athoracsur.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VY, Houghtaling C, Fallon J, et al. Use of a diode laser balloon ablation catheter to generate circumferential pulmonary venous lesions in an open-thoracotomy caprine model. Pacing Clin Electrophysiol. 2004;27:52–7. doi: 10.1111/j.1540-8159.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 16.Sie HT, Beukema WP, Elvan A, Ramdat Misier AR. Long-term results of irrigated radiofrequency modified maze procedure in 200 patients with concomitant cardiac surgery: six years experience. Ann Thorac Surg. 2004;77:512–6. doi: 10.1016/S0003-4975(03)01466-8. discussion 516–7. [DOI] [PubMed] [Google Scholar]
- 17.Melby SJ, Gaynor SL, Lubahn JG, et al. Efficacy and safety of right and left atrial ablations on the beating heart with irrigated bipolar radiofrequency energy: a long-term animal study. J Thorac Cardiovasc Surg. 2006;132:853–60. doi: 10.1016/j.jtcvs.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 18.Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–96. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–7. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 20.Melby SJ, Zierer A, Voeller RK, et al. Wide Variations in Energy Delivery Using an Impedance-Controlled Algorithm in Bipolar Radiofrequency Ablation: Evidence Against Fixed Time Ablation. Innovations. 2007;2:67–72. doi: 10.1097/IMI.0b013e31803c9b11. [DOI] [PubMed] [Google Scholar]
- 21.Gaynor SL, Ishii Y, Diodato MD, et al. Successful performance of Cox-Maze procedure on beating heart using bipolar radiofrequency ablation: a feasibility study in animals. Ann Thorac Surg. 2004;78:1671–7. doi: 10.1016/j.athoracsur.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 22.Prasad SM, Maniar HS, Diodato MD, Schuessler RB, Damiano RJ., Jr Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: a chronic animal study. Ann Thorac Surg. 2003;76:836–41. doi: 10.1016/s0003-4975(03)00716-1. discussion 841–2. [DOI] [PubMed] [Google Scholar]
- 23.Prasad SM, Maniar HS, Schuessler RB, Damiano RJ., Jr Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002;124:708–13. doi: 10.1067/mtc.2002.125057. [DOI] [PubMed] [Google Scholar]
- 24.Klein GJ, Harrison L, Ideker RF, et al. Reaction of the myocardium to cryosurgery: electrophysiology and arrhythmogenic potential. Circulation. 1979;59:364–72. doi: 10.1161/01.cir.59.2.364. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher JJ, Sealy WC, Anderson RW, et al. Cryosurgical ablation of accessory atrioventricular connections: a method for correction of the pre-excitation syndrome. Circulation. 1977;55:471–9. doi: 10.1161/01.cir.55.3.471. [DOI] [PubMed] [Google Scholar]
- 26.Harrison L, Gallagher JJ, Kasell J, et al. Cryosurgical ablation of the A-V node-His bundle: a new method for producing A-V block. Circulation. 1977;55:463–70. doi: 10.1161/01.cir.55.3.463. [DOI] [PubMed] [Google Scholar]
- 27.Gage AM, Montes M, Gage AA. Freezing the canine thoracic aorta in situ. J Surg Res. 1979;27:331–40. doi: 10.1016/0022-4804(79)90149-5. [DOI] [PubMed] [Google Scholar]
- 28.Edgerton JR, Edgerton ZJ, Weaver T, et al. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg. 2008;86:35–8. doi: 10.1016/j.athoracsur.2008.03.071. discussion 39. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Suarez S, Claysset B, Botta L, et al. Surgery for atrial fibrillation with radiofrequency ablation: four years experience. Interact Cardiovasc Thorac Surg. 2007;6:71–6. doi: 10.1510/icvts.2006.136663. [DOI] [PubMed] [Google Scholar]
- 30.Matsutani N, Takase B, Ozeki Y, Maehara T, Lee R. Minimally invasive cardiothoracic surgery for atrial fibrillation: a combined Japan-US experience. Circ J. 2008;72:434–6. doi: 10.1253/circj.72.434. [DOI] [PubMed] [Google Scholar]
- 31.McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1289–95. doi: 10.1111/j.1540-8167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 32.Suwalski P, Suwalski G, Doll N, Majstrak F, Kurowski A, Suwalski KB. Epicardial beating heart “off-pump” ablation of atrial fibrillation in non-mitral valve patients using new irrigated bipolar radiofrequency technology. Ann Thorac Surg. 2006;82:1876–9. doi: 10.1016/j.athoracsur.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Adragao P, Queiros e Melo J, Aguiar C, et al. Evaluation of bilateral pulmonary vein isolation for the treatment of atrial fibrillation: value of postoperative electrophysiological study. Rev Port Cardiol. 2002;21:1281–93. [PubMed] [Google Scholar]
- 34.Knaut M, Tugtekin SM, Matschke K. Pulmonary vein isolation by microwave energy ablation in patients with permanent atrial fibrillation. J Card Surg. 2004;19:211–5. doi: 10.1111/j.0886-0440.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- 35.Gillinov AM, Bhavani S, Blackstone EH, et al. Surgery for permanent atrial fibrillation: impact of patient factors and lesion set. Ann Thorac Surg. 2006;82:502–13. doi: 10.1016/j.athoracsur.2006.02.030. discussion 513–4. [DOI] [PubMed] [Google Scholar]
- 36.Ghavidel AA, Javadpour H, Shafiee M, Tabatabaie MB, Raiesi K, Hosseini S. Cryoablation for surgical treatment of chronic atrial fibrillation combined with mitral valve surgery: a clinical observation. Eur J Cardiothorac Surg. 2008;33:1043–8. doi: 10.1016/j.ejcts.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Itoh A, Kobayashi J, Bando K, et al. The impact of mitral valve surgery combined with maze procedure. Eur J Cardiothorac Surg. 2006;29:1030–5. doi: 10.1016/j.ejcts.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Bando K, Kasegawa H, Okada Y, et al. Impact of preoperative and postoperative atrial fibrillation on outcome after mitral valvuloplasty for nonischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2005;129:1032–40. doi: 10.1016/j.jtcvs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 39.Bando K, Kobayashi J, Kosakai Y, et al. Impact of Cox maze procedure on outcome in patients with atrial fibrillation and mitral valve disease. J Thorac Cardiovasc Surg. 2002;124:575–83. doi: 10.1067/mtc.2002.124392. [DOI] [PubMed] [Google Scholar]
- 40.Melo J, Santiago T, Aguiar C, et al. Surgery for atrial fibrillation in patients with mitral valve disease: results at five years from the International Registry of Atrial Fibrillation Surgery. J Thorac Cardiovasc Surg. 2008;135:863–9. doi: 10.1016/j.jtcvs.2007.08.069. [DOI] [PubMed] [Google Scholar]
- 41.Cox JL. Atrial fibrillation I: a new classification system. J Thorac Cardiovasc Surg. 2003;126:1686–92. doi: 10.1016/j.jtcvs.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Ad N, Cox JL. The Maze procedure for the treatment of atrial fibrillation: a minimally invasive approach. J Card Surg. 2004;19:196–200. doi: 10.1111/j.0886-0440.2004.4036_1.x. [DOI] [PubMed] [Google Scholar]