Abstract
Truncation of the cytoplasmic tail of membrane-bound IgE in vivo results in lower serum IgE levels, decreased numbers of IgE-secreting plasma cells and the abrogation of specific secondary immune responses. Here we present mouse strain KN1 that expresses a chimeric ε-γ1 BCR, consisting of the extracellular domains of the ε gene and the trans-membrane and cytoplasmic domains of the γ1 gene. Thus, differences in the IgE immune response of KN1 mice reflect the influence of the “γ1-mediated signalling” of mIgE bearing B cells. KN1 mice show an increased serum IgE level, resulting from an elevated number of IgE-secreting cells. Although the primary IgE immune response in KN1 mice is inconspicuous, the secondary response is far more robust. Most strikingly, IgE-antibody secreting cells with “γ1-signalling history” migrate more efficiently towards the chemokine CXCL12, which guides plasmablasts to plasma cell niches, than IgE-antibody secreting cells with WT “ε-signalling history”. We conclude that IgE plasmablasts have an intrinsic, lower chance to contribute to the long-lived plasma cell pool than IgG1 plasmablasts.
Keywords: B cells, Chemokines, Immunoglobulins, Knockout mice, Memory cells
Introduction
Serum antibodies, in particular the isotypes other than IgM, bear the individual humoral immunological memory. They are produced by plasma cells, relatively long-lived cells, which integrate the recent immunological history into a longer-lasting, protective shield [1, 2]. Unfortunately, in predisposed individuals, they also perpetuate the production of unwanted, harmful antibodies, like IgE in allergy and autoantibodies in autoimmune diseases [3]. The plasma cell, the final cell type in a long B-cell differentiation process, can be identified based on the expression of specific markers. Several markers that are specific for the B-cell lineage are down-regulated upon plasma cell differentiation, including major histocompatibility complex class II, CD19, CD21, CD22 and CD45 [4]. In contrast, the proteoglycan syndecan-1 (syn-1 or CD138) is up-regulated and serves as an identifying surface marker for plasma cells. Although some plasma cells persist in the spleen, most of them return to their “place of birth” and home to the bone marrow or inflamed tissues where they persist for up to several months in survival niches as resident, immobile cells [5, 6]. Longevity of the plasma cell is influenced by a broad panel of stimuli, including cytokines like IL-5, IL-6, TNF-α, GM-CSF and, also, the chemokine CXCL12 [7, 8]. It is believed that the contact with stroma cells in the bone marrow provides further adhesion-dependent signals supporting plasma cell longevity [9]. The lifespan of plasma cells is limited by the immigration of newly formed migratory plasmablasts that compete with old plasma cells for space in the survival niches [3].
The migration of plasmablasts to the bone marrow is a critical differentiation step to long-lived plasma cells. Chemokines and their receptors are crucially involved in the control of lymphocyte trafficking. Hauser and coworkers [10, 11] showed that migratory plasmablasts lose responsiveness to many chemokines. The expression of the chemokine receptors CXCR5 and CCR7 is decreased on plasma cells, which impairs their migration to B- and T-cell zones in secondary lymphoid organs [12]. On the other hand, the chemokine receptor CXCR4 is highly expressed, guiding the plasma cells into CXCL12 (stromal-cell-derived factor 1-alpha)-expressing organs, including splenic red pulp, lymph nodes and bone marrow [13]. Muehlinghaus et al. [14] first noticed the importance of the BCR in modulating the migration behaviour of memory B cells and showed that the chemokine receptor CXCR3 was preferentially expressed on a fraction of human memory B cells that also expressed mIgG1.
The functional importance of the BCR in the generation of humoral immunological memory became apparent in experiments in which γ1 and ε cytoplasmic tails were deleted in the mouse germ line, and a profound deficiency in the development of IgG1- or IgE-antibody responses was observed [15, 16]. Truncation of the IgE and IgG1 cytoplasmic tail diminished the secretion of antigen-specific IgE or IgG1 by >10–50-fold during primary and secondary immune responses, showing that the tail is necessary for a memory response. These results were complemented and extended by a study in which the transgenic expression of a γ1 or μ/γ1 hybrid heavy chain together with a transgenic κ light chain which conferred a particular antigenic specificity, led to an enhanced generation of memory and plasma cells upon antigenic challenge [17, 18]. These in vivo studies clearly established that the cytoplasmic tails of class-switched BCR dramatically enhance or reduce B-cell antibody responses, although the molecular mechanism remains to be defined.
Here we show that the final fate of a plasma cell is determined to a large extent by the immunoglobulin isotype that forms the BCR. IgE-antibody secreting cells (ASC) carrying a γ1 tail mature faster and migrate more effectively towards a CXCL12 chemokine gradient than IgE-ASC carrying an ε tail. This implies that the isotype-specific BCR causes a specific functional differentiation, i.e. chemokine receptor sensitivity. We concluded that “γ1-ness” of the signalling gives the plasma cell a competitive advantage over “ε-ness” in the quest for plasma cell niches in the bone marrow.
Results
IgE-ASC migrate less efficiently towards a CXCL12 gradient than IgG1-ASC
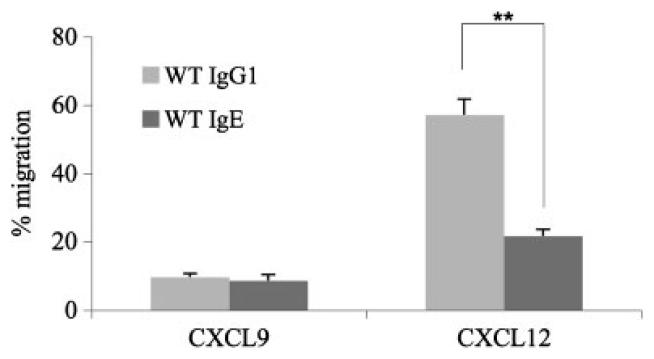
We first investigated the migration behaviour of IgG1-ASC and IgE-ASC towards a gradient of CXCL9 and CXCL12. IgM+ cells were enriched from the spleens of three normal, WT mice, using an FITC-labelled rat α-mouse IgM antibody, followed by magnetic separation with α-FITC beads. IgM+ cells were activated with LPS and IL-4 for 4 days, inducing a class switch to IgG1 or IgE. Equal numbers of activated cells were then seeded onto transwell plates. In preparatory experiments, a concentration of 10 nM for CXCL12 and 100 nM for CXCL9 was found to be optimal. After 90 min, migrated ASC were collected and counted by ELISPOT. The number of potentially migrating ASC after activation, but without exposure to chemokines, served as 100% value. Supporting Information Fig. 1 shows one representative ELISPOT analysis of pooled activated cells originating from three WT mice after migration. Figure 1 shows the statistical analysis of three independent experiments with the individual analysis of three WT mice each. Migration of IgE- and IgG1-ASC towards CXCL9 was very poor and showed a comparable value of 7 and 8.75%, respectively. This result is in agreement with the data of Muehlinghaus et al. [14] who showed that CXCR3− B cells up-regulate CXCR3 only when they are co-stimulated with interferon-γ, but not with IL-4. In contrast, migration towards a gradient of CXCL12 was much more extensive and significantly differed between IgE-ASC and IgG1-ASC. While 54% of the IgG1-ASC actively migrated towards a CXCL12 gradient, only 20% of IgE-ASC migrated within 90 min towards 10 nM CXCL12 (Fig. 1). These observations suggest that the migration behaviour of ASC is dependent on the immunoglobulin isotype that forms the BCR.
Figure 1.
Migration assay of IgE- and IgG1-ASC. IgG1-ASC and IgE-ASC were exposed to the chemokine CXCL9 and CXCL12 in a Transwell culture plate and their migratory behaviour was tested (see also Supporting Information Fig. 1). This figure summarizes data from an experiment repeated three times using three mice per group. Potentially migrating cells of each subclass were counted by ELISPOT and set as 100%. Using the paired t-test, statistical significance at the level of **p<0.001 was calculated.
Construction of knock-in mouse strain “KN1”
To validate the observation that IgG1-ASC more efficiently migrate towards a CXCL12 gradient than IgE-ASC we constructed a mouse strain, in which the membrane exons of the ε heavy chain gene were replaced by those of the γ1 heavy chain with the technique of homologous recombination in embryonic stem cells (see the Materials and methods; Supporting Information Fig. 2A). This mouse line, KN1, expresses a chimeric ε-γ1 antigen receptor. Bona fide homologous recombination was tested by nested PCR and Southern blot analysis (Supporting Information Fig. 2B). Chimeric mice were crossed with WT BALB/c mice. Heterozygous mice were identified by PCR and bred to homozygocity. We feel certain that the downstream neomycine-resistance expression cassette, driven by the thymidine kinase promoter, does not bias our results. First, it is relatively remote from the γ1 membrane exons; second, reading frames are in the same sense and third, Seidl et al. [19] showed that the herpes simplex virus thymidine kinase promoter did not influence class switch recombination to upstream constant Ig heavy chain exons. We thus exclude interfering side effects on the phenotypes described for KN1.
Continually increasing serum IgE levels in KN1 mice
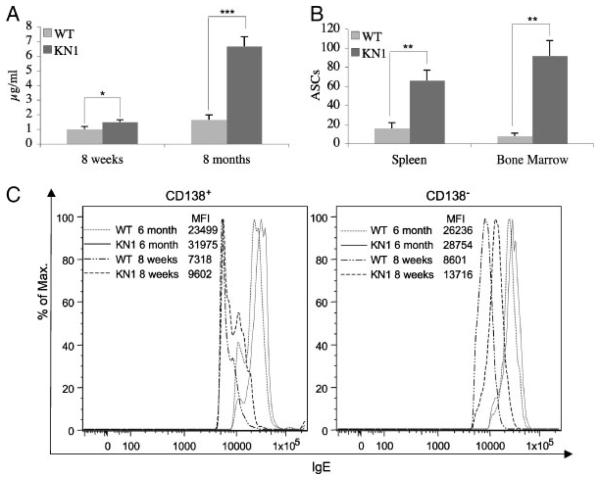
We compared the total serum IgE level of five young (8 wk) and five old (8 months), unprimed WT and KN1 mice. In WT young mice, we measured a mean serum IgE level of 1 μg/mL, which increased to 1.7 μg/mL in 8-month-old mice. KN1 mice showed an increase of the mean serum IgE from 1.6 μg/mL in young mice to 6.6 μg/mL in 8-month-old mice (Fig. 2A), indicating a four- to sixfold elevated mean serum IgE level (***p<0.0001).
Figure 2.
IgE serum levels, ELISPOT and flow cytometric analysis of bone marrow and spleen of WT and KN1 mice. (A) Total serum IgE levels of five young (8 wk) and five old (8-months) WT and KN1 mice. (B) ELISPOT analysis of IgE-ASC originating from pooled spleen and bone marrow cells from five WT and five KN1 mice. For statistical analysis, IgE-ASC were determined individually and extrapolated for 106 spleen or bone marrow cells. Values are means±SD of five mice per time point. (A,B) Data from the described experiments repeated three times, five mice per group. (*p<0.05; **p<0.001; ***p<0.0001). (C) Flow cytometric analysis of bone marrow cells of 8 wk and 6-month-old WT and KN1 mice. Bone marrow cells were incubated with ice-cold stripping buffer followed by incubation with rat anti-CD138 and rat-anti IgE. Cells were pre-gated for living lymphocytes. The lower cutoff value for mIgE+ cells was determined with corresponding isotype controls (see Fig. 6B).
To determine whether the elevated IgE titres in KN1 were caused by enhanced levels of IgE production per cell or by a higher number of cells that produce IgE, we performed individual ELISPOT analyses of spleen and bone marrow cells (Fig. 2B, Supporting Information Fig. 3) of the five 8-month-old mice. The number of IgE-ASC in the spleen and the bone marrow were in full agreement with the serum IgE measurements. In WT mice we counted 16 (±6) IgE-ASC per 106 spleen cells and 8 (±3) IgE-ASC per 106 bone marrow cells, while in KN1 66 (±11) IgE-ASC per 106 spleen cells and 92 (±16) IgE-ASC per 106 bone marrow cells were detected (Fig. 2B). Interestingly, the increase was not equally distributed between spleen and bone marrow. When compared with WT, the increase in IgE-ASC in spleen cells of KN1 mice was on average 4-fold (**p<0.001), whereas in the bone marrow the difference was 7–11-fold (**p<0.001).
Flow cytometric analysis of B cells in the marrow supported these findings. In young WT mice very few, if any CD138+, mIgE+ plasma cells could be detected (Fig. 2C, left panel), in KN1 a minor fraction of CD138+, mIgEdull cells was found. In contrast, in old (6 month) mice, both of KN1 and WT origin, a clear population of CD138+, mIgE+ cells was identified. In KN1, but not in WT young mice a CD138− , IgE+ subset of B cells was seen, which we consider as being early plasmablasts or IgE memory cells (Fig. 2C, right panel). As expected, this CD138−, IgE+ subpopulation was present in older (6 month) mice of both origins. Always, the MFI, and therewith the mIgE-density of the mIgE+-BCR on B cells was higher on B cells of KN1 mice than on those of WT mice.
Summarizing, the increased serum IgE level in 8-month-old KN1 mice is thus most likely explained by the increase in the number of IgE-ASC in the spleen and the bone marrow of these mice.
Increased serum IgE level in KN1 mice is not the result of a different secretion rate
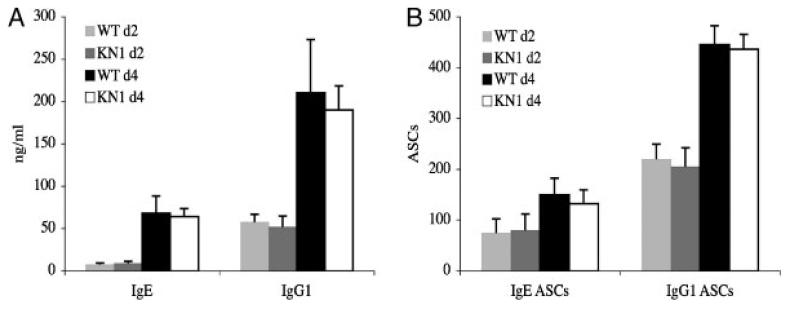
To determine if the increased IgE titre in KN1 mice is the result of an increased secretion rate, IgM+ B cells were activated as described in the section IgE-ASC migrate less efficiently towards a CXCL12 gradient than IgG1-ASC. At days 2 and 4, IgE and IgG1 concentrations were measured in the supernatant of the activated cells (Fig. 3A). Thereafter, a rapidly beginning cell death prevented further observation. The cells were transferred to MultiScreen™ plates and ELISPOT analyses for detection of IgG1-ASC and IgE-ASC were performed (Fig. 3B). For IgE, a similar number of IgE-ASC per 105 activated cells were found at days 2 and 4 in WT (80±21 at day 2 and 150±27 at day 4, respectively) and KN1 (85±25 at day 2 and 132±29 at day 4, respectively) mice, also the amount of IgE in the supernatant was comparable in both types of mice. For IgG1, three fold more IgG1-ASC (220±25 at day 2 and 446±32 at day 4 for WT and 200±50 at day 2 and 436±37 at day 4 for KN1), than IgE-ASC were observed, also reflected in a two- three fold higher concentration of IgG1 in the supernatant. There was no indication for different kinetics in the development of ASC and secretion rates of ASC for the two subclasses in both types of mice.
Figure 3.
In vitro differentiation potential of activated IgM+ cells of WT and KN1 mice. In vitro activated IgM+ cells of WT and KN1 mice were cultured for 2 and 4 days with LPS and IL-4. (A) Supernatants were analysed for IgE and IgG1 content by ELISA. (B) Cells were analysed for IgE and IgG1 secretion by ELISPOT. Values for A and B are means±SD of three independent experiments with three mice each.
We conclude that the amount of immunoglobulin secreted per cell is not different between IgG1-ASC and IgE-ASC and is therefore independent of the isotype of the membrane anchor of the BCR. Equal Ig secretion rates of human plasma cells for all isotypes were also reported before [20].
The specific IgE response in KN1 mice is significantly up-regulated
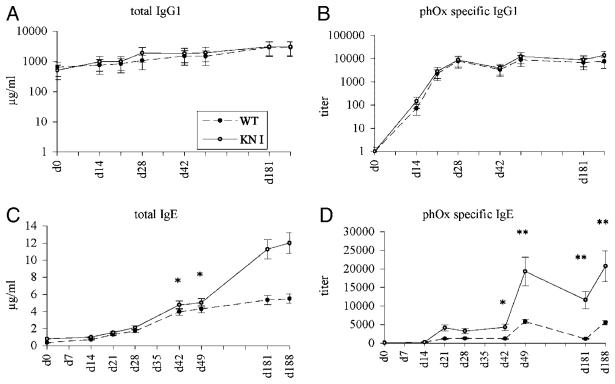
Five WT and five KN1 mice were immunized with the T-cell-dependent antigen phenyloxazolone coupled to ovalbumin (phOx-OVA). Serum levels of total and specific IgG1 were comparable in WT and KN1 mice (Fig. 4A and B) during the whole course of immunization. Total IgE levels of KN1 mice were not significantly elevated at the beginning of the immunization period, but the levels increased at a higher rate in KN1 mice especially after the second booster immunization (Fig. 4C). phOx-specific IgE antibodies in WT and KN1 mice were barely detectable within the first 2 wk, which is in agreement with the initial development of ASC, as described in the previous section. However, after the first booster immunization titres increased and a clear secondary response, as characterized by a strong and fast rise in specific antibody titre, was found (Fig. 4D). Comparing the kinetics of the IgE response following the booster immunizations (d42 and d181), it is obvious that the IgE response was more robust in KN1 than in WT mice.
Figure 4.
Time course of immunization I. Five WT and five KN1 mice were immunized with the T-cell dependent antigen phOx-OVA at days 0, 14, 21, 42 and 181. Immunoglobulin levels were measured by ELISA. (A) Total serum IgG1; (B) phOx-specific IgG1; (C) total IgE; (D) phOx-specific IgE. Values are means±SD of three immunizations with five mice per time point. p-values for time points d42, d49, d181 and d188 for total and specific IgE were significant (*p<0.05 and **p<0.001).
We interpret these observations as reflecting a more efficient development or recruitment of IgE-ASC in general and of IgE-plasma cells in particular in KN1 mice. The boost of specific IgE, following the fourth round of immunization, most likely reflects a higher frequency of IgE memory cells in KN1 mice.
Chimeric IgE-ASC develop more efficiently into non-dividing resident plasma cells
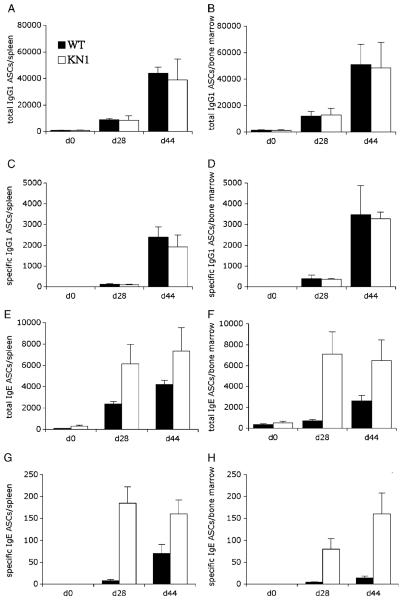
Five WT and five KN1 mice per group were immunized i.p. with 10 μg of OVA, precipitated in alum. Fourteen and 21 days later, mice received a booster immunization with 10 μg OVA. To determine the number of total and OVA-specific IgE- and IgG1-ASC, bone marrow and spleen were obtained for ELISPOT analysis at days 0, 28 and 44 (Fig. 5A–H). For IgG1, a significant number of antigen-specific ASC could be detected in spleen and bone marrow from day 28 onwards (Fig. 5C and D), accompanied by a substantial increase in total IgG1-ASC in both tissues (Fig. 5A and B). In KN1, but not in WT mice, a similar and significant number of antigen-specific IgE-ASC was found in spleen and bone marrow on day 28 (Fig. 5G and H). Later in the response in these mice, the number of IgE-ASC still increased in the bone marrow (Fig. 5H), but at a lower pace than the number of IgG1-ASC (Fig. 5C and D). In WT mice, antigen-specific IgE-ASC were only detectable at day 44 in spleen, but hardly in bone marrow (Fig. 5G and H). A similar pattern was seen when comparing KN1 and WT mice for the total number of IgE-ASC (Fig. 5E and F).
Figure 5.
Time course of immunization II. Five WT and five KN1 mice were immunized with OVA. IgE- and IgG1-ASC of spleen and bone marrow were detected with ELISPOT assays. The total and specific humoral IgE response at day 28 of KN1 mice was significantly up-regulated in the spleen (E and G) and even more in the bone marrow (F and H). Corresponding measurement of IgG1-ASC (A–D) served as control. Values are means±SD of three immunization experiments with five mice per time point.
We conclude from these experiments that the presence of a γ1 tail in the BCR causes an earlier expansion or recruitment of ASC to spleen and bone marrow.
Chimeric IgE-ASC of KN1 resemble the migration behaviour of IgG1-ASC
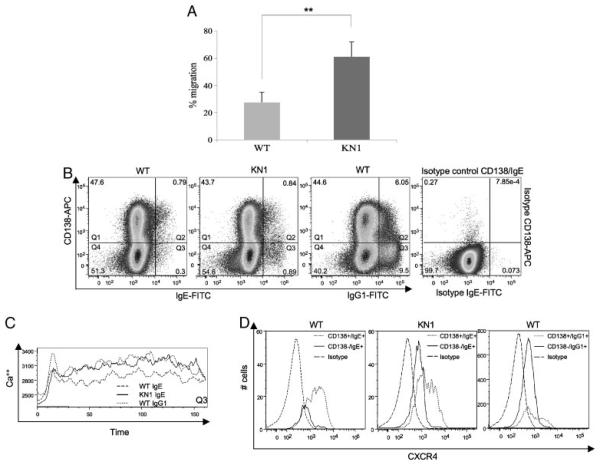
We investigated whether chimeric IgE-ASC of KN1 mice migrate with comparable speed towards a gradient of CXCL12 as do WT IgG1-ASC. The experiment was performed as described in Fig. 1. Equal numbers of activated cells were seeded into transwell plates; the number of potentially migrating cells (100%) was set by establishing the number of IgE- and IgG1-ASC without exposure to chemokines. Figure 6A represents the statistical analysis of three WT and three KN1 mice. The 23±8.9% IgE-secreting cells of WT origin and around 60±11.1% IgE-secreting cells of KN1 origin actively migrated towards a CXCL12 gradient. Thus, the chimeric receptor caused a 2.5-fold increase in the number of IgE-ASC migrating towards the CXCL12 gradient.
Figure 6.
Transwell migration assay of WT- and KN1-derived IgE-ASC/evaluation of the surface co-expression of mIgE, CD138 and CXCR4 and functional activity of CD138low, mIgE(mIgG1)+ cells. (A) Summary of an experiment repeated three times, with three mice per group, investigating the migration frequency of IgE-ASC towards CXCL12. Values are means±SD (**p<0.001). (B) To visualize mIgE+ populations co-expressing CD138, 2 × 106 activated lymphocytes were acquired. The lower cutoff value for mIgE+ cells was determined with corresponding isotype controls and competition stainings. Numbers in the quadrants represent % of the cell populations. (C) Ca2+ fluxes of CD138low, mIg+ B cells (see Quadrant Q3 of Fig. 6B). (D) CD138low, mIgE+ and CD138high, mIgE+ populations were gated and further analysed for CXCR4 expression.
Summarizing, the migration frequency of chimeric IgE-ASC of KN1 mice was significantly influenced by the chimeric receptor situation and resembled the migration frequency of WT IgG1-ASC (54±4.6%).
CD138+ cells from WT and KN1 mice express mIgE at different levels
To investigate whether quantitative differences in receptor density are responsible for our observations, we determined the mIgE-antigen receptor expression in WT and KN1 mice. The activation protocol was the same as used for the cells that were studied for migration behaviour. Frequencies of cell populations were calculated from three independent experiments. Figure 6B displays one representative experiment. CD138+, mIgE+ double positive cells were found in WT and KN1 mice at a frequency of about 0.8±0.22% for both (Quadrants Q2). Remarkably, CD138low, mIgE+ cells, which we consider memory cells or early plasmablasts, were found with a higher frequency in KN1 mice (0.3±0.09% in WT and 0.9±0.15% in KN1 mice, respectively (Quadrants Q3). Additionally, the mIgE+ early plasmablasts of KN1 mice had a higher expression of the (chimeric) IgE-BCR as those of WT mice with MFI of 23 324 and 17 979 for KN1 mice and WT mice, respectively.
Summarizing, (i) CD138low, early plasmablasts express mIgE; (ii) in KN1 mice, more CD138low, mIgE+cells arise than in WT mice, (iii) CD138low, early plasmablasts of KN1 mice express higher levels of (chimeric) mIgE receptor than WT mice; (iv) CD138high plasma cells of WT and KN1 mice still express the IgE- and the chimeric-IgE-antigen receptor.
CD138low, mIgE+ B cells of KN1 migrate similarly to CD138low, mIgG1+ B cells of WT mice
The quantitatively and qualitatively different expression of mIgE on CD138low, mIgE+ cells in KN1 and WT mice raised questions whether the quality and quantity of the signal induced by BCR ligation in this cell type would be different between both mouse strains. We used Ca2+ mobilization as read-out. B cells were activated as in Fig. 1; on day 4 of culture the responsiveness of CD138low, mIgE+ cells from WT and KN1 mice and CD138low, mIgG1+ cells from WT mice, corresponding to the cells in Quadrant Q3 of Fig. 6B was tested. We consider these cells as being memory B cells and early plasmablasts and consider them maximally responsive to BCR ligation. All cell types studied responded to BCR ligation with an early Ca2+ flux from intracellular stores (Fig. 6C), The early response of CD138low, mIgE+ cells from WT and KN1 mice was not apparently different, and lower than the response of CD138low, mIgG1+ cells. However, there was a clear difference between chimeric mIgE+ cells of KN1 mice and mIgG1+ cells at the one hand and mIgE+ cells of WT mice at the other hand: the first show a much higher mobilization of Ca2+ from the extracellular space.
We conclude that the Ca2+ response of cells carrying a chimeric IgE-BCR is quantitatively and qualitatively different from those carrying a WT IgE-BCR.
The frequency of mIgE+, CXCR4low cells is increased in KN1 mice
We next asked whether the different signalling behaviour of the WT and chimeric IgE-BCR could result in a different expression pattern of relevant surface receptors, e.g. chemokine CXCR4, the receptor for CXCL12. The 46.3±1.2% of the CD138high, mIgE+ population in WT mice stained positive for CXCR4 and only a slightly enhanced CXCR4 expression (48.9±1.1%) was measured in KN1 mice. Again, a more significant difference was observed for the CD138low, mIgE+ cells. While 2.8±0.3% of the WT population expressed CXCR4, KN1-derived CD138low, mIgE+ cells expressed CXCR4 with a frequency of 10.2±1.4%. In comparison, 45.8±1.6% of the CD138high, mIgG1+ and 13.5±1.9% CD138low, mIgG1+ population expressed CXCR4. Most strikingly, the absolute number of CD138low, mIgE+ cells in KN1 mice was much higher than the corresponding WT population resembling the situation of CD138low, mIgG1+ cells (Fig. 6D). This supports the data observed above: emerging plasmablasts of KN1 mice carry the highest number of (chimeric) mIgE and are thus sensitive to isotype-specific (i.e. γ1-specific) signalling.
Summarizing, emerging plasmablasts of KN1 mice show the highest expression of the (chimeric) IgE-BCR, show a more γ1-like signalling pattern and express the chemokine receptor CXCR4 at a higher frequency, allowing them to respond to the chemokine CXCL12, which guides plasma cells to the bone marrow.
Discussion
Compared with the other immunoglobulin classes, which are present in serum in concentrations of up to milligrams per millilitre, the titre of IgE ranges between ng- to mg/mL in the plasma of normal healthy individuals and laboratory mouse strains. Although the biological function of IgE is still a matter of speculation, to date IgE is best known for its strong, unwanted effector functions manifesting as allergic symptoms [21]. These can range from local symptoms like hay fever to life-threatening systemic reactions culminating in anaphylactic shock, underlining the potential hazard of high systemic IgE titres. In the recent past others and we have described several B-cell specific control mechanisms that affirm a tight control of the IgE response, with the inherent biological aim to restrain potentially dangerous IgE responses:
IgE has the shortest free serum half-life of all immunoglobulins averaging 12 h in mice [22] and 1–5 days in humans [23, 24].
Studies with CD23-deficient mice clearly demonstrated the role of CD23 as negative feedback regulator of IgE production [25].
Mice lacking the ε membrane exons [15] revealed the key function of the IgE BCR in the regulation of the quality and quantity of IgE expression in vivo [26, 27].
Recently, we published that alternative polyadenylation restricts membrane IgE expression and thus influences serum IgE production [28]. This latter mechanism appears to set a high transcriptional threshold for IgE expression. Because expression of the membrane form of IgE is necessary for the survival or recruitment of IgE-secreting cells, low expression of the mRNA for mIgE most likely limits the number of IgE-secreting cells and thereby limits the magnitude of an IgE-mediated immune response.
In the present manuscript we identified a further mechanism limiting the IgE response. Comparing WT mice with KN1 mice, in which the ε membrane exons were exchanged for those of γ1, leading to the production of an chimeric IgE-IgG1 BCR, we found that IgE-ASC migrate slower towards a CXCL12 gradient than IgG1-ASC, a difference that was caused by a different signalling behaviour of γ1-versus ε-anchored BCR.
In KN1 mice, IgE serum titres continually increased during life. In 8 wk-old mice the difference in the IgE titres between WT and KN1 mice was not significant, but 6 months later a four- to sixfold increase of serum IgE was observed. ELISPOT analyses showed a corresponding increase in the number of IgE-ASC. Therefore, the enhanced IgE level in KN1 mice was the reflection of higher numbers of IgE-secreting cells. Remarkably, IgE-ASC were not distributed equally between spleen and bone marrow. A significantly higher number of IgE-ASC was found in the bone marrow. While IgE-ASC detected in the spleen mainly belong to early and short-lived plasma cells, the IgE-secreting population found in the bone marrow most likely represented non-dividing, resident, long-lived plasma cells (reviewed in [3]). Our interpretation of this observation is that during plasma cell development γ1-like signalling enhances the formation of Ig-secreting plasmablasts and facilitates homing of these cells to the bone marrow, allowing them to become non-dividing, resident, long-lived plasma cells. This is reflected in KN1 mice by a greater number of IgE-ASC in spleen and even more in bone marrow, leading to an overall enhanced IgE response. This implies that IgG1 responses mature faster and are therefore much more efficient than IgE responses. Evidence for the latter notion was obtained when we studied the kinetics of IgE- and IgG1-ASC formation in spleen and bone marrow during an immune response. In KN1 mice, after immunization antigen-specific IgE-ASC were found from day 28 onwards in spleen and bone marrow, whereas in WT mice the first IgE-ASC were detectable in spleen around day 44. Similarly, in not intentionally immunized mice, CD138+, mIgE+ cells were detected at a higher frequency in 8 wk-old KN1 mice than in WT mice of the same age, underlining that specific development of IgE-ASC is reflected in general development of these cells in KN1 mice. Also precursors to plasma cells, CD138low, mIgE+ cells, were detected earlier and at a higher frequency in the bone marrow of KN1 mice than in that of WT mice.
Also memory formation was affected by γ1-like signalling. After the second booster immunization (day 42) a far more robust rise in phOx-specific IgE-antibody titre was measured in KN1 mice compared with WT mice.
These results were not caused by an increased secretion rate of IgE-ASC with a γ1-like signalling history or a different rate of class switch recombination: After in vitro activation, there was no difference in class switch recombination towards ε in KN1 mice, as compared with WT mice, neither did KN1-derived IgE-ASC produce more IgE per cell than did those of WT mice. Further, when comparing the secretion rate of IgE-ASC with that of IgG1-ASC, we found comparable rates. We did not expect a higher switch recombination rate to ε in KN1 mice, because class switch to ε is in mice an inherent feature of the Sε switch region, and is not influenced to a major extent by “trans-switching” from μ to γ1 to ε [29].
A substantive point was observed in the in vitro activated cultures of B cells: more CD138low, mIgE+ cells arose in the KN1-derived cultures when compared with those derived from WT mice. This can be explained by a more efficient recruitment of activated B cells, in particular, cell population, or by an extended lifespan of these cells. The latter possibility was less likely, because we did not see an increased expression of the survival protein Bcl2 (data not shown). Furthermore, CD138low, mIgE+ cells, which we consider to be similar to germinal centre B cells and precursors to memory cells and early plasmablasts, of KN1 mice expressed a higher number of IgE-BCR per cell. The enhanced expression may well be caused by a more efficient polyadenylation of the chimeric mRNA [28] as our unpublished data has shown. Of course, the increased density of BCR bore the potential of an altered, c.q. stronger signal raised by receptor ligation. This we could experimentally show: The signal generated by the chimeric, KN1-derived IgE-BCR resembled an IgG1-driven response in that it showed an increased, late mobilization of Ca2+ ions from the extracellular space. However, the early mobilization from intracellular stores was similar in WT- and KN1-derived CD138low, mIgE+ cells, and lower than that in mIgG1+ cells.
Functionally, the increased expression of the chimeric IgE-BCR on CD138low, mIgE+ cells of KN1 mice was accompanied by an increased expression of the chemokine receptor CXCR4. In view of this, it is not surprising that IgE-ASC of KN1 mice show an increased mobility towards the ligand of CXCR4, the chemokine CXCL12. It was reported before that CXCR4 expression alone does not necessarily predict a more efficient migration behaviour [30] of ASC. Currently, we therefore cannot exclude that factors activated in an isotype-specific fashion and different from CXCR4 further explain our observations. Because similar results were obtained when comparing the mobility towards a CXCL12 gradient of IgG1-ASC and IgE-ASC, we conclude that γ1-like signalling prepares early plasmablasts better for reactivity towards the chemokine CXCL12 than does ε-like signalling, giving plasmablasts with a γ1-like signalling history an competitive edge over those with an ε-like signalling history in their quest for niches in the bone marrow.
In our experiments we have used a BCR-independent activation of B cells (LPS and IL-4), albeit after ligation of the BCR with anti-IgM antibodies, used in the enrichment procedure of B cells. We assume, that in a normal immune response, the B cell is triggered by its nominal antigen, an interaction, which is probably of a low affinity character. Therefore, our activation schedule may well be of physiological nature. We assume that after activation, the B cell proceeds on its differentiation pathway independently of the initiating cue, most likely helped in vitro by various autoantibodies, that are induced upon polyclonal stimulation. More significantly, our in vitro data are mirrored and corroborated by in vivo observations.
Our data lead to the following conclusions. Firstly, γ1-like signalling by the chimeric receptor during all times of the germinal centre reaction in KN1 mice leads to a higher number of (chimeric) IgE-specific memory B cells or to a more efficient recruitment of B cells with a chimeric receptor to the pool of IgE-secreting plasma cells. These hypotheses are not mutually exclusive, but both focus on the fact that the exchange of the ε transmembrane and cytoplasmic domains of mIgE with those of γ1 in KN1 mice influences the magnitude and quality of the IgE immune response and recall the notion that IgG1 memory responses in WT mice are superior over IgE memory responses [27]. Similar conclusions, comparing IgG1 and IgM responses, were suggested by Martin and Goodnow [18]. In their study, a transgenic expression of γ1 or μ/γ1 hybrid chains led to an enhanced generation of memory and plasma cell progeny upon antigenic challenge [17, 18]. By means of transfer experiments, Martin and Goodnow[18] could perfectly dissect whether their observations were the result of a more efficient generation of memory or plasma cells, or a more efficient survival of them. Based on expression levels of Bcl2, which is the same in mIgE+ cells derived from WT or KN1 mice, we believe we can exclude different survival rates determined by either the normal or the chimeric IgE-BCR. Unfortunately, the cell number for IgE expressing cells, also after immunizations, were too low to perform meaningful transfer experiments.
Secondly, BCR-mediated signalling continues in the plasmablast stage and has an isotype-specific component: γ1-like signalling by the chimeric receptor leads to a specific expression pattern, exemplified by the expression of the chemokine receptor CXCR4. Reactivity to its ligand CXCL12 gives the plasmablasts with a γ1-like signalling history a competitive edge over those with an ε-like signalling history.
Our observations also lead to the conclusion that during a Th2-mediated immune response, in normal, WT mice, IgE plasmablasts have an intrinsic, lower chance to contribute to the long-lived plasma cell pool and thus to humoral immunologic memory than IgG1 plasmablasts. Apparently, an IgE immune response is in all stages of the response less efficiently regulated, leading to a poor response. The IgE antibodies may have strong effector functions, but the IgE response is slow and limited in developing memory responses.
Materials and methods
Construction of mouse strain KN1
The γ1 gene was isolated by PCR using genomic DNA of BALB/c ES cells as template. The sense-primer (5′gtcctggtgatttctgagat3′) was located 3′ to spoly(A). The antisense-primer (5′gtggaggacagactgaggacc3′) was located 3′ of the mpoly(A). The target vector pKN1 (Supporting Information Fig. 2A) consists of 8745 bp of genomic DNA, starting inside CH3 of the ε gene. A BamH1 site was inserted 3′ to spoly(A). The ε-membrane locus (ranging from 3′ of spoly(A) to 3′of the third cryptic “membrane” poly(A) site) was replaced by the γ1-membrane locus (3686 bp), followed by additional 1000 bp of ε-genomic sequence. The herpes simplex virus thymidine kinase gene (HSV-Tk) was inserted 5′of CH3. For positive selection a neomycin resistance gene, expressed by a thymidine kinase promoter (Tk-NEO), was inserted. BALB/c ES cells for gene targeting were transfected with 30 μg of linearized target vector and cultured and selected as described before [15, 31]. Resistant clones were screened by nested PCR with primer combination 257–258 and 259–260:
257: 5′AGTTGGCCTTTTCTGGGTTCT3′
258: 5′ATGGCCGCTTTTCTGGATTC3′
259: 5′CAACTACGCCCCGGAGCCATCAG3′
260: 5′CGCATCGCCTTCTATCG3′
Positive targeting events were confirmed by Southern blotting, using a 5′external, a 3′ external and a neo probe (Supporting Information Fig. 2B). Positive ES-cell clones were injected in C57/Bl6 blastocysts. Chimeric mice were bred for heterozygocity and later on for homozygocity. Genotypes were finally confirmed by Southern blot analysis.
Enzyme-linked immunosorbent assay, activation of spleen cells with LPS and IL-4
Spleen cells for in vitro induction were activated with LPS and IL-4 as described elsewhere [32]. The ELISA assay for IgE quantification was performed as described before [15, 26, 31].
ELISPOT analysis
Single cell suspensions from bone marrow and spleen were filtered through a 70 μm cell strainer and re-suspended in RPMI 1640 medium supplemented with 10% FCS. MultiScreen™-IP (Millipore) plates were coated with rat α-mouse IgE, rat α-mouse IgG1 or OVA and incubated over night with defined numbers of spleen cells in RPMI medium. Antibodies for coating and detection are described in [15]. Spots were detected with NBT/BCIP (Roche) tablets.
FACS analysis
Spleen and bone marrow cells were isolated and erythrocyte lysis was performed. To remove CD23-bound IgE, cells were incubated with ice-cold stripping buffer (0.05 M NaAcetate, 0.01 M EDTA, 0.005 M KCl, 0.085 M NaCl, pH = 4) for 1 min. Antibodies used were rat anti-CD184(CXCR4), -CD138, -IgE(R35–72), -CD45R(B220) (all BD-Pharmingen, labels are indicated in the figures). Appropriate isotype controls (all BD-Pharmingen) were used.
Dead cells were excluded by 7-AAD staining. Data were acquired with a CANTO II device (BD) and analysed with FlowJo 8.5.3.
Transwell migration assay
Spleen cells were enriched for IgM+ cells using an FITC-labelled rat α-mouse IgM antibody, followed by a separation with magnetically labelled α-FITC beads. Subsequent FACS analysis showed enrichment for IgM+ cells of 99%. Afterwards, these cells were activated with LPS and IL-4, inducing class switch to IgG1 and IgE. Equal numbers of activated cells were then seeded onto transwell plates. The migration assay was performed according to Hauser et al. [33]. Migration towards the ligands mouse CXCL12/SDF-1a (460-SD Biomedica) and mouse CXCL9 (492-MM Biomedica) at 10 and 100 nM concentration was analysed. After 90 min, migrated ASC were distributed onto ten wells and ELISPOT detection was performed. For CXCL12 we also tried concentrations of 1 and 50 nM. However, with 1 nM no migration was observed and with 50 nM we observed the same migration frequency as with 10 nM.
Immunization protocol with phOx-OVA
Five WT and five KN1 mice were immunized s.c. with 20 μg of phOx11-OVA precipitated in alum with Bordetella pertussis (1 × 107). Exactly, 14, 21, 42 and 181 days later, the mice received a booster immunization with 20 μg of phOx11-OVA s.c. and 20 μg of phOx11-OVA i.p. Serum was taken at day 0 (d0), d7, d14, d21, d28, d42, d49, d181 and d188. Individual serum samples were used to detect total and phOx-specific IgG1 and IgE antibodies with ELISA.
Supplementary Material
Acknowledgements
Experimental work was supported by the FWF Hertha Firnberg Fellowship T166, the FWF project P-19017, the OENB grant 11710, the Swiss National Science Foundation (grant 31000-114634/1) and by the OPO-Pharma Foundation, Zürich.
Abbreviations
- ASC
antibody secreting cells
- phOx
phenyloxazolone
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Supporting Information for this article is available at www.wiley-vch.de/contents/jc_2040/2008/38456_s.pdf
References
- 1.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams MG. B cells as effectors. Curr. Opin. Immunol. 2003;15:354–361. doi: 10.1016/s0952-7915(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 3.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 4.Calame KL. Plasma cells: finding new light at the end of B cell development. Nat. Immunol. 2001;2:1103–1108. doi: 10.1038/ni1201-1103. [DOI] [PubMed] [Google Scholar]
- 5.Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 6.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 7.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 8.Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, et al. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 2001;31:2726–2732. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 10.Hauser AE, Muehlinghaus G, Manz RA, Cassese G, Arce S, Debes GF, Hamann A, et al. Long-lived plasma cells in immunity and inflammation. Ann. N. Y. Acad. Sci. 2003;987:266–269. doi: 10.1111/j.1749-6632.2003.tb06059.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyster JG. Homing of antibody secreting cells. Immunol. Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 14.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser A,E, Hiepe F, et al. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 15.Achatz G, Nitschke L, Lamers MC. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276:409–411. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 16.Kaisho T, Schwenk F, Rajewsky K. The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science. 1997;276:412–415. doi: 10.1126/science.276.5311.412. [DOI] [PubMed] [Google Scholar]
- 17.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J. Exp. Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol. 2002;3:182–188. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 19.Seidl KJ, Manis JP, Bottaro A, Zhang J, Davidson L, Kisselgof A, Oettgen H, Alt FW. Position-dependent inhibition of class-switch recombination by PGK-neor cassettes inserted into the immunoglobulin heavy chain constant region locus. Proc. Natl. Acad. Sci. USA. 1999;96:3000–3005. doi: 10.1073/pnas.96.6.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner-Favre C, Matthes T, Barnet M, Zubler RH. High IgE secretion capacity of human plasma cells. Eur. J. Immunol. 1993;23:2038–2040. doi: 10.1002/eji.1830230849. [DOI] [PubMed] [Google Scholar]
- 21.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 22.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 23.Meno-Tetang GM, Lowe PJ. On the prediction of the human response: a recycled mechanistic pharmacokinetic/pharmacodynamic approach. Basic Clin. Pharmacol. Toxicol. 2005;96:182–192. doi: 10.1111/j.1742-7843.2005.pto960307.x. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann TA, Iio A, Ogawa M, McIntyre OR. Strober, W., The metabolism of IgE. Studies in normal individuals and in a patient with IgE myeloma. J. Immunol. 1976;117:1139–1144. [PubMed] [Google Scholar]
- 25.Yu P, Kosco-Vilbois M, Richards M, Kohler G, Lamers MC. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994;369:753–756. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]
- 26.Achatz G, Luger E, Geisberger R, Achatz-Straussberger G, Breiten-bach M, Lamers M. The IgE antigen receptor: a key regulator for the production of IgE antibodies. Int. Arch. Allergy Immunol. 2001;124:31–34. doi: 10.1159/000053661. [DOI] [PubMed] [Google Scholar]
- 27.Luger E, Lamers M, Achatz-Straussberger G, Geisberger R, Infuhr D, Breitenbach M, Crameri R, Achatz G. Somatic diversity of the immunoglobulin repertoire is controlled in an isotype-specific manner. Eur. J. Immunol. 2001;31:2319–2330. doi: 10.1002/1521-4141(200108)31:8<2319::aid-immu2319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Karnowski A, Achatz-Straussberger G, Klockenbusch C, Achatz G, Lamers MC. Inefficient processing of mRNA for the membrane form of IgE is a genetic mechanism to limit recruitment of IgE-secreting cells. Eur. J. Immunol. 2006;36:1917–1925. doi: 10.1002/eji.200535495. [DOI] [PubMed] [Google Scholar]
- 29.Jung S, Siebenkotten G, Radbruch A. Frequency of immunoglobulin E class switching is autonomously determined and independent of prior switching to other classes. J. Exp. Med. 1994;179:2023–2026. doi: 10.1084/jem.179.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, Cyster JG. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achatz-Straussberger G, Geisberger R, Oberndorfer I, Infuhr D, Luger E, Fallon P, Lamers M, Achatz G. Construction of an sIgE:FLAG-mIgE:GFP reporter mouse strain. Int. Arch. Allergy Immunol. 2003;130:280–287. doi: 10.1159/000070215. [DOI] [PubMed] [Google Scholar]
- 32.Kracker S, Radbruch A. Immunoglobulin class switching: in vitro induction and analysis. Methods Mol. Biol. 2004;271:149–159. doi: 10.1385/1-59259-796-3:149. [DOI] [PubMed] [Google Scholar]
- 33.Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.