Abstract
We performed a comprehensive analysis of innate and adaptive immune responses in dual-virus infected pigs to understand whether a pre-existing immunomodulatory respiratory viral infection affects the overall immunity to a subsequent porcine respiratory coronavirus (PRCV) infection in pigs. Pigs were either mock-infected or infected with porcine reproductive and respiratory syndrome virus (PRRSV), a virus known to cause immunosuppressive respiratory disease, and then pigs were co-infected with PRCV, which normally causes subclinical respiratory infection. We collected samples for six independent experiments from 178 pigs that were also used for pathological studies. We detected a significant reduction in innate NK-cell-mediated cytotoxic function in PRRSV-infected pigs, which was synergistically further decreased in pigs co-infected with PRCV. Subsequently, in association with clinical signs we observed elevated levels of proinflammatory (IL-6), Th-1 (IL-12), and regulatory (IL-10 and TGF-β) cytokines. Increased frequencies of CD4CD8 double-positive T lymphocytes and myeloid cells, in addition to the elevated Th-1 and proinflammatory cytokines in dual-infected pigs, contributed to the severity of lung disease in pigs. The results of our study clarify how each virus modulates the host innate and adaptive immune responses, leading to inflammatory reactions and lung pathology. Thus measurements of cytokines and frequencies of immune cells may serve as indicators of the progression of respiratory viral co-infections, and provide more definitive approaches for treatment.
Introduction
Cytokines are key regulators in governing the host defense against pathogens and are produced following microbial infections. They are potent immunomodulatory molecules that act as mediators of inflammation and the immune response. Proinflammatory cytokines (TNF-α, IL-1, IL-6, and IL-8) are produced early in viral infection, triggering the production of the Th-1 cytokines (IFN-γ and IL-2) involved in cellular immune responses. Both IL-10 and TGF-β suppress the host's cell-mediated immune response by reducing cell recruitment and downregulation of cytokine production by innate immune cells (14). Natural killer (NK) cells are populations of lymphocytes recognized for their ability to provide a first line of innate defense against viral pathogens (8). Pigs possess relatively more NK cells (up to 10% of lymphocytes) than other species of animals and humans (16).
We used a porcine reproductive and respiratory syndrome virus (PRRSV) infection to understand how immune modulation induced by a prior respiratory viral pathogen influences a subsequent porcine respiratory coronavirus (PRCV) respiratory infection. Coronaviruses (CoVs) are members of the family Coronaviridae and the order Nidovirales (34,38). They are enveloped viruses with a single-stranded positive-sense RNA. The coronaviruses infect a broad range of vertebrates and cause a variety of disorders, including gastroenteritis and respiratory tract disease. In this study we used PRCV, a deletion mutant of transmissible gastroenteritis virus (TGEV) of pigs (44). PRCV alone causes mostly subclinical respiratory tract infection in pigs, but in conjunction with other viral or bacterial infections, it causes severe respiratory disease in swine (44). PRCV replicates in epithelial cells of the nasal mucosa and lung (13,15,32). PRRSV is an enveloped RNA virus and a member of the family Arteriviridae and the order Nidovirales (28). PRRSV has a specific tropism for macrophages in the lung and other tissues (7,17,35), and infected pigs have weak and delayed adaptive immune responses, as suggested by low levels and deferred generation of IFN-γ-secreting cells (27). PRRSV is a strong inducer of the immunoregulatory cytokine IL-10 in the lungs (40). The IL-10 is produced by antigen-presenting cells (APCs) and lymphocytes, which are also important targets of IL-10 (23). Overall, the immune responses against PRRSV are ineffective in resolving the infection completely, and they induce immune modulation, resulting in prolonged viremia and persistent infection in lung and lymphoid tissues, potentiating the effects of other swine pathogens (31).
To determine if dual-virus infections, compared to single-virus infections, result in enhanced clinical manifestations in pigs, Van Reeth's group carried out experiments, and detected more persistent fever and growth retardation in PRRSV/PRCV and PRRSV/SIV dual-virus infected pigs than in pigs with single-virus infections (45). In another study, the cytokine analysis of PRRSV, PRCV, and SIV single-virus infected pigs revealed that changes in proinflammatory cytokine levels are associative, and do not demonstrably cause viral respiratory disease in pigs (43,44). Suppressed innate immune responses to TGEV infection in pigs were associated with a reduced NK-cell response (35). However, comprehensive immunological responses to cytokines in systemic and local mucosal sites of lungs, and lymphoid and myeloid cell populations in the dual respiratory virus–infected pigs were unexplored. The aim of our study was to understand how viral co-infections modulate innate and adaptive immune responses, and how these responses relate to the clinical outcome in pigs.
Materials and Methods
Virus inoculation and management of pigs
Conventional Large White-Duroc crossbred specific-pathogen-free piglets (n = 178) were weaned at 16–20 d of age, and transported to animal facilities at the Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, Ohio. The swine herd was seronegative for antibodies to PRRSV, PRCV, TGEV, and porcine circovirus type 2 (PCV2). The piglets were bled on arrival, and pre-bleed sera were tested to confirm the absence of neutralizing antibody to PRCV using a fluorescent focus neutralization test (48), and for PRRSV antibody screening, serum samples were sent to the Animal Disease Research and Diagnostic Laboratory, South Dakota State University, for serological study, and confirmed as negative. The pigs were allowed to acclimate for an additional week before the initiation of the experiments. Six sequential batches of pigs were obtained for six individual trials. The piglets were randomly assigned to one of four treatment groups: mock-infected (n = 43); PRRSV-infected (n = 39), PRCV-infected (n = 48), and PRCV/PRRSV-infected (n = 48).
The ISU-1 strain of PRCV (PRCV-ISU-1) (18), and the PRRSV strain SD23983, were used to infect pigs in this study. To establish PRCV infection after progressive PRRSV disease (subsequent to PRRSV viremia), the pigs were inoculated intranasally (IN) with 3 × 104 TCID50, and intramuscularly (IM) with 2 × 104 TCID50, of the PRRSV SD23983 strain. At 10 d after PRRSV infection, Telazol®-anesthetized pigs were inoculated IN with 4 × 106 plaque-forming units (PFU) and intratracheally with 6 × 106 PFU of the PRCV ISU-1 strain. Mock-infected control pigs received 5 mL of DMEM by similar routes to those described for the respective viruses. After virus inoculation, we assessed clinical signs, body weight gains, breathing rates, and rectal temperatures every other day as previously described (19). Nasal swabs and blood were collected every other day throughout the experimental period for detection of virus shedding and serum cytokine and antibody analysis. The pigs were maintained in accordance with the standards of the Institutional Laboratory Animal Care and Use Committee, The Ohio State University.
Determination of cytokine concentrations in serum and lung by ELISA
The lung tissue from all of the euthanized pigs was collected and lung lysates were prepared in DMEM without serum. Approximately 2–5 g of lung tissue of individual pigs was minced into tiny pieces. To make homogenates, the tissues were blended for 2 min in a Stomacher 400 laboratory blender (Seward, Long Island, NY), and clarified by centrifugation. Then the supernatant collected was aliquoted and frozen at −20°C until subjected to cytokine analysis. Pigs were bled on PRCV/PRRSV post-infection days (PID) −10/0, −6/4, −2/8, 0/10, 2/12, 4/14, 6/16, 8/18, 10/20, 12/22, 14/24, 16/26, 18/28, and 21/31, and serum samples were aliquoted and frozen at −20°C until used for cytokine analysis. Representative proinflammatory (IL-6), Th-1 (IL-12p35 and p40), and anti-inflammatory/T-regulatory (IL-10 and TGF-β) cytokine levels in serum and lung were determined by ELISA as previously described (4,20,49).
Isolation of PBMCs and flow cytometric analysis of lymphoid and myeloid cell populations
For the isolation of peripheral blood mononuclear cells (PBMCs), blood was collected in acid-citrate dextrose solution from the euthanized pigs and processed as previously described (47). The PBMCs (1 × 106 cells/well) were incubated with optimal dilutions of fluorophore-conjugated antibodies in staining buffer (0.02% PBS, 0.5% sodium azide, and bovine serum albumin) for 30 min at 4°C. For CD4+ and CD8+ cell staining, the cells were incubated with fluorescein isothiocyanate-conjugated mouse anti-pig CD4α or CD8α mAb (BD Biosciences, San Jose, CA). For CD3+ staining, biotin-conjugated mouse anti-pig CD3ɛ mAb (Southern Biotechnology Associates, Birmingham, AL) was used, followed by incubation with streptavidin-PerCP (peridinin-chlorophyll-protein complex) conjugate (BD Biosciences). The CD172+ cells were stained with mouse anti-pig CD172 R-phycoerythrin (R-PE; Southern Biotechnology). Fifty thousand PBMCs were analyzed out of the 1 × 106 cells stained for each sample by flow cytometry. Lymphocytes were defined by their light-scatter characteristics (36). For discrimination of positive and negative populations, quadrant markers were set, and these were controlled by non-stained samples and samples incubated only with isotype control antibodies. Flow cytometry data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). The frequency of each individual type of lymphocyte or CD172+ cell was expressed as the frequency (percentage) of these cells within the 50,000 PBMCs counted.
Lymphocyte subpopulations were separated initially by CD3+ and CD3− gates. Based on CD4 and CD8 staining characteristics, each population was then further grouped as: CD3−CD4−CD8+ as NK cells (16); CD3+CD4+CD8− as T-helper cells; CD3+CD4−CD8+ as CTL; and CD3+CD4+CD8+ double-positive T cells. All of the CD172+ cells were grouped as total myeloid cell population.
Natural killer cell cytotoxicity assay
The basic assay for colorimetric determination of NK-cell cytotoxicity has been previously described by others (24,25), and by us (20). In brief, the target cell line, K-562 (human myeloblastoid leukemia cell line), was maintained in RPMI 1640 medium supplemented with FBS. The PBMCs isolated from mock-infected and virus-infected pigs were used as effectors (source of NK cells). The cells were washed three times using medium-199 containing HEPES buffer, gentamicin, and bovine serum albumin (assay medium), to remove free lactate dehydrogenase (LDH), and then the cells were resuspended in assay medium. The effectors were transferred to a 96-well round-bottom plate and twofold dilutions were performed. Fixed numbers of target cells were added to attain different effector:target cell ratios. Appropriate controls were included in each plate, such as: only targets; lysed only targets; only effectors; and medium control. The plates were incubated at 37°C in a CO2 incubator overnight. Control target cells were lysed with 2% Triton-X 100 for 15 min. All the plates were centrifuged briefly, and 100 μL of the supernatant was transferred to a fresh 96-well flat-bottom plate, and then equal amounts of LDH substrate [5.4 × 10−2 M L(+) lactic acid, 6.6 × 10−4 M 2-p-idophenyl-3-p-nitrophenyl tetrazolium chloride, 2.8 × 10−4 M phenazine methosifate, and 1.3 × 10−3 M NAD (Sigma-Aldrich, St Louis, MO) in 0.2 M Tris buffer, pH 8.2] was added to all of the wells and incubated at room temperature. To measure the amount of LDH released into the supernatant, the plates were read in a microtiter plate reader at 490 nm after 5–10 min. The quantity of LDH released into the supernatant is directly proportional to NK-specific lysis of targets in experimental wells (24). The percentage of NK cell-specific lysis was calculated as follows: [(ODE+T− ODE − ODT-spon)/ODT-total − ODT-AM)] × 100, where E = effectors, T = targets, AM = assay medium, T-total = targets with 0.5% NP40, T-AM = targets with assay medium, and T-spon = T-AM minus the OD of AM control wells.
Data analysis
Statistical analyses were performed for each PID among the four experimental groups using the nonparametric Kruskal-Wallis test, and in addition, for serum samples a repeated-measures ANOVA was used due to repeated analyses of sera from the same animals. Statistical significance was set at p < 0.05.
Results
Prior PRRSV infection exacerbates PRCV-induced clinical illness in co-infected pigs
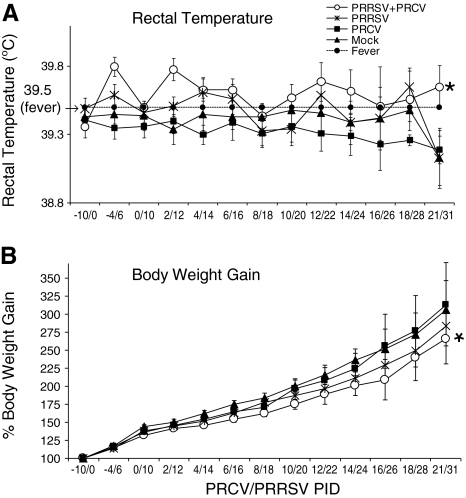
Pigs were infected first with PRRSV, and subsequently on day 10 with PRCV. We recorded clinical signs (body temperature, sneezing, coughing, polypnea, and anorexia) for all of the pigs, either mock-infected or infected with either single virus (PRRSV or PRCV), or co-infected with both the viruses (PRRSV and PRCV), on alternate days over a period of 30 d. To minimize handling stress for the pigs, which may affect the immune responses to viral infection, the pigs were handled by experienced personnel, and were bled by a veterinarian with vast experience. Even so, a minor effect on the overall immune responses in virus-infected pigs could not be ruled out. More severe clinical signs were observed in the pigs with co-infection than with either single-virus infection. Rectal temperatures of >39.5°C were considered to be febrile responses. A significantly higher incidence of fever (p < 0.05) was evident in more than 70% of dual virus–infected pigs compared to pigs infected with PRRSV alone (52%). Fever appeared in PRCV/PRRSV-infected pigs on PID 2/12, and persisted until PID 21/31 (Fig. 1A). However, the pigs infected only with PRRSV had sporadic fever, beginning at PID 4 until PID 31, and in contrast to this finding, pigs infected with PRCV alone had no fever. The PRRSV-only-infected pigs had less body weight gain than PRCV-infected and mock-infected pigs. However, the dual virus–infected pigs had significantly decreased body weight gain compared to pigs infected with PRCV (p < 0.05) or PRRSV alone (Fig. 1B).
FIG. 1.
Clinical responses in pigs infected with PRRSV, and then infected with PRCV 10 d later. Clinical signs of pigs mock-infected (n = 43), PRRSV-infected (n = 39), PRCV-infected (n = 48), or infected with both PRRSV and PRCV (n = 48) were recorded on the indicated post-inoculation days (PID). (A) Rectal temperature. (B) Body weight gain. Each data point represents the mean ± SEM of 5–48 pigs. A significantly higher incidence of fever was observed in PRCV/PRRSV co-infected pigs than in PRRSV-alone pigs (p = 0.027), and a significantly higher proportion had less body weight gain in the PRCV/PRRSV co-infected pigs than in those infected with PRRSV alone (p = 0.005), as indicated by the asterisks. Statistical analysis was performed using Fischer's exact test to evaluate the proportions of pigs with fever and decreased body weight gain, and p < 0.05 was considered statistically significant.
Suppressed innate NK-cell cytotoxicity occurred in the dual-infected pigs
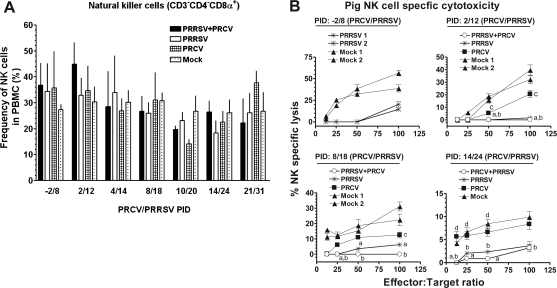
Innate immunity is the first line of defense and is essential for effective adaptive immune responses and protection against pathogens. To examine the systemic innate immune responses in the blood of PRCV/PRRSV co-infected pigs, the NK cell population and its cytotoxic function were analyzed. Only marginal changes were detected in the frequency of total NK cells (CD3−CD4−CD8+) in single-virus and co-infected pigs (Fig. 2A). The NK-cell-specific lysis seen at different time points post-infection indicated that PRCV infection alone resulted in only a 10–30% reduction in NK cell lytic activity, whereas PRRSV infection alone resulted in significant reductions (50–80%) at different post-infection days. In dual virus-infected pigs, NK cell-mediated cytotoxicity was synergistically reduced by 80–100% (Fig. 2B). The percentage reduction in NK-cell cytotoxicity was calculated in relation to mock-infected pigs (100%) at the respective post-infection days. The reductions in NK-cell cytotoxic function in dual-infected pigs was statistically significant on PRCV/PRRSV PID 2/12, 8/18, and 14/24, compared to mock-infected pigs, and compared to pigs infected with PRCV alone at PID 14/24. Significant reductions in NK-cell function were detected at a higher effector:target cell ratio in PRCV-only-infected pigs at PRCV/PRRSV PID 2/12 and 8/18, compared to mock-infected pigs. Thus, prior infection with PRRSV suppressed the innate NK-cell cytotoxic function in pigs, which resulted in a further synergistic reduction in NK-cell-mediated cytotoxicity following PRCV infection. Interestingly, the NK-cell cytotoxicity observed was independent of their frequency in virus-infected pigs.
FIG. 2.
Significant reductions occurred in NK-cell cytotoxicity despite a lack of significant changes in NK-cell populations following PRRSV and PRCV co-infection in pigs. (A) Percentages of CD3−CD4−CD8α+ lymphocytes (NK cells) in PBMCs of pigs were evaluated in mock-infected, PRRSV-infected, PRCV-infected, or dual-infected (PRCV/PRRSV) pigs on the indicated post-inoculation days (PID) by flow cytometric analysis. Each bar represents the mean ± SEM of NK cells from 4–8 pigs, and total numbers of pigs at each PRCV/PRRSV PID: −2/8 (n = 17); 2/12 (n = 18); 4/14 (n = 18); 8/18 (n = 20); 10/20 (n = 20); 14/24 (n = 21); and 21/31 (n = 21). (B) Percentages of NK-specific cytotoxicity were measured using pig PBMCs (effectors) harvested from mock-infected or infected pigs as described above against target cells (K-562). Effectors and targets at the different indicated ratios were cultured together, and the supernatants harvested were analyzed for amounts of LDH released using its substrate at 490 nm. Each line is from one pig, and each data point on the line is the mean of triplicate readings ± SEM at the respective E:T ratios tested. Altogether there were 19 pigs from one independent experimental trial (a total of four PIDs). A similar trend in NK-specific lysis was detected in another two independent experimental trials comprised of the same numbers of pigs. Statistical analyses were performed from the pooled results of all three independent experimental trials, and “a” denotes a statistically significant difference (p < 0.05) between the mock- and dual-infected groups; “b” denotes a statistically significant difference between the mock- and PRRSV-infected groups; “c” denotes a statistically significant difference between the mock- and PRCV-infected groups; and “d” denotes a statistically significant difference between the PRCV- and dual-infected groups, as analyzed by the nonparametric Kruskal-Wallis test.
Co-infection of pigs with PRRSV and PRCV resulted in elevated Th-1 and proinflammatory cytokines in pigs
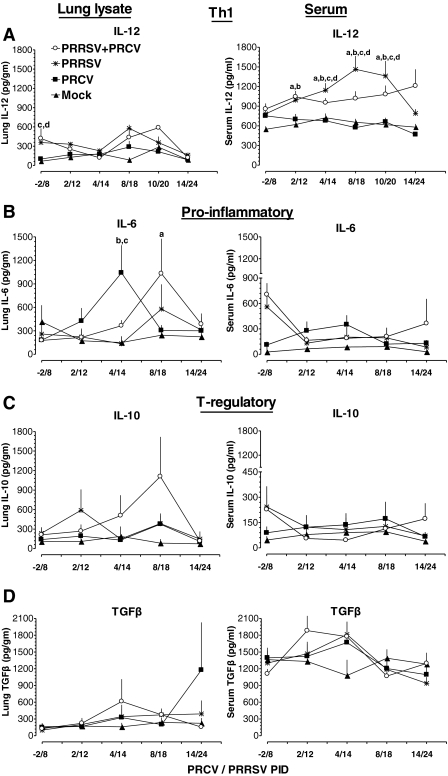
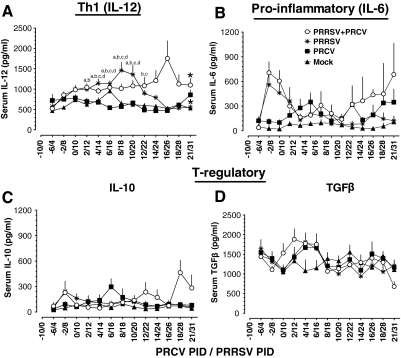
Effective adaptive immune responses are important for protection against viral infections. To measure Th-1 (IL-12) and proinflammatory (IL-6) cytokine responses in pigs infected with PRCV and/or PRRSV, we collected serum at 14 different time points over a period of 30 d post-infection. To measure cytokine responses in the lung, lung lysates were prepared from all of the euthanized pigs and used in the assay. In both lung and serum, levels of the Th-1 cytokine IL-12 in PRCV-only-infected pigs remained low compared to the other groups (Fig. 3A). In PRRSV-alone-infected pigs, IL-12 was detected at higher levels at both middle and later time points post-infection (PID 8–24) in both lung and serum compared to the PRCV-alone and mock-infected groups, and it was significantly higher at PID 14–20 (Fig. 3A). In dual virus–infected pigs, serum IL-12 levels were significantly higher at both early and later time points post-infection (PRCV/PRRSV PID 2/12 to 21/31), compared to the mock-infected and PRCV-alone-infected groups (Fig. 3A and 4A). In the lungs of dual virus–infected pigs, substantially higher levels of IL-12 were detected only during the middle period of infection (PRCV/PRRSV PID 8/18 and 10/20), compared to the mock-infected and PRCV-alone-infected groups (Fig. 3A).
FIG. 3.
Serum and lung cytokine levels in PRRSV- and PRCV-infected pigs. Pigs were mock infected or infected with the indicated viruses as described in the legend to Fig. 2. Serum samples and lung lysates were assayed for different cytokines by ELISA. (A) IL-12, (B) IL-6, (C) IL-10, and (D) TGF-β. Each data point represents the average cytokine levels of 5–8 pigs from each indicated PID ± SE. “a” denotes a statistically significant difference (p < 0.05) between the mock- and dual-infected groups; “b” denotes a statistically significant difference (p < 0.05) between the PRCV- and dual-infected groups; “c” denotes a statistically significant difference (p < 0.05) between the PRCV- and PRRSV-infected groups; and “d” denotes a statistically significant difference (p < 0.05) between the mock- and PRRSV-infected groups, as analyzed by the nonparametric Kruskal-Wallis test.
FIG. 4.
Serum cytokine levels in PRRSV- and PRCV-infected pigs. Pigs were mock-infected (n = 43), or infected with PRRSV (n = 39), PRCV (n = 48), or PRCV/PRRSV (n = 48), and serum was collected on the indicated post-inoculation days and assayed for different cytokines by ELISA. (A) IL-12, (B) IL-6, (C) IL-10, and (D) TGF-β. Each data point represents the average cytokine levels of 5–48 pig serum samples collected on the indicated post-inoculation days ± SEM. “a” denotes a statistically significant difference (p < 0.05) between the mock- and dual-infected groups; “b” denotes a statistically significant difference (p < 0.05) between the PRCV- and dual-infected groups; “c” denotes a statistically significant difference (p < 0.05) between the PRCV- and PRRSV-infected groups; and “d” denotes a statistically significant difference (p < 0.05) between the mock- and PRRSV-infected groups, as analyzed by the nonparametric Kruskal-Wallis test. Asterisks indicate a statistically significant difference for repeated sera collected from the respective pig groups (those infected with PRRSV alone and those infected with both viruses), as analyzed by repeated-measures ANOVA.
Interestingly, higher levels of the proinflammatory cytokine IL-6 were detected in both lungs and serum of pigs infected with PRCV alone on PRCV PID 2 and 4 than in the other three groups (Fig. 3B). In contrast, in pigs infected with PRRSV alone, IL-6 levels remained low in lungs and serum throughout the study period compared to other groups (Fig. 3B and 4B). However, in the dual virus–infected pigs, the IL-6 levels in the lungs were increased during the middle period of infection (PRCV/PRRSV PID 4/14 and 8/18), compared to both single-virus-infected groups (Fig. 3B).
Co-infection of pigs with PRRSV and PRCV resulted in elevated T-regulatory cytokines, IL-10, and TGF-β
The immunosuppressive cytokines IL-10 and TGF-β are important regulators of inflammation. In pigs infected with PRCV alone, IL-10 levels were low in serum at both early and later periods of infection compared to dual virus–infected pigs. But in pigs infected with PRRSV alone, higher levels of IL-10 were detected at PRRSV PID 4–12 in both serum and lungs, than in the other groups (Fig. 3C and 4C). Interestingly, in contrast, dual-infected pigs had higher levels of IL-10 during PRCV/PRRSV PID 2/12 to 8/18 in the lungs, and during PID 10/20 to 21/31 in the serum (Fig. 3C and 4C), than the other groups. TGF-β was detected at higher levels in PRCV-infected pigs on PRCV PID 4 in both lungs and serum than in mock-infected pigs, and on PRCV PID 14 in the lungs, than in the other groups (Fig. 3D). In dual-infected pigs, TGF-β levels were higher at PRCV/PRRSV PID 4/14 and 8/18 in lungs, and in serum at PID 2/12 to 4/12, than in the other groups (Fig. 3D). In dual virus–infected pigs, we detected increased TGF-β levels until PRCV/PRRSV PID 6/16, which were associated with concurrently low levels of the Th-1 cytokine IL-12, in pig serum. Subsequently, after PID 6/16, the opposite trend was observed (Figs. 3A and D and 4A and D). We have shown side-by-side comparisons of lung and serum cytokines at corresponding days (Fig. 3), and serum cytokine levels at 14 different time points post-infection (Fig. 4) separately, to better illustrate the mucosal (local) and systemic immune responses seen throughout the study period.
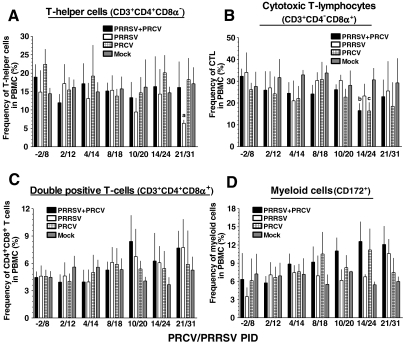
Dual-infected pigs had increased lymphocyte and myeloid cell populations
Flow cytometric analysis was performed to evaluate different lymphocyte subpopulations (lymphoid cells), and monocyte, macrophage, and granulocyte (myeloid cells) populations in PBMCs of mock- and virus-infected pigs. The amount of BAL cells collected from half of the lungs (the other half was used for the pathology studies) was insufficient to perform flow cytometry, so we used PBMCs for this purpose. We did not detect any significant changes in the frequency of T-helper cell populations in most of the groups, except for PRRSV-alone-infected pigs at PID 10/20 and 21/31 (p < 0.05), representing a substantial decrease compared to the other groups (Fig. 5A). The frequencies of cytotoxic T lymphocyte (CTL) populations in both single-virus and dual virus–infected pigs was lower at PID 4/14 and 14/24 (p < 0.05), than in mock-infected pigs (Fig. 5B). In swine, both activated and memory T-helper cells belong to the CD4+CD8+ double-positive population (37). Functional analysis suggested that some subsets of CD4+CD8α+ T cells also had cytolytic activity (22). Interestingly, increased CD4+CD8+ T-cell subpopulations were detected in both the single- and dual-infected pig groups from PRCV/PRRSV PID 10/20 to PID 21/31 compared to mock-infected pigs (Fig. 5C). No major changes were seen in the frequency of myeloid cells (CD172+) in PBMCs until PID 4/14, but thereafter substantial increases were seen, especially in dual-infected pigs, compared to other groups (Fig. 5D).
FIG. 5.
Variable frequencies of lymphoid and myeloid cell populations in PBMCs of pigs were detected following PRRSV and PRCV co-infection. Pigs were mock-infected or infected with the indicated viruses as described in the legend to Fig. 2. Percentages of CD3+ lymphocyte subpopulations in PBMCs of pigs on the indicated post-inoculation days (PID) were evaluated by flow cytometric analysis, and then further grouped based on CD4- and CD8-specific staining: (A) T-helper cells (CD3+CD4+CD8α−); (B) cytotoxic T lymphocytes (CD3+CD4−CD8α+); and (C) double-positive T cells (CD3+CD4+CD8α+). (D) Total myeloid cell populations (CD172+) in PBMCs were also evaluated by flow cytometric analysis. Each bar represents the mean ± SEM from 3–8 pigs, and the total numbers of pigs at each PRCV/PRRSV PID were: −2/8 (n = 17); 2/12 (n = 18); 4/14 (n = 18); 8/18 (n = 20); 10/20 (n = 20); 14/24 (n = 21); and 21/31 (n = 12). On each PID, 4–6 pigs were euthanized, representing all four treatment groups. “a” denotes a statistically significant difference (p < 0.05) between the PRRSV- and mock-infected groups; “b” denotes a statistically significant difference (p < 0.05) between the dual- and mock-infected groups; and “c” denotes a statistically significant difference (p < 0.05) between the PRCV- and mock-infected groups, as analyzed by the nonparametric Kruskal-Wallis test.
Due to large variations in cytokine levels among the individual outbred pigs, the differences were not statistically significant in many of the combinations, but the trends observed were consistent and reproducible within the independent trials and treatment groups.
Discussion
The innate immune responses are crucial to protect against local pathogens, and are also involved in the maintenance of homeostasis of mucosal tissues; dysregulation of these responses by repeated infections is likely to have a major impact on the outcome of an infectious disease (14). We hypothesized that a pre-existing immunomodulatory respiratory virus (e.g., PRRSV) infection compromises innate and adaptive immune responses, and exacerbates the severity of a subsequent mild respiratory virus (e.g., PRCV) infection.
Normally, NK cells are activated to mediate innate antiviral cytotoxic activity following infection. Cytotoxicity and cytokine production, both features of activated NK cells, do not necessarily coincide. Differential regulation of these functions has been demonstrated in virus-infected animals (12,33,42). The PRRSV suppresses production of IFN-α and other important cytokines at the site of infection (31), and even concurrent infection of PRRSV-infected pigs with TGEV failed to rescue IFN-α production, whereas alone, PRCV and even UV-treated TGEV induced strong IFN-α production (2,43,49). So far our knowledge related to NK-cell cytotoxicity in CoV-infected individuals is limited. In PRRSV-infected pigs a marginal increase in NK-cell frequency in BAL fluid was detected after 3 wk of infection (39), and in our study in PRRSV infected pigs suppressed blood NK-cell cytotoxicity was detected with no appreciable changes in their frequency in blood (20). A total innate immunosuppression was demonstrated in our viral co-infection model, whereby synergistic suppression of NK-cell cytotoxicity and IFN-γ production were detected, compared to either single-virus infection alone.
In our PRCV/PRRSV co-infection model, we evaluated the cytokine and cellular immune responses as a measure of adaptive immune responses. In the lungs of PRCV-infected pigs, we and others have detected more Th-1 cytokines at early time points post-infection (3). In PRCV/PRRSV co-infected pigs, increased Th-1, proinflammatory, and T-regulatory cytokines were detected. An elevated level of the proinflammatory cytokine (IL-6) was detected following PRCV infection of pigs (49), and also in the acute phase of SARS infection in humans (10). IL-6 has a pleiotropic nature, and is secreted by professional APCs, fibroblasts, epithelial cells, and astrocytes (11,21). This cytokine is found at increased levels at sites of inflammation. An interaction between IL-6 and macrophage colony-stimulating factor switches monocyte differentiation to macrophages rather than dendritic cells (11).
Co-infected pigs had a higher frequency of T-regulatory cytokines in lungs than in serum, suggestive of active immunomodulation at the site of infection. The proinflammatory cytokines (IL-6, TNF-α, IL-1, IL-8, and IFN-α) are pyrogenic (44,46). We detected higher levels of IL-6 at both the early and late stages of co-infection in both serum and lung, which were also associated with high fever and reduced body weight gain, in addition to more severe lung pathology (20). We also detected increased frequencies of CD4+CD8+ T lymphocytes and myeloid cells in the dual virus-infected pigs during the later stages of infection. This elevated myeloid cell pool may represent macrophages rather than dendritic cells, and was potentially mediated by IL-6. Indeed, the peak of PRRSV antigen in the lungs of dual virus–infected pigs was at PID 8/18, coinciding with the peak of IL-6, and more apoptotic alveolar macrophages were detected by in situ TUNEL assay in all dual-infected pigs (20).
The importance of cytokines as mediators of virus-induced inflammatory responses was recognized in this study and by others, and resulted in severe lung inflammation and disease (29,30). The mechanism of tolerance in the lung is governed by the production of IL-10, which drives the generation of IL-10-producing Tregs in draining lymph nodes, which are capable of suppressing subsequent responses to antigenic challenges at airway mucosal surfaces (1). The PRRSV has immunomodulatory effects and upregulates IL-10 production in the lungs of infected pigs (26). The sources of IL-10 in lung include monocytes, macrophages, and Tregs (6,9). Many viruses that can readily establish persistence induce IL-10 production. We also detected elevated IL-10 production in the lungs of dual-infected pigs (induced by PRRSV), with concomitant increased frequencies of myeloid cells and double-positive lymphocytes, compared to either of the single-virus-infected pig groups.
The reciprocal regulation of Th-1 and T-regulatory cytokines was evident in our study by increased IL-10 and TGF-β at PID 4/14, when Th-1 cytokine responses were the lowest. In this study, variability in immune responses was encountered, and this was attributed to the use of conventional outbred pigs, which reflects a similar scenario to that among the human population. We used large numbers of pigs in multiple independent trials in an attempt to assess the overall significance, and to mimic the variability observed in human viral co-infections. Although a trend for the experimental parameters assessed was reproducible among the different pig trials, statistically significant differences were only achieved for some cytokines analyzed at certain time points after the data from all six experiments were combined.
Both PRRSV and PRCV cause infections of the lung, but with different cell tropisms. In our study, the use of lung tissue homogenates to detect lung cytokines yielded satisfactory results. This method could be useful to assess lung cytokines, because cytokine levels in BAL fluid are usually low to undetectable for certain important cytokines. This approach with experimental animals may allow us to better understand the actual disease situation in lung infections constituting both acute and chronic respiratory viral diseases. Recently, cytokines in the lungs of swine influenza virus–infected pigs were analyzed using lung tissue homogenates (5).
Increased T-cell populations and myeloid cells in the dual-infected pigs might have contributed to the trend toward elevated Th-1 and proinflammatory cytokines, which was coincident with severe pneumonia. In addition, in the early stages of infection in lungs of dual-infected pigs, lower PRRSV titers and antigen were detected, compared to those seen with PRRSV infection alone (20). In the same pig group during the later stages of infection, elevated PRRSV titers and antigen were detected, which may be due to high levels of IL-10 or IL-6 in the lung, with the latter influencing the generation of macrophages (11,41), the target cells for PRRSV infection.
In conclusion, our findings suggest that antiviral innate immune responses mediated through NK cells and early IFN-α responses are important to reduce the viral burden of respiratory viral infections. Elevated levels of Th-1 proinflammatory and T-regulatory cytokines, and reduced Th-2 cytokine levels, in addition to continued lower NK cell-mediated cytotoxicity, resulted in persistent fever and reduced body weight gain in co-infected pigs. Measurements of cytokine levels and immune cell populations could serve as potential indicators of the progression of respiratory viral co-infections, and may provide more definitive approaches for treatment.
Acknowledgments
We thank Ms. Ruthi Patterson for tissue processing, and technical assistance with the LDH assay, ELISA, and statistical analysis; Ms. Kathryn Dodson for tissue processing and data analysis; Ms. Peggy Lewis and Dr. Yuxin Tang for help in tissue processing; and Dr. Xinsheng Zhang for his assistance with an initial animal trial. We also thank Dr. Juliette Hanson, Todd Root. Aaron Higgins, April Eyster, Morgan Chapman, Justin Dickey, and Thales DeNardo, for assistance with animal care and sample collection.
Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, grant R01 AI060739 to L.J. Saif, and the U.S. National Pork Checkoff to G.J.R.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Akbari O. DeKruyff RH. Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 2.Albina E. Carrat C. Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res. 1998;18:485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- 3.Atanasova S. Van Gucht S. Barbe F. Lefebvre DJ. Chiers K. Van Reeth K. Lung cell tropism and inflammatory cytokine-profile of porcine respiratory coronavirus infection. Open Vet Sci J. 2008;2:117–126. [Google Scholar]
- 4.Azevedo MS. Yuan L. Pouly S. Gonzales AM. Jeong KI. Nguyen TV. Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80:372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbe F. Atanasova K. Van Reeth K. Cytokines, acute phase proteins associated with acute swine influenza infection in pigs. Vet J. 2010 doi: 10.1016/j.tvjl.2009.12.012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglia M. Gianfrani C. Gregori S. Roncarolo MG. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann NY Acad Sci. 2004;1029:142–153. doi: 10.1196/annals.1309.031. [DOI] [PubMed] [Google Scholar]
- 7.Beyer J. Fichtner D. Schirrmeier H. Polster U. Weiland E. Wege H. Porcine reproductive and respiratory syndrome virus (PRRSV): kinetics of infection in lymphatic organs and lung. J Vet Med B Infect Dis Vet Public Health. 2000;47:9–25. doi: 10.1046/j.1439-0450.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biron CA. Nguyen KB. Pien GC. Cousens LP. Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 9.Charerntantanakul W. Platt R. Roth JA. Effects of porcine reproductive and respiratory syndrome virus-infected antigen-presenting cells on T cell activation and antiviral cytokine production. Viral Immunol. 2006;19:646–661. doi: 10.1089/vim.2006.19.646. [DOI] [PubMed] [Google Scholar]
- 10.Chien JY. Hsueh PR. Cheng WC. Yu CJ. Yang PC. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11:715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomarat P. Banchereau J. Davoust J. Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 12.Cousens LP. Orange JS. Su HC. Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox E. Hooyberghs J. Pensaert MB. Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res Vet Sci. 1990;48:165–169. doi: 10.1016/S0034-5288(18)30984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didierlaurent A. Goulding J. Hussell T. The impact of successive infections on the lung microenvironment. Immunology. 2007;122:457–465. doi: 10.1111/j.1365-2567.2007.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frieman M. Baric R. Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation. Microbiol Mol Biol Rev. 2008;72:672–685. doi: 10.1128/MMBR.00015-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerner W. Kaser T. Saalmuller A. Porcine T lymphocytes and NK cells—An update. Dev Comp Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Halbur PG. Miller LD. Paul PS. Meng XJ. Huffman EL. Andrews JJ. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of three-week-old colostrum-deprived pigs. Vet Pathol. 1995;32:200–204. doi: 10.1177/030098589503200218. [DOI] [PubMed] [Google Scholar]
- 18.Hill HT. Biwer JD. Wood RD. Wesley RD. Porcine respiratory coronavirus isolated from two U.S. swine herds. In: Neuzil TA, editor. Proceedings of the American Association of Swine Practitioners. American Association of Swine Practitioners; Des Moines, IA: pp. 333–335. [Google Scholar]
- 19.Jung K. Alekseev KP. Zhang X. Cheon DS. Vlasova AN. Saif LJ. Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus. J Virol. 2007;81:13681–13693. doi: 10.1128/JVI.01702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung K. Renukaradhya GJ. Alekseev KP. Fang Y. Tang Y. Saif LJ. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamimura D. Ishihara K. Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 22.Kitchen SG. Jones NR. LaForge S, et al. CD4 on CD8(+) T cells directly enhances effector function and is a target for HIV infection. Proc Natl Acad Sci USA. 2004;101:8727–8732. doi: 10.1073/pnas.0401500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klenerman P. Ludewig B. Virus scores a perfect 10. Nat Med. 2006;12:1246–1248. doi: 10.1038/nm1106-1246. [DOI] [PubMed] [Google Scholar]
- 24.Knoblock KF. Canning PC. Modulation of in vitro porcine natural killer cell activity by recombinant interleukin-1 alpha, interleukin-2 and interleukin-4. Immunology. 1992;76:299–304. [PMC free article] [PubMed] [Google Scholar]
- 25.Korzeniewski C. Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 26.Mateu E. Diaz I. The challenge of PRRS immunology. Vet J. 2008;177:345–351. doi: 10.1016/j.tvjl.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier WA. Galeota J. Osorio FA. Husmann RJ. Schnitzlein WM. Zuckermann FA. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003;309:18–31. doi: 10.1016/s0042-6822(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 28.Meulenberg JJ. Hulst MM. de Meijer EJ, et al. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murtaugh MA. Ma KN. Benson J. Curtin K. Caan B. Slattery ML. Antioxidants, carotenoids, and risk of rectal cancer. Am J Epidemiol. 2004;159:32–41. doi: 10.1093/aje/kwh013. [DOI] [PubMed] [Google Scholar]
- 30.Murtaugh MP. Baarsch MJ. Zhou Y. Scamurra RW. Lin G. Inflammatory cytokines in animal health and disease. Vet Immunol Immunopathol. 1996;4:45–55. doi: 10.1016/s0165-2427(96)05698-x. [DOI] [PubMed] [Google Scholar]
- 31.Murtaugh MP. Xiao Z. Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002;15:533–547. doi: 10.1089/088282402320914485. [DOI] [PubMed] [Google Scholar]
- 32.O'Toole D. Brown I. Bridges A. Cartwright SF. Pathogenicity of experimental infection with ‘pneumotropic’ porcine coronavirus. Res Vet Sci. 1989;47:23–29. doi: 10.1016/S0034-5288(18)31226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orange JS. Biron CA. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 34.Peiris JS. Lai ST. Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raymond C. Wilkie BN. Natural killer cell frequency and function in pigs selectively bred for high or low antibody and cell-mediated immune response: response to vaccination with modified live transmissible gastroenteritis virus. Nat Immun. 1998;16:18–26. doi: 10.1159/000069426. [DOI] [PubMed] [Google Scholar]
- 36.Rota PA. Oberste MS. Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 37.Saalmuller A. Werner T. Fachinger V. T-helper cells from naive to committed. Vet Immunol Immunopathol. 2002;87:137–145. doi: 10.1016/s0165-2427(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 38.Saif LJ. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev Sci Tech. 2004;23:643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- 39.Samsom JN. de Bruin TG. Voermans JJ. Meulenberg JJ. Pol JM. Bianchi AT. Changes of leukocyte phenotype and function in the broncho-alveolar lavage fluid of pigs infected with porcine reproductive and respiratory syndrome virus: a role for CD8(+) cells. J Gen Virol. 2000;81:497–505. doi: 10.1099/0022-1317-81-2-497. [DOI] [PubMed] [Google Scholar]
- 40.Suradhat S. Thanawongnuwech R. Poovorawan Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84:453–459. doi: 10.1099/vir.0.18698-0. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T. Akira S. Yoshida K, et al. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 42.Une C. Andersson J. Eloranta ML. Sunnemark D. Harris RA. Orn A. Enhancement of natural killer (NK) cell cytotoxicity and induction of NK cell-derived interferon-gamma (IFN-gamma) display different kinetics during experimental infection with Trypanosoma cruzi. Clin Exp Immunol. 2000;121:499–505. doi: 10.1046/j.1365-2249.2000.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Reeth K. Labarque G. Nauwynck H. Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res Vet Sci. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Reeth K. Nauwynck H. Proinflammatory cytokines and viral respiratory disease in pigs. Vet Res. 2000;31:187–213. doi: 10.1051/vetres:2000113. [DOI] [PubMed] [Google Scholar]
- 45.Van Reeth K. Nauwynck H. Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Reeth K. Van Gucht S. Pensaert M. Correlations between lung proinflammatory cytokine levels, virus replication, and disease after swine influenza virus challenge of vaccination-immune pigs. Viral Immunol. 2002;15:583–594. doi: 10.1089/088282402320914520. [DOI] [PubMed] [Google Scholar]
- 47.VanCott JL. Brim TA. Simkins RA. Saif LJ. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol. 1993;150:3990–4000. [PubMed] [Google Scholar]
- 48.Welch SK. Saif LJ. Monoclonal antibodies to a virulent strain of transmissible gastroenteritis virus: comparison of reactivity with virulent and attenuated virus. Arch Virol. 1988;101:221–235. doi: 10.1007/BF01311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X. Alekseev K. Jung K. Vlasova A. Hadya N. Saif LJ. Cytokine responses in porcine respiratory coronavirus-infected pigs treated with corticosteroids as a model for severe acute respiratory syndrome. J Virol. 2008;82:4420–4428. doi: 10.1128/JVI.02190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]