Abstract
In a number of clinical studies researchers have reported that acute hyperglycemia is associated with increased mortality and worsened neurological outcome in patients with traumatic brain injury (TBI). In contrast, it has been demonstrated that intensive insulin therapy to lower blood glucose can lead to an increased frequency of hypoglycemic episodes and poor outcome. Consistent with this, experimental and clinical studies have shown that TBI causes a “metabolic crisis” in the injured brain, suggesting that a reduction in glucose availability may exacerbate brain damage. We therefore examined the consequences of hyperglycemia on cognitive and pathological measures. Using a rodent model of TBI, we find that when acute hyperglycemia is induced in animals prior to injury, there is little to no change in motor and cognitive performance, contusion volume, or cerebral edema. To examine the consequences of persistent hyperglycemia (as seen in diabetic patients), animals were treated with streptozotocin (STZ) to induce type 1 diabetes. We find that the presence of persistent STZ-induced hyperglycemia results in a reduction of brain edema. Insulin therapy to reduce blood glucose reverses this beneficial effect of hyperglycemia. Taken together, our results indicate that an acute increase in blood glucose levels may not be harmful, and that intervention with insulin therapy to lower blood glucose levels in TBI patients may increase secondary brain damage.
Key words: cerebral edema, diabetes, hyperglycemia, metabolic distress, secondary brain injury
Introduction
A number of retrospective clinical studies have reported that transient hyperglycemia following traumatic brain injury (TBI) is associated with elevated intracranial pressure (ICP) and increased mortality (Jeremitsky et al., 2005; Lam et al., 1991; Liu-DeRyke et al., 2009; Rovlias and Kotsou 2000). For instance, a blood glucose level above 200 mg/dL in the acute post-injury period has been associated with a 3.6-fold increase in mortality in patients with severe TBI (Griesdale et al., 2009). This association is not necessarily related to a pre-existing diabetic state, as in TBI studies researchers have found a worsened outcome in non-diabetic patients with hyperglycemia in the acute post-injury period (Jeremitsky et al., 2005; Rovlias and Kotsou, 2000). Beyond the relationship between high blood glucose levels in the immediate post-injury period and decreased survival, acute hyperglycemia has also been reported to be associated with poorer neurological outcome (Jeremitsky et al., 2005; Lam et al., 1991; Rovlias and Kotsou 2000; Young et al., 1989). Furthermore, it has previously been demonstrated that elevated blood glucose at the time of ischemia exacerbates secondary pathology (Ginsberg et al., 1980; Pulsinelli et al., 1982; Siemkowicz and Hansen, 1978). Similarly, in a rodent model of TBI, Cherian and colleagues (1998) observed that glucose administration 20 min prior to, but not 20 min after, cortical impact injury resulted in an increase in cortical contusion volume.
Despite the apparent relationship between high blood glucose and poor outcome, several studies have demonstrated that tight management of blood glucose in head-injured patients by intensive insulin therapy leads to an increased incidence of hypoglycemia (Bilotta et al., 2008, 2009; Vespa et al., 2006). Further, a microdialysis study conducted with patients 1–5 days post-injury found that low levels of extracellular glucose were correlated with decreased neurological outcome at 6 months post-injury (Vespa et al., 2003). These studies indicate that after the initial injury, there is a brief period of hypermetabolism that is followed by a sustained period of hypometabolism, suggesting that a reduction in glucose availability may exacerbate brain damage. Consistent with this, administration of a low dose of glucose immediately prior to behavioral training improved the performance of injured animals on the Morris water maze task (Kokiko-Cochran et al., 2008). However, the influence of acute hyperglycemia on learning and memory in injured animals has not been investigated.
Using a rodent model of TBI, we examined the consequences of increased blood glucose on post-injury outcomes. In order to mimic the conditions associated with TBI in persons with pre-existing hyperglycemia/diabetes, glucose was administered prior to injury. We find that when acute hyperglycemia is induced in animals prior to injury, there is no detrimental change in behavior, contusion volume, or cerebral edema. As an additional measure, we investigated whether the presence of an extended hyperglycemic state would result in a worsening of outcome in the acute post-injury period. We find that sustained high blood glucose levels results in a decrease in cerebral edema, which was reversed by post-injury insulin therapy. Taken together, our results suggest that increased blood glucose levels, whether acute or chronic, are not detrimental and may reduce secondary pathologies.
Methods
Animals and materials
Adult male C57/BL6 mice weighing 25–35 g and Sprague-Dawley rats weighing 350–400 g were purchased from Charles River Laboratories (Wilmington, MA). The animals were group housed and maintained on a 12-h light/dark cycle with access to food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee, and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals.
Glucose and streptozotocin (STZ) were purchased from Sigma-Aldrich (St. Louis, MO).
Controlled cortical impact injury
The mice were initially anesthetized with 5% isoflurane and a 1:1 mixture of N2O/O2. While being maintained under anesthesia (2% isoflurane in a 1:1 mixture of N2O/O2), the mice were placed in a stereotaxic frame and a 5-mm craniotomy (halfway between the bregma and the lambda, 0.5 mm lateral to the midline) was performed. A heating pad was used to maintain body temperature at 37°C. Using a 3-mm-diameter impact tip and an electromagnetic cortical impact device, a single impact was delivered to the parietal association cortex at an angle of 10° from the vertical plane. The injury depth for the mice was 1.6 mm at a velocity of 4.0 m/sec. For all rat experiments, the injury was performed using a pneumatic injury device, with a depth of 1.5 mm at a velocity of 4.9 m/sec. The diameter of the impact tip for the pneumatic device was 5 mm. After injury, the scalp was sutured closed with stainless steel surgical staples. The duration of suppression of the righting response was then measured. The righting response was defined as the animal's ability to right itself three times consecutively after being placed on its back. After the completion of assessment of the righting response, the animals were given time to recuperate in a warming chamber before being returned to their home cages. The animals were weighed daily after the injury for the first 3 days, then weekly thereafter.
Blood glucose testing
For blood draws, the mice were placed in a restraining device, and approximately 0.6 μL of blood was extracted from the tail vein with a 25-gauge needle. In the rats, because the sustained hyperglycemia study required multiple readings from the same animal, the lateral saphenous vein was used as the source of blood draws in order to minimize discomfort. This also allowed us to minimize handling, as we were able to read blood glucose levels and administer insulin subcutaneously in a single session. An Aviva Accu-check monitor (Roche Diagnostics Corp., Indianapolis, IN) was used in both experiments to analyze blood glucose levels from each sample.
Induction of hyperglycemia
In the initial experiment examining the effect of acute hyperglycemia on behavioral and neurological outcome, mice were used. Rats were utilized in the subsequent experiments involving the effects of chronic hyperglycemia, because the use of streptozotocin has been more extensively characterized in this species (Junod et al., 1969; McNeill, 1999).
Acute hyperglycemia
A previous TBI study has shown that administration of a dose of glucose 2.2 g/kg prior to injury results in significant hyperglycemia at the time of injury (Cherian et al., 1998). Guided by that study, we employed the 2.2-g/kg dose of glucose 15 min prior to controlled cortical impact injury. The animals were administered glucose in saline, or saline only i.p.
Chronic hyperglycemia
The rats were administered a one-time 65-mg/kg dose of STZ i.p. to induce type 1 diabetes. This drug exerts its effects through a dose-dependent destruction of the pancreatic β cells (Junod et al., 1969), as well as through tissue-specific decreases in insulin-receptor signaling (Kadowaki et al., 1984; Plaschke and Hoyer, 1993). The drug was prepared in a sodium citrate solution with pH 4.5 immediately prior to injection. The animals were fasted for 4 h prior to injection of either STZ or vehicle (sodium citrate buffer alone). In order to avoid sudden hypoglycemic shock, a 10% sucrose solution was placed in the water bottle in each cage overnight. Hyperglycemia in rats receiving STZ was confirmed 2 days later through the blood glucose readings taken from the lateral saphenous vein. Rats subjected to STZ treatment were removed from the study if their blood glucose level was less than 270 mg/dL, or if they experienced any weight gain. The animals were maintained in a hyperglycemic state for 1 week prior to injury.
Insulin administration
Beginning 30 min after injury, the rats were subcutaneously administered a dose of undiluted long-acting insulin Levemir (15 U/kg; Novo Nordisk, Bagsvaerd, Denmark) every 12 h thereafter until sacrifice.
Motor skills testing
Vestibulomotor and motor skills were tested using the beam balance and foot-fault tasks, respectively (Adelson et al., 1997; Chen et al., 1997; Whalen et al., 1999; Phillips et al., 1994). These tests were conducted on days 2, 3, 4, and 8 post-injury. Three testing trials were given daily and averaged for each animal. The animals were placed on a cylindrical metal beam (1 cm diameter) and the time spent balancing was recorded. Paw placement was evaluated by placing the animal on a wire grid (1 × 1 cm opening size) and counting the number of foot faults out of a total of 50 steps (Dash et al., 2002). A foot fault was defined as when a front paw misses and appears below the plane of the grid. Paw placement testing was repeated three times and averaged for each animal. Uninjured animals were tested in the same manner prior to the beginning of the study to establish baseline values for each measure.
Morris water maze task
Mice were trained in the standard Morris water maze (MWM) task as previously described (Dash et al., 1995; Hamm et al., 1992; Raghupathi and Huh, 2007; Smith et al., 1994). Over 8 days, the animals were trained to find the location of a stationary, hidden platform with four separate trials per day. In each trial, the mouse was placed in the tank facing the wall, and then allowed to search for the submerged platform for 60 sec. If the animal did not find the platform during this time period, it was led to it by the investigator. The animal was then required to stay on the platform for 30 sec before removal from the tank. During the 4-min inter-trial interval, the animals were placed in a 37°C warming cage. Probe trials were conducted 30 min and 24 h after the completion of training. In probe trials, the hidden platform was removed from the tank and the animals were allowed to search for it for 60 sec. The search path was monitored using a tracking device connected to a video camera. The data were analyzed for latency to the first platform crossing, distance traveled to the platform, swimming speed, quadrant preference, and number of platform crossings. For quadrant preference analysis, which is used to analyze a group's ability to remember the general spatial location of the platform, the tank was divided in the tracking program into four equal quadrants. Within each group, the data were then analyzed to determine the amount of time spent searching for the platform in each quadrant.
Contusion volume measurement
Following the completion of the behavioral studies, approximately 32 days after injury, the mice were deeply anesthetized with sodium pentobarbital (100 mg/kg), and then transcardially perfused with 100 mL of phosphate-buffered saline, followed by 100 mL of 4% paraformaldehyde. The brains were removed, post-fixed overnight in perfusant, and then cryoprotected in a 30% sucrose solution. Contusion volume was estimated using previously described methods (Sullivan et al., 1999). In brief, cryosections (40-μm thick) spanning the rostral-caudal extent of the injured cortex were selected and stained with cresyl violet. The area of cortical tissue loss for each section was carefully outlined using Image J software from the National Institutes of Health (NIH). Contusion volume was calculated using the equation: A1(0.5X1) + A2(0.5X1 + 0.5X2) + An − 1 (0.5Xn − 1 + 0.5Xn) + An(0.5Xn), where A is the area (mm2) of the contusion for each slice, and X is the distance (mm) between two sequential slices. Group differences were then assessed.
Cerebral edema measurement
The animals were decapitated, and the brains were removed as quickly as possible in order to avoid water loss (Zhang and Shohami 1998). The cerebellum was removed, and then the brain was bisected into the ipsilateral and contralateral hemispheres, relative to the site of injury. These were quickly weighed three times each in order to yield an average wet weight, and then placed in an oven. Three days later, the brains were re-weighed in order to produce a dry weight. At 24 h after this, the brains were weighed again in order to ensure that the dry weight was stable. The percentage of tissue water was calculated using the following formula: [(wet wt – dry wt)/wet wt × 100]. This measure was calculated both for the ipsilateral and contralateral hemispheres.
Statistical analysis
In all experiments, data collected from the same animal with one or more factors, such as blood glucose level analysis, latency to platform, and probe trial quadrant preference, was subjected to repeated measures (RM) ANOVA. Data comparing only one factor between groups, including probe trial data analysis, contusion volume, and edema levels in the acute hyperglycemia experiment and the vehicle and STZ-only experiment, was subjected to t-tests. In cases in which data did not pass the Shapiro-Wilk normality test, a non-parametric rank-sum test (ANOVA or t-test, depending on the data to be analyzed) was performed. These cases are noted in the text. Significant differences were determined at p < 0.05.
Results
Acute elevation of blood glucose does not affect recovery of vestibulomotor or motor skill performance
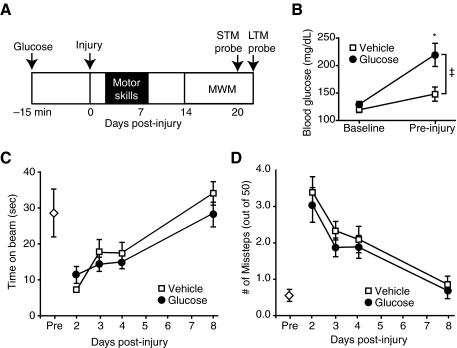
To investigate if acutely elevated glucose at the time of injury affected subsequent measures of motor performance and cognition, we administered either 2.2 g/kg glucose (n = 10), or vehicle (n = 11), IP 15 min prior to TBI. A schematic diagram of the experimental timeline is presented in Figure 1A. As expected, administration of this dose led to a significant increase in blood glucose in the treated mice (two-way RM ANOVA for time-group interaction, p = 0.027; Fig. 1B). No significant difference was found between glucose and vehicle animals in the rate of recovery of vestibulomotor faculties, as assessed by beam balance testing (Fig. 1C). Similarly, foot-fault testing demonstrated that there was no difference between the two groups in motor skill recovery (Fig. 1D). In both cases, the performance of the animals returned to pre-injury levels by 8 days post-injury.
FIG. 1.
Hyperglycemia at the time of injury did not affect recovery of motor skills. (A) Schematic diagram showing the paradigm for glucose injection, injury, motor assessment, and behavioral testing.(B) Intraperitoneal injection of glucose led to a significant increase in blood glucose immediately prior to injury. Post-injury, there was no difference between vehicle (n = 11) and glucose (n = 10) animals in performance of (C) the balance beam task, or in (D) the foot fault task (open diamond = task performance pre-injury; data are presented as mean ± standard error of the mean; *p < 0.05; MWM, Morris water maze; STM, short-term memory; LTM, long-term memory).
Acute elevation of blood glucose does not impair neurocognitive outcome
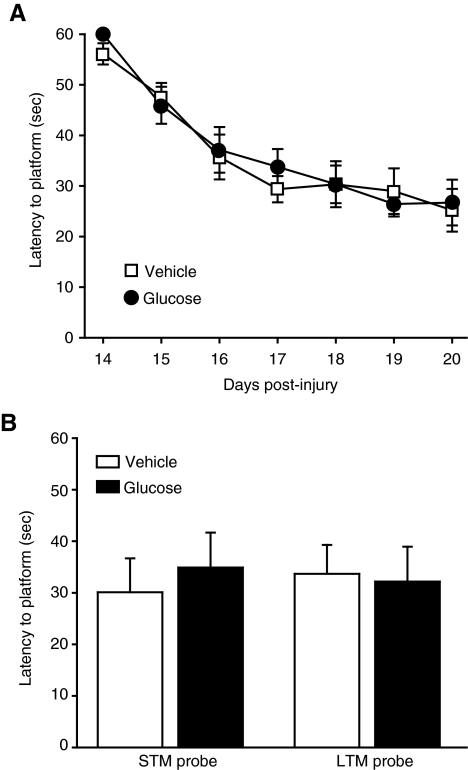
The effect of acute hyperglycemia at the time of injury on learning and memory was assessed 14 days post-TBI. Animals that received glucose 15 min before injury did not show any significant difference in their performance in the MWM, as evidenced by similar latencies to platform in both groups across all days of training (two-way RM ANOVA, p = 0.724; Fig. 2A). This was further confirmed by similar latency to platform in both the short-term and long-term probe trials, designed to test short-term memory (STM) and long-term memory (LTM), respectively (Fig. 2B).
FIG. 2.
Pre-injury acute elevation of blood glucose did not result in overt differences in behavioral performance. Treatment with glucose did not result in a change in behavior in either the (A) acquisition of the Morris water maze (MWM) test, or (B) in latency to platform during short-term memory (STM) 30 min following the completion of training, and long-term memory (LTM) 24 h after the completion of testing (vehicle n = 11; glucose n = 10; data are presented as mean ± standard error of the mean.
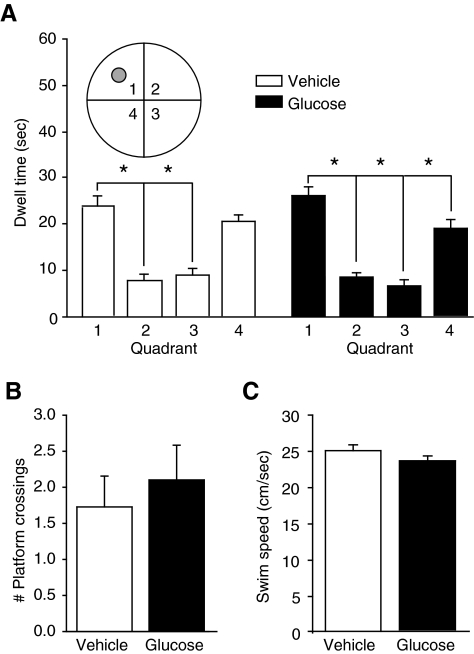
Next, we analyzed the quadrant preference of the animals during both LTM and STM probe trial performance (STM data, Fig. 3A). Neither group showed a significant quadrant preference during the LTM probe trials (vehicle, p = 0.138; glucose, p = 0.530). However, both groups showed an increased quadrant preference during the STM probe trials (one-way RM ANOVA rank-sum for both groups, p < 0.001). A post-hoc Holm-Sidak pairwise comparison revealed that the glucose animals spent significantly more time in the quadrant containing the platform than any other quadrant, while the vehicle animals preferred that quadrant and an adjacent quadrant equally. Despite this difference in preference, there was no significant difference in the number of platform crossings (t-test, p = 0.569; Fig. 3B). There was also no significant difference in swim speed during the STM probe (t-test, p = 0.482; Fig. 3C).
FIG. 3.
Pre-injury administration of glucose caused the animals to demonstrate an increased ability to remember general spatial location during short-term memory (STM) probe trials. (A) Glucose animals (n = 10) spent significantly more time in the quadrant containing the platform than any other quadrant (Q1), while the vehicle animals (n = 11) spent increased time in both Q1 and Q4 (the inset shows a schematic depiction of the quadrants; grey circle = platform location). (B) Despite a difference in quadrant preference, glucose administration did not significantly alter the number of platform crossings during the STM probe trial (data presented as mean ± standard error of the mean; *p < 0.05). (C) There was also no significant difference in swim speed during the STM probe trial.
Increased pre-injury blood glucose does not affect contusion volume
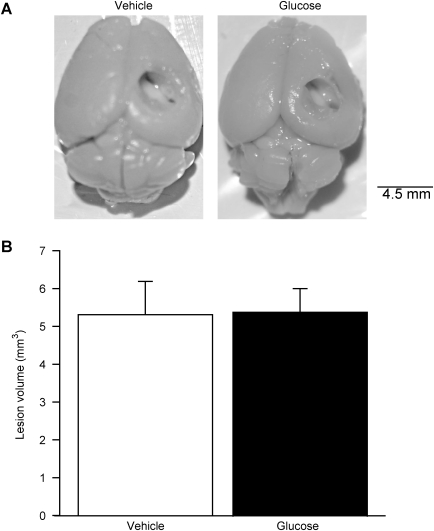
To examine if acute hyperglycemia exacerbates brain damage, cortical contusion volume of each injured animal was measured as described in the methods section (vehicle, n = 4; glucose, n = 5). As evidenced by photographs of animals representative of each group, injury resulted in no gross differences in contusion volume (Fig. 4A). Further histological measurement of contusion volume shown in Figure 4B demonstrated that acute hyperglycemia did not significantly increase contusion volume (t-test, vehicle = 5.3 ± 0.88 mm3, glucose = 5.4 ± 0.63 mm3; p = 0.937).
FIG. 4.
Contusion volume was not significantly affected by the administration of 2.2 g/kg of glucose 15 min before injury. (A) A representative photograph of an animal brain from each group shows no visible difference in contusion volume (scale bar = 4.5 mm). (B) Histological measurement of contusion volumes in the animals further confirmed that hyperglycemia at the time of injury did not have a significant effect (vehicle, n = 4; glucose, n = 5; data are presented as mean ± standard error of the mean).
Acute hyperglycemia at the time of injury does not affect cerebral edema
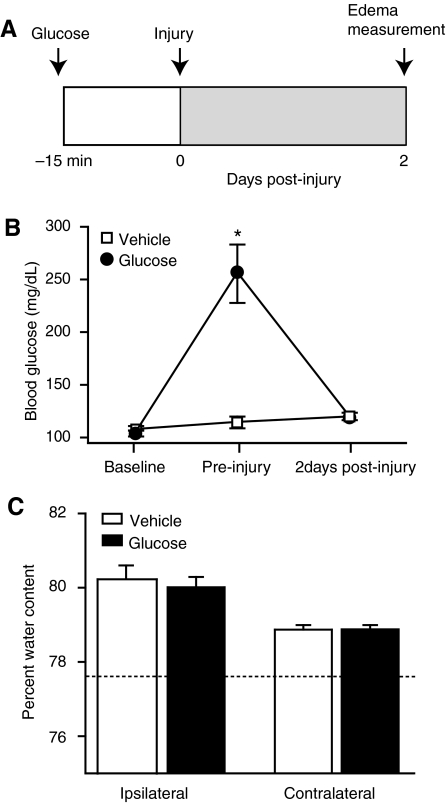
In the clinical literature, evidence has suggested a correlation between maximum ICP and postoperative levels of blood glucose in patients undergoing trauma-related neurosurgery (Rovlias and Kotsou, 2000). A primary cause for TBI-associated mortality is cerebral edema, which can give rise to elevated ICP (Marmarou, 1994). We therefore investigated whether there was a relationship between edema and an acute episode of hyperglycemia in rats through IP administration of 2.2 g/kg of glucose or vehicle 15 min prior to injury (n = 8/group), followed by sacrifice and measurement of brain water content 48 h post-injury (Fig. 5A). Again, this dose of glucose resulted in an increase in blood glucose immediately prior to injury in the glucose-treated animals, which was significantly different from the vehicle group (two-way RM ANOVA for group-time interaction, p < 0.001; Fig. 5B). At the time of sacrifice at 2 days post-injury, the blood glucose levels in both groups were not significantly different than they were at pre-injury baseline. We found that significantly increased blood glucose at the time of injury did not have an influence on cerebral edema at 48 h post-injury (rank-sum t-test, ipsilateral, p = 0.597; contralateral, p = 0.941; Fig. 5C). The dashed line in Figure 5C illustrates the mean percentage of brain water present in uninjured animals.
FIG. 5.
Cerebral edema was not significantly affected by administration of 2.2 g/kg of glucose 15 min before injury. (A) Schematic timeline of the edema experiment. (B) Administration of this dose of glucose to rats significantly raised blood glucose prior to injury. Two days later, this level had returned to normal. (C) At 48 h post-injury, there was no significant difference in the level of cerebral edema between animals with elevated glucose at the time of injury and injured vehicle animals (n = 8/group). The dotted line represents the average level of brain water content previously observed in uninjured animals (data are presented as mean ± standard error of the mean; *p < 0.05).
Chronic hyperglycemia reduces TBI-induced cerebral edema
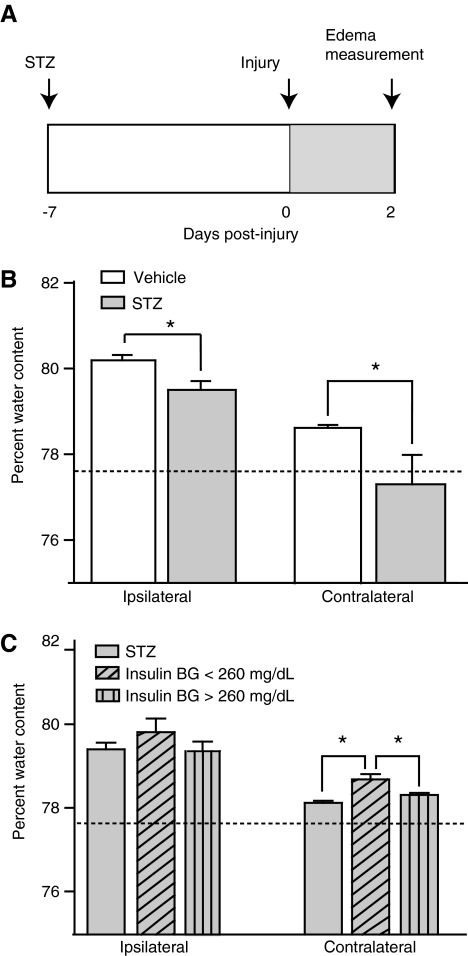
To determine whether the length of hyperglycemia has an impact on post-injury outcome, we decided to examine the effect of inducing persistently high glucose levels in animals prior to brain injury. To examine the effect of chronic hyperglycemia on cerebral edema, we treated rats with streptozotocin (STZ), a chemical known to kill pancreatic β cells and induce diabetes. We conducted this experiment using the same injury magnitude and sacrifice time point as those described for assessment of the effect of acute hyperglycemia on edema. A schematic outline of this experiment is shown in Figure 6A. The measurement of blood glucose levels verified the induction of hyperglycemia in STZ-treated animals (n = 8), with a significant increase in mean levels from a baseline level of 103 ± 3.1 mg/dL to 428 ± 14.3 mg/dL 1 week later (two-way RM ANOVA for group-time interaction, p < 0.001). There was no significant change in the blood glucose level of vehicle animals (n = 9) over time. Following injury, the recovery of the righting response, which measures the animal's ability to right itself three times following injury, was equivalent for both groups (t-test, p = 0.925).
FIG. 6.
Persistent hyperglycemia post-TBI resulted in a decrease in cerebral edema, which was partially reversed by insulin administration. (A) Schematic diagram showing the paradigm for streptozotocin (STZ) administration, injury, and outcome analysis. (B) Administration of STZ 1 week prior to injury resulted in significant changes in tissue water content at 48 h post-injury, as measured by the hemisphere relative to the site of injury. The tissue water percentage was decreased in the STZ-treated animals (n = 8), relative to the vehicle group (n = 9), for all measures. (C) In a separate experiment in which a subset of STZ animals was treated with insulin post-TBI, animals responsive to insulin treatment (blood glucose [BG] level < 260 mg/dL; n = 6) displayed significantly greater edema in the contralateral hemisphere than untreated STZ animals (n = 7), or those that did not respond to insulin treatment (n = 6). The dotted line represents the average level of brain water content previously seen in uninjured animals (data are presented as mean ± standard error of the mean; *p < 0.05; TBI, traumatic brain injury).
In order to examine the effect of persistent hyperglycemia on TBI-induced cerebral edema, we measured the percentage of brain water present at 48 h. Figure 6B shows that STZ-treated animals exhibited significantly less edema than the injured animals receiving vehicle. Edema in both the ipsilateral (rank-sum t-test, p = 0.011), and contralateral hemisphere (rank-sum t-test, p < 0.001; Fig. 6B), were decreased.
Reduction of blood glucose levels in streptozotocin-treated animals post-injury results in a partial increase in cerebral edema
In order to further examine the relationship between blood glucose levels and edema, we repeated the previous experiment with an additional group: STZ rats receiving insulin treatment post-injury. These animals were treated every 8 h with a long-acting insulin analog, and were assessed using intermittent blood glucose recordings. The timeline of this experiment also follows that shown in Figure 6A, with insulin administration taking place in the shaded post-injury period.
Prior to the injury and subsequent insulin administration, there was no significant baseline difference in blood glucose between the vehicle-treated (n = 10) and STZ-treated animals (n = 19). Initial STZ treatment resulted in a significant increase in blood glucose (two-way RM ANOVA for group-time interaction, p < 0.001; STZ pre-injury blood glucose = 423 ± 14.4 mg/dL). Following injury, recovery of the righting response was equivalent for the vehicle-treated and STZ-treated animals (rank-sum t-test, p = 0.708). When insulin treatment was applied to the designated subset of the STZ animals post-injury (n = 12), we found that some animals had a greater response to the drug than others. For this reason, when we analyzed the impact of blood glucose on edema, we divided the insulin-receiving animals into two groups: those animals with blood glucose under 260 mg/dL (n = 6), and animals with blood glucose greater than 260 mg/dL (n = 6). This value closely corresponded with the median blood glucose level for the two groups (median = 268.5 mg/dL). Immediately prior to injury, there was no significant difference between these two groups in blood glucose level (t-test, p = 0.485). In order to eliminate any confounding variables associated with the STZ treatment itself, we compared these animals directly to the animals receiving STZ and subcutaneous vehicle injection (n = 7), rather than the non-STZ-treated vehicle animals. There was no significant difference between the level of cerebral edema observed in the STZ-treated animals in this experiment and the previous experiment (rank-sum t-test, ipsilateral p = 0.591; contralateral p = 0.152).
When the groups were compared in this manner, we found that STZ-treated animals that responded to insulin therapy prior to sacrifice (blood glucose <260 mg/dL; mean blood glucose = 157 ± 29.3 mg/dL) had significantly greater edema in the contralateral hemisphere than both the animals that were not as responsive (blood glucose >260 mg/dL; mean blood glucose = 321 ± 35.4 mg/dL), and the STZ-only group (Fig. 6C; 1-way ANOVA of ranks, p = 0.002). The group with blood glucose >260 mg/dL was not significantly different from the STZ-only group. There was also no significant group effect in the ipsilateral cortex (p = 0.322).
Discussion
Using a rodent model of TBI, we have examined the consequences of acute and sustained pre-existing hyperglycemia on outcome after TBI. The results from this study revealed three key findings. First, in contrast to a previous report, hyperglycemia at the time of TBI did not increase cortical contusion volume (Cherian et al., 1998). Second, no adverse effect of acute hyperglycemia was observed on motor skills or learning and memory. Interestingly, it improved short-term memory, as indicated by enhanced quadrant preference in the MWM task. And third, sustained hyperglycemia reduced cerebral edema in injured animals. Insulin treatment that resulted in significantly decreased serum glucose reversed this effect. Taken together, these findings suggest that neither acute nor sustained hyperglycemia negatively influences TBI outcome, and supports the studies that question the utility of intensive insulin therapy to regulate blood glucose.
A number of clinical studies have found that hyperglycemia is associated with poor neurological outcome after TBI (Griesdale et al., 2009; Lam et al., 1991; Liu-DeRyke et al., 2009; Rovlias and Kotsou, 2000; Young et al., 1989). The general consensus among caretakers is that blood glucose should be maintained below 180 mg/dL (Oddo et al., 2008). However, recent studies have provided evidence that although blood glucose above a certain level may be harmful, strict management of serum glucose levels through use of intensive insulin therapy is also not ideal for TBI patients (Bilotta et al., 2008, 2009; Vespa et al., 2006). TBI increases the cellular demand for glucose, and Vespa and colleagues (2006) associated this metabolic shift with a decrease in extracellular glucose and an increase in glutamate concentrations within the brain. These changes can persist in patients sustaining moderate to severe injury (Glenn et al., 2003; Kato et al., 2007). Therefore, a decrease in blood glucose level in TBI patients can increase the risk of secondary brain damage. Consistent with this, in a recent study researchers reported that early metabolic dysfunction can lead to chronic brain atrophy in TBI patients (Xu et al., 2009).
In both clinical and experimental studies, it has been reported that the injured brain undergoes a metabolic crisis: an acute period of hyperglycemia followed by sustained hypoglycemia (Bergsneider et al., 1997, 2000; Jiang et al., 2000; Yoshino et al., 1991). In order to examine the consequences of acute hyperglycemia, investigations have utilized glucose injections prior to, or immediately following, different models of TBI. Of these studies, Cherian and associates (1998) and Kinoshita and associates (2002) both reported that acute hyperglycemia increases cortical tissue loss, an effect not replicated in the present study. Unfortunately, those investigators did not examine if this enhanced tissue loss was associated with altered motor or cognitive function. Using a fluid percussion model of brain injury, Vink and colleagues (1997) reported that acute hyperglycemia does not influence motor function, as indicated by the finding of no significant change in composite neurological scores between normoglycemic and hyperglycemic injured animals. Consistent with this observation, our results show no adverse or beneficial effect of acute hyperglycemia on either motor or vestibulomotor function following cortical impact injury (Fig. 1C and 1D). For the beam-balance task, a metal rod was used instead of a wooden beam. Although uninjured mice had a high degree of variability in this task (likely due to the inability of the animals to always recover from a misstep because of the composition of the beam), the statistical comparison between the glucose-treated and vehicle-treated animals was carried out only using the post-injury measures. When these animals were tested in the MWM, a modest improvement in short-term memory, as indicated by enhanced quadrant preference during a short-term memory probe trial, was observed. Although no significant increase was found in the number of platform crossings, the enhanced quadrant preference suggests that the glucose-treated animals have enhanced short-term memory for the general location of the platform.
Understanding the complications presented by treating patients with high glucose levels has become an increasing concern in various clinical settings, as the number of diabetic and pre-diabetic persons in the U.S. is now estimated at over 40% (Cowie et al., 2009). Individuals with pre-diabetes, who often remain untreated for high blood glucose levels, comprise 29.5% of the population. Currently, the impact of chronically high blood glucose on TBI outcome is poorly understood. Our experimental results suggest that the presence of a persistent state of hyperglycemia is associated with a decreased level of cerebral edema. This finding is in contrast to a previous report, in which STZ administration was associated with an increase in cerebral edema (Elangovan et al., 2000). Although the reason for this difference is unclear, the duration of hyperglycemia (7 days versus 4 weeks) may have been a contributing factor. One possible explanation for the results of our edema experiment in animals with sustained hyperglycemia is that glucose present in high serum amounts could act as an osmotic agent, creating a gradient that enhances efflux of water from the injured brain. However, it has been previously demonstrated that STZ treatment and the resultant hyperglycemia leads to equivalent increases in the concentration of glucose in both the brain and in the plasma (Shram et al., 1997). Consistent with this, it has been shown that STZ-induced diabetes does not have any effect on glucose uptake or the abundance of luminal GLUT1 transporters in the brain (Simpson et al., 1999). Thus, despite an overall increase in glucose levels, it is unlikely that this would result in an osmotic gradient that would reduce cerebral edema. We recognize that the results of our edema studies of the impact of sustained hyperglycemia would be stronger if they were supported by behavioral data. It has been reported that rats maintained in an uncontrolled diabetic state are lethargic and suffer from extreme weight loss (McNeill, 1999). As we observed similar effects of sustained hyperglycemia, long-term behavioral studies were not performed. Previously it has been demonstrated that in the chronic phase of injury, glucose supplementation may be beneficial. Kokiko-Cochran and colleagues reported that daily administration of a small amount of glucose (100 mg/kg), while insufficient to alter learning and memory in sham-operated controls, enhances water maze performance in TBI animals (Kokiko-Cochran et al., 2008).
We acknowledge a number of weaknesses of the present study. Most reports on the adverse effects of hyperglycemia use data from patients who had sustained severe TBI or were critically ill. In these studies, one of the primary outcomes associated with hyperglycemia was mortality. The injury level employed in the present study was moderate, since the injury did not result in the death of any of our experimental animals. Also, severe TBI patients often have injuries to other organs and/or experience hemorrhagic shock (Clifton et al., 1980; Gennarelli et al., 1989; Neugebauer et al., 2000), unlike the isolated brain injury model used in this study. Future experiments are needed to address if acute hyperglycemia adversely affects outcome in these situations using models of polytrauma (Hauser, 2005; Tsukamoto and Pape 2009).
Acknowledgment
The authors would like to thank Anthony Moore and Sara Orsi for their technical assistance and critical reading of the manuscript. This work was supported by grants from the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson P.D. Dixon C.E. Robichaud P. Kochanek P.M. Motor and cognitive functional deficits following diffuse traumatic brain injury in the immature rat. J. Neurotrauma. 1997;14:99–108. doi: 10.1089/neu.1997.14.99. [DOI] [PubMed] [Google Scholar]
- Bergsneider M. Hovda D.A. Lee S.M. Kelly D.F. McArthur D.L. Vespa P.M. Lee J.H. Huang S.C. Martin N.A. Phelps M.E. Becker D.P. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J. Neurotrauma. 2000;17:389–401. doi: 10.1089/neu.2000.17.389. [DOI] [PubMed] [Google Scholar]
- Bergsneider M. Hovda D.A. Shalmon E. Kelly D.F. Vespa P.M. Martin N.A. Phelps M.E. McArthur D.L. Caron M.J. Kraus J.F. Becker D.P. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bilotta F. Caramia R. Cernak I. Paoloni F.P. Doronzio A. Cuzzone V. Santoro A. Rosa G. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit. Care. 2008;9:159–166. doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- Bilotta F. Caramia R. Paoloni F.P. Delfini R. Rosa G. Safety and efficacy of intensive insulin therapy in critical neurosurgical patients. Anesthesiology. 2009;110:611–619. doi: 10.1097/ALN.0b013e318198004b. [DOI] [PubMed] [Google Scholar]
- Chen Y. Lomnitski L. Michaelson D.M. Shohami E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997;80:1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- Cherian L. Goodman J.C. Robertson C.S. Effect of glucose administration on contusion volume after moderate cortical impact injury in rats. J. Neurotrauma. 1998;15:1059–1066. doi: 10.1089/neu.1998.15.1059. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Grossman R.G. Makela M.E. Miner M.E. Handel S. Sadhu V. Neurological course and correlated computerized tomography findings after severe closed head injury. J. Neurosurg. 1980;52:611–624. doi: 10.3171/jns.1980.52.5.0611. [DOI] [PubMed] [Google Scholar]
- Cowie C.C. Rust K.F. Ford E.S. Eberhardt M.S. Byrd-Holt D.D. Li C. Williams D.E. Gregg E.W. Bainbridge K.E. Saydah S.H. Geiss L.S. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K. Mach S.A. Moore A.N. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–767. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Moore A.N. Dixon C.E. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J. Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan V. Kohen R. Shohami E. Neurological recovery from closed head injury is impaired in diabetic rats. J. Neurotrauma. 2000;17:1013–1027. doi: 10.1089/neu.2000.17.1013. [DOI] [PubMed] [Google Scholar]
- Gennarelli T.A. Champion H.R. Sacco W.J. Copes W.S. Alves W.M. Mortality of patients with head injury and extracranial injury treated in trauma centers. J. Trauma. 1989;29:1193–1201. doi: 10.1097/00005373-198909000-00002. discussion 1201–1202. [DOI] [PubMed] [Google Scholar]
- Ginsberg M.D. Welsh F.A. Budd W.W. Deleterious effect of glucose pretreatment on recovery from diffuse cerebral ischemia in the cat. I. Local cerebral blood flow and glucose utilization. Stroke. 1980;11(4):347–54. doi: 10.1161/01.str.11.4.347. [DOI] [PubMed] [Google Scholar]
- Glenn T.C. Kelly D.F. Boscardin W.J. McArthur D.L. Vespa P. Oertel M. Hovda D.A. Bergsneider M. Hillered L. Martin N.A. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Griesdale D.E. Tremblay M.H. McEwen J. Chittock D.R. Glucose control and mortality in patients with severe traumatic brain injury. Neurocrit Care. 2009. [Epub ahead of print]. [DOI] [PubMed]
- Hamm R.J. White-Gbadebo D.M. Lyeth B.G. Jenkins L.W. Hayes R.L. The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurgery. 1992;31:1072–1077. doi: 10.1227/00006123-199212000-00013. discussion 1078. [DOI] [PubMed] [Google Scholar]
- Hauser C.J. Preclinical models of traumatic, hemorrhagic shock. Shock. 2005;24(Suppl. 1):24–32. doi: 10.1097/01.shk.0000191387.18818.43. [DOI] [PubMed] [Google Scholar]
- Jeremitsky E. Omert L.A. Dunham C.M. Wilberger J. Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J. Trauma. 2005;58:47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- Jiang X.B. Ohno K. Qian L. Tominaga B. Kuroiwa T. Nariai T. Hirakawa K. Changes in local cerebral blood flow, glucose utilization, and mitochondrial function following traumatic brain injury in rats. Neurol. Med. Chir. (Tokyo) 2000;40:16–28. doi: 10.2176/nmc.40.16. discussion 28–29. [DOI] [PubMed] [Google Scholar]
- Junod A. Lambert A.E. Stauffacher W. Renold A.E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J. Clin. Invest. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T. Kasuga M. Akanuma Y. Ezaki O. Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J. Biol. Chem. 1984;259:14208–14216. [PubMed] [Google Scholar]
- Kato T. Nakayama N. Yasokawa Y. Okumura A. Shinoda J. Iwama T. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J. Neurotrauma. 2007;24:919–926. doi: 10.1089/neu.2006.0203. [DOI] [PubMed] [Google Scholar]
- Kinoshita K. Kraydieh S. Alonso O. Hayashi N. Dietrich W.D. Effect of posttraumatic hyperglycemia on contusion volume and neutrophil accumulation after moderate fluid-percussion brain injury in rats. J Neurotrauma. 2002;19(6):681–92. doi: 10.1089/08977150260139075. [DOI] [PubMed] [Google Scholar]
- Kokiko-Cochran O.N. Michaels M.P. Hamm R.J. Delayed glucose treatment improves cognitive function following fluid-percussion injury. Neurosci. Lett. 2008;436:27–30. doi: 10.1016/j.neulet.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Lam A.M. Winn H.R. Cullen B.F. Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J. Neurosurg. 1991;75:545–551. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- Liu-DeRyke X. Collingridge D.S. Orme J. Roller D. Zurasky J. Rhoney D.H. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit. Care. 2009;11:151–157. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- Marmarou A. Traumatic brain edema: an overview. Acta Neurochir. Suppl. (Wien.) 1994;60:421–424. doi: 10.1007/978-3-7091-9334-1_114. [DOI] [PubMed] [Google Scholar]
- McNeill J.H., editor. Streptozotocin-Induced Diabetes: Induction, Mechanism(S), and Dose Dependency. CRC Press; Boca Raton, FL: 1999. [Google Scholar]
- Neugebauer E. Hensler T. Rose S. Maier B. Holanda M. Raum M. Rixen D. Marzi I. [Severe craniocerebral trauma in multiple trauma. An assessment of the interaction of local and systemic mediator responses] Unfallchirurg. 2000;103:122–131. doi: 10.1007/s001130050023. [DOI] [PubMed] [Google Scholar]
- Oddo M. Schmidt J.M. Mayer S.A. Chiolero R.L. Glucose control after severe brain injury. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:134–139. doi: 10.1097/MCO.0b013e3282f37b43. [DOI] [PubMed] [Google Scholar]
- Phillips L.L. Lyeth B.G. Hamm R.J. Povlishock J.T. Combined fluid percussion brain injury and entorhinal cortical lesion: a model for assessing the interaction between neuroexcitation and deafferentation. J. Neurotrauma. 1994;11:641–656. doi: 10.1089/neu.1994.11.641. [DOI] [PubMed] [Google Scholar]
- Plaschke K. Hoyer S. Action of the diabetogenic drug streptozotocin on glycolytic and glycogenolytic metabolism in adult rat brain cortex and hippocampus. Int. J. Dev. Neurosci. 1993;11:477–483. doi: 10.1016/0736-5748(93)90021-5. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W.A. Levy D.E. Duffy T.E. Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol. 1982;11(5):499–502. doi: 10.1002/ana.410110510. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Huh J.W. Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. J. Neurotrauma. 2007;24:1596–1608. doi: 10.1089/neu.2007.3790. [DOI] [PubMed] [Google Scholar]
- Rovlias A. Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–342. doi: 10.1097/00006123-200002000-00015. discussion 342–343. [DOI] [PubMed] [Google Scholar]
- Shram N.F. Netchiporouk L.I. Martelet C. Jaffrezic-Renault N. Cespuglio R. Brain glucose: voltammetric determination in normal and hyperglycaemic rats using a glucose microsensor. Neuroreport. 1997;8:1109–1112. doi: 10.1097/00001756-199703240-00009. [DOI] [PubMed] [Google Scholar]
- Siemkowicz E. Hansen A.J. Clinical resitution following cerebral ischemia in hypo-, normo- and hyperglycemic rats. Acta Neurol Scand. 1978;58(1):1–8. [PubMed] [Google Scholar]
- Simpson I.A. Appel N.M. Hokari M. Oki J. Holman G.D. Maher F. Koehler-Stec E.M. Vannucci S.J. Smith Q.R. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J. Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Lowenstein D.H. Gennarelli T.A. McIntosh T.K. Persistent memory dysfunction is associated with bilateral hippocampal damage following experimental brain injury. Neurosci. Lett. 1994;168:151–154. doi: 10.1016/0304-3940(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Bruce-Keller A.J. Rabchevsky A.G. Christakos S. Clair D.K. Mattson M.P. Scheff S.W. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T. Pape H.C. Animal models for trauma research: what are the options? Shock. 2009;31:3–10. doi: 10.1097/SHK.0b013e31817fdabf. [DOI] [PubMed] [Google Scholar]
- Vespa P. Boonyaputthikul R. McArthur D.L. Miller C. Etchepare M. Bergsneider M. Glenn T. Martin N. Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit. Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. McArthur D. O'Phelan K. Glenn T. Etchepare M. Kelly D. Bergsneider M. Martin N.A. Hovda D.A. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J. Cereb. Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Vink R. Golding E.M. Williams J.P. Mcintosh T.K. Blood glucose concentration does not affect outcome in brain trauma: A 31P MRS study. J Cereb Blood Flow Metab. 1997;17(1):50–3. doi: 10.1097/00004647-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Whalen M.J. Clark R.S. Dixon C.E. Robichaud P. Marion D.W. Vagni V. Graham S.H. Virag L. Hasko G. Stachlewitz R. Szabo C. Kochanek P.M. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 1999;19:835–842. doi: 10.1097/00004647-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Xu Y. McArthur D.L. Alger J.R. Etchepare M. Hovda D.A. Glenn T.C. Huang S. Dinov I. Vespa P.M. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;30:883–894. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A. Hovda D.A. Kawamata T. Katayama Y. Becker D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Young B. Ott L. Dempsey R. Haack D. Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann. Surg. 1989;210:466–472. doi: 10.1097/00000658-198910000-00007. discussion 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. Shohami E. Beit-Yannal E. Bass R. Trembovler V. Samuni A. Mechanism of brain protection by nitroxide radicals in experimental model of closed-head injury. Free Radic Biol Med. 1998;24(2):332–40. doi: 10.1016/s0891-5849(97)00267-0. [DOI] [PubMed] [Google Scholar]