Abstract
Arginine vasopressin (AVP) has previously been shown to promote disruption of the blood−brain barrier, exacerbate edema, and augment the loss of neural tissue in various forms and models of brain injury. However, the mechanisms underlying these AVP actions are not well understood. These mechanisms were studied in AVP-deficient Brattleboro rats (Avpdi/di), and their parental Long-Evans strain, using a controlled cortical impact model of traumatic brain injury (TBI). The increased influx of inflammatory cells into the injured cortex in wild-type versus Avpdi/di rats was associated with higher levels of cortical synthesis of the CXC and CC chemokines found in wild-type versus Avpdi/di rats. These chemokines were predominantly produced by the cerebrovascular endothelium and astrocytes. In astrocyte and brain endothelial cell cultures, AVP acted synergistically with tumor necrosis factor-α (TNF-α) to increase the TNF-α-dependent production of CXC and CC chemokines. These AVP actions were mediated by c-Jun N-terminal kinase (JNK), as shown by Western blotting and pharmacological inhibition of JNK activity. The activity of JNK was increased in response to injury, and the differences in the magnitude of its post-traumatic activation between Avpdi/di and wild-type rats were observed. These data demonstrate that AVP plays an important role in exacerbating the brain inflammatory response to injury.
Key words: arginine vasopressin, c-Jun N-terminal kinase, CXC and CC chemokines, monocytes, neutrophils, traumatic brain injury
Introduction
Over the years experimental evidence has accumulated demonstrating that arginine vasopressin (AVP) plays an important pathophysiological role in brain injury. By using a genetic model of AVP deficiency, the Brattleboro rat, or employing a pharmacological approach in wild-type rodents involving the use of the V1a receptor (AVPR1A) or AVPR1A/V1b receptor (AVPR1B) antagonists, it has been shown that AVP promotes disruption of the blood−brain barrier (BBB), exacerbates cerebral edema, and augments the loss of neural tissue in various forms and models of brain injury, such as cerebral ischemia, intracerebral hemorrhage, and cryogenic or traumatic cortical injury (Bemana and Nagao, 1999; Dickinson and Betz, 1992; Rosenberg et al., 1992; Shuaib et al., 2002; Trabold et al., 2008; Vakili et al., 2005). The AVPR1A is broadly distributed throughout the rodent and primate brains (Ostrowski et al., 1994; Young et al., 1999). It is expressed in the cerebral cortex, hippocampus, and numerous subcortical regions. The V2 receptor (AVPR2) is not expressed in the adult rodent brain (Hirasawa et al., 1994), which is consistent with observations that AVPR2 antagonists were ineffective in abrogating the pathophysiological actions of AVP on an injured brain (Trabold et al., 2008; Vakili et al., 2005). Brain injury results in a dramatic increase in expression of AVPR1A on astrocytes and cerebrovascular endothelium (Szmydynger-Chodobska et al., 2004), suggesting that these two types of parenchymal cells are the primary targets for AVP in the injured brain. In rodent models of cerebral ischemia and traumatic brain injury (TBI), the augmented synthesis of AVP in the hypothalamus and elevated plasma hormone levels, as well as increased AVP expression in the injured cortex, have been demonstrated (Chang et al., 2006; Liu et al., 2000; Pascale et al., 2006). Consistent with data from animal experiments, clinical studies have shown that the concentrations of AVP in plasma and cerebrospinal fluid of patients with ischemic stroke, subarachnoid hemorrhage, and TBI are elevated (Barreca et al., 2001; Mather et al., 1981; Sørensen et al., 1985; Xu et al., 2007).
The aim of this study was to gain an insight into the cellular and molecular mechanisms underlying the pathophysiological actions of AVP on an injured brain. Using the controlled cortical impact (CCI) model of TBI in AVP-deficient Brattleboro rats, which were compared with their parental Long-Evans strain, we have shown that AVP augments the post-traumatic brain inflammatory response. Vasopressin acts synergistically with tumor necrosis factor-α (TNF-α) to increase the TNF-α-dependent production of proinflammatory mediators, such as the neutrophil chemoattractants CXCL1 and CXCL2, and the monocyte chemoattractant CCL2, in brain endothelium and astrocytes. These actions of AVP are mediated by c-Jun N-terminal kinase (JNK), and lead to increased influx of inflammatory cells into the injured brain.
Methods
Reagents and antibodies
ThermoScript RNase H– reverse transcriptase, and the RNase inhibitor RNaseOut, were obtained from Invitrogen (Carlsbad, CA). HotStart Taq DNA polymerase was purchased from Qiagen (Valencia, CA). Recombinant rat TNF-α was obtained from R&D Systems (Minneapolis, MN), whereas synthetic AVP was purchased from Bachem (Torrance, CA). A selective JNK inhibitor, SP600125, was obtained from Biomol (Plymouth Meeting, PA). Low-endotoxin bovine serum albumin (BSA; 81-068) was obtained from Millipore (Billerica, MA).
The following rabbit polyclonal antibodies were used: anti-rat CXCL1 (1 μg/ml), CXCL2 (2 μg/mL), and CCL2 (2 μg/mL), from Antigenix America (Huntington Station, NY), and anti-human phosphorylated (p)-JNK (0.2 μg/mL) from Biomol. The other rabbit polyclonal antibodies were anti-human von Willebrand factor (10 μg/mL) from Dako (Glostrup, Denmark), and anti-human ubiquitin C-terminal hydrolase/protein gene product 9.5 (PGP9.5, diluted 1:500) from Chemicon International (Temecula, CA). The rabbit monoclonal antibodies were as follows: anti-rat JNK3 (clone C05T, diluted 1:1000 or 1:4000) from Upstate (Lake Placid, NY), and anti-human c-Jun (clone 60A8; diluted 1:200 or 1:500) and activating transcription factor 2 (ATF2; clone 20F1, diluted 1:200), both from Cell Signaling Technology (Danvers, MA). The following mouse monoclonal antibodies were used: anti-human JNK1 (clone 228601) and JNK2 (clone 252320) (both used at 1 or 2 μg/mL) from R&D Systems; anti-human p-c-Jun (KM-1; 0.4 or 1 μg/mL) and p-ATF2 (Thr71; 1 μg/mL), from Santa Cruz Biotechnology (Santa Cruz, CA); and p-JNK (clone G9; used for immunoprecipitation) from Cell Signaling. The other mouse monoclonal antibodies were anti-human JNK (D-2; 0.2 μg/mL) and p-JNK (G-7; used for immunohistochemistry at 2 μg/mL), both from Santa Cruz Biotechnology; anti-rat RECA-1 (clone HIS52; 5 μg/mL) and anti-rat ED1 (1 μg/mL), both from Serotec (Oxford, U.K.); anti-porcine glial fibrillary acidic protein (GFAP; clone GA5; 0.1 μg/mL) from Chemicon; and anti-rat trans-Golgi network protein 38 (TGN38; clone 2F7.1; diluted 1:2000) from Novus Biologicals (Littleton, CO). For detection on Western blots, horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies from goat (Cell Signaling) were used diluted 1:5000. Secondary antibodies for immunohistochemistry were obtained from Molecular Probes (Eugene, OR). These were goat anti-rabbit and anti-mouse antibodies conjugated with Alexa Fluor 488 or 594. They were used at 2 μg/mL.
The rat model of TBI
Adult male Long-Evans and homozygous AVP-deficient Brattleboro rats (Avpdi/di) weighing 250–350 g (Harlan Laboratories, Indianapolis, IN) were used. The surgical and animal care procedures were in accordance with the guidelines of the Animal Care and Use Committee of Rhode Island Hospital, and conformed to international guidelines on the ethical use of animals. The CCI model of TBI was used as previously described (Szmydynger-Chodobska et al., 2009). In brief, the rats were anesthetized with intraperitoneal pentobarbital sodium (60 mg/kg) and a 4-mm craniotomy was performed on the right side of the skull to expose the dura, with the center of the opening located 3 mm posterior to the bregma and 2.5 mm lateral to the midline. The velocity of impact was 5 m/sec, and the duration of impact was 50 msec. The diameter of impactor's tip was 2.5 mm, and the depth of brain deformation was set at 3 mm. In sham-injured animals, the same surgical procedures were performed, but the injury was not produced. Samples of the cerebral cortex adjacent to the post-traumatic lesion, and those of the contralateral cortex from the corresponding region, were collected at 2, 4, and 6 h, and 1, 2, and 4 days after TBI. Samples of the cerebral cortex were also collected from sham-injured rats.
Cell cultures
Immortalized rat brain microvascular endothelial cells, SV-RBEC, and astrocytes, SV-NRA, (Garberg et al., 2005) were kindly provided by Dr. Danica Stanimirovic (Institute for Biological Sciences, National Research Council, Ottawa, Canada). The SV-RBEC cells were seeded into 6-well cell culture plates pre-coated with collagen I and grown to confluence, whereas the SV-NRA cells were seeded into 12- or 6-well plates pre-coated with poly-L-lysine and grown to subconfluence. The cells were cultured at 37°C in a humidified atmosphere of 5% CO2/95% air in high-glucose Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. The cells were serum starved in serum-free medium containing 0.1% BSA for 24 h prior to experimentation. On the day of the experiment, the cells were exposed to TNF-α or AVP alone, or the combination of TNF-α and AVP. To inhibit JNK activity, the cells were pre-incubated for 1 h with the selective JNK inhibitor SP600125, at a concentration of 50 μM. SP600125 was also added to culture media when the cells were exposed to TNF-α and AVP.
Real-time reverse-transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany). First-strand cDNAs were synthesized using oligo(dT)20 primer (50 pmol), and 15 U of ThermoScript RNase H– reverse transcriptase. The reverse transcription reactions also contained 40 U of RNase inhibitor, RNaseOut. For each reaction, 0.5 or 1 μg of total RNA was used, and the reactions were carried out for 1 h at 50°C. Real-time PCR was performed using TaqMan chemistry. The sequences of primers and TaqMan probes are given in Table 1. Cyclophilin A was used for the normalization of the data obtained. The 50-μL PCR reaction mixtures contained 0.2 mM mixed dNTPs, 0.2 μM each primer, 0.1 μM TaqMan probe, 5 mM MgCl2, 2 U (AVP) or 1 U (other genes) of HotStart Taq DNA polymerase, and 1/20 of the reverse transcription reaction product. For cyclophilin A, 1/2000 of the reverse transcription reaction product was used. The reaction mixtures were heated to 95°C for 15 min, and were then subjected to 45 cycles of denaturation (15 sec) at 96°C (AVP) or 94°C (other genes), and annealing/extension (60°C for 45–60 sec).
Table 1.
The Sequences of Primers and TaqMan Probes, and the Predicted Sizes of the PCR Products
| Gene | Primer/probe | Sequence | Predicted size of PCR product (base pairs) |
|---|---|---|---|
| CXCL1 | F | 5′-TGTCCAAAAGATGCTAAAGGG-3′ | 89 |
| R | 5′-AGAAGCCAGCGTTCACCA-3′ | ||
| P | 5′-AAGATAGATTGCACCGATGGCGTC-3′ | ||
| CXCL2 | F | 5′-CTGAACAAAGGCAAGGCTAAC-3′ | 120 |
| R | 5′-CATCAGGTACGATCCAGGCT-3′ | ||
| P | 5′-CCTGGAAAGGAAGAACATGGGCTC-3′ | ||
| CCL2 | F | 5′-TGTCTCAGCCAGATGCAGTTA-3′ | 181 |
| R | 5′-CATTCCTTATTGGGGTCAGC-3′ | ||
| P | 5′-ATGCCCCACTCACCTGCTGCTA-3′ | ||
| MMP9 | F | 5′-CTTGAAGTCTCAGAAGGTGGAT-3′ | 200 |
| R | 5′-GCAGGAGGTCATAGGTCACG-3′ | ||
| P | 5′-AGTTCTCTGGCGTGCCCTGGA-3′ | ||
| AVP | F | 5′-CCGAGTGTCGAGAGGGTTT-3′ | 135 |
| R | 5′-CAGAATCCACGGACTCTTGTG-3′ | ||
| P | 5′-CGGGAGCAGAGCAACGCCA-3′ | ||
| TNF-α | F | 5′-ATTTCCAACAACTACGATGCTC-3′ | 112 |
| R | 5′-GAGTTCCGAAAGCCCATTG-3′ | ||
| P | 5′-CTGGATTGCGGGCTGCTCAT-3′ | ||
| Cyclophilin A | F | 5′-GGTGAAAGAAGGCATGAGCA-3′ | 152 |
| R | 5′-GCTACAGAAGGAATGGTTTGATG-3′ | ||
| P | 5′-TTTGGGTCCAGGAATGGCAAGAC-3′ |
F, forward primer; R, reverse primer; P, probe; MMP9, matrix metalloproteinase 9; TNF-α, tumor necrosis factor-α; AVP, arginine vasopressin; PCR, polymerase chain reaction.
Immunoprecipitation and immunoblotting
Proteins from cortical tissue or cultured cells were extracted using isotonic lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.4], 2 mM EDTA, and 1% Triton X-100), containing protease inhibitors (1 mM benzamidine, 100 U/mL aprotinin, 20 μg/mL antipain, 20 μg/mL leupeptin, 1 μg/mL pepstatin A, and 1 mM PMSF), and phosphatase inhibitors (10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 mM β-glycerophosphate). For extraction of nuclear proteins, the samples of cortical tissue (∼30 mg) were homogenized using a Dounce homogenizer in 3 mL of buffer A (10 mM HEPES-potassium salt, 10 mM KCl, 1.5 mM MgCl2, and 0.5 mM DTT) containing protease and phosphatase inhibitors. The homogenate was incubated for 15 min on ice with constant agitation, and the nuclei were pelleted by centrifugation for 10 min at 1000g at 4°C. The pellet was resuspended in buffer B (20 mM HEPES-potassium salt, 420 mM NaCl, 1.5 mM MgCl2, 25% glycerol, 0.2 mM EDTA, and 0.5 mM DTT) containing protease and phosphatase inhibitors, and incubated at 4°C with constant agitation. After 45 min of salt extraction and subsequent centrifugation for 15 min at 14,000g at 4°C, the supernatant was collected and stored at −80°C.
For immunoprecipitation, 1 μg of rabbit (Biomol) or 2 μL of mouse (Cell Signaling) anti-pJNK antibody was initially incubated with 40 μL of agarose-immobilized protein G (Pierce, Rockford, IL) in isotonic lysis buffer containing 0.5% BSA for 4 h at 4°C with constant agitation. Then 120 μg of total protein from cortical tissue were added, and the incubation was continued overnight at 4°C. The recovered immune complexes were resolved via SDS-polyacrylamide gel electrophoresis (4–12%) under reducing conditions, and the proteins were transferred onto 0.2-μm nitrocellulose membranes (Invitrogen). After blocking with 5% ECL Advance blocking agent (GE Healthcare, Little Chalfont, U.K.) for 1 h at room temperature, the membranes were incubated with primary antibodies overnight at 4°C. The membranes were subsequently incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody for 1 h at room temperature. For detection, SuperSignal West Dura extended duration (Pierce), or ECL Advance (GE Healthcare) chemiluminescence substrate and the Bio Imaging System Chemi Genius2 (Syngene, Frederick, MD) were used. In the analysis of the optical density of the bands on the immunoblots, the levels of phosphorylated JNK, c-Jun, and ATF2 were normalized to the levels of total phosphorylated and non-phosphorylated forms of these proteins. This analysis was performed using ImageJ software (http://rsb.info.nih.gov/ij/).
Immunohistochemistry
Rats (2–4 animals per group) were perfused transcardially with ice-cold 0.9% NaCl, followed by ice-cold 4% paraformaldehyde in 0.05 M phosphate-buffered saline (pH 7.4). Vibratome- (100 μm) or cryostat-cut (10 μm) coronal brain sections were used. Immunohistochemical procedures were performed at room temperature, except for the incubation with primary antibodies, that was completed at 4°C. To minimize non-specific staining, the brain sections were incubated for 30 min with 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). Four percent of normal goat serum was also added when the sections were incubated with primary and secondary antibodies. After the initial blocking step, the sections were incubated overnight with primary antibodies, and then were incubated for 1 h with secondary antibodies. The sections were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
The assessment of the extent of edema, loss of neural tissue, and magnitude of influx of inflammatory cells
Brain water content was determined at 24 h post-TBI. The samples of the cerebral cortex adjacent to the post-traumatic lesion, and those of the contralateral cortex from the corresponding region, were quickly collected. Changes in brain water content were assessed by measuring the difference between the wet and dry weight of the cortical tissue.
The loss of neural tissue was assessed at 4 weeks post-TBI. The rats were perfused transcardially with 0.9% NaCl, followed by 4% paraformaldehyde, as described above. The brains were removed and post-fixed for 2 days in 4% paraformaldehyde. Coronal brain sections were then cut on a vibratome at 100 μm and stained with cresyl violet. The slides were scanned and the images were analyzed using ImageJ software. The volume of the post-traumatic lesion was assessed as previously described (Longhi et al., 2009).
To assess the magnitude of post-traumatic influx of neutrophils, the myeloperoxidase (MPO) activity assay was performed as previously described (Szmydynger-Chodobska et al., 2009). In brief, at 24 h post-TBI the rats were perfused transcardially with ice-cold 0.9% NaCl and the cortical samples were collected. The activity of MPO was assayed spectrophotometrically. One unit of MPO activity was defined as the degradation of 1 μmol of H2O2 per min. The results are expressed as units of MPO activity per gram of tissue.
The magnitude of influx of monocytes into the traumatized cortex was assessed at 24 h post-TBI. The brains were processed for immunohistochemistry as described above. Coronal brain sections were stained with anti-ED1 antibody, and the images of the ipsilateral cerebral cortex were acquired using a fluorescence microscope. The number of ED1-positive cells per 1000 μm2 were counted in randomly selected areas in the cerebral cortex adjacent to the post-traumatic lesion.
Statistical analysis
For statistical evaluation of data, except those on monocyte influx, ANOVA was used, followed by the Newman-Keuls test for multiple comparisons among means. The Mann-Whitney U test was employed to evaluate the data on monocyte influx. The results are presented as mean values ± SEM, with p < 0.05 considered statistically significant.
Results
Vasopressin deficiency results in a reduction of post-traumatic edema, decreased loss of neural tissue, and smaller magnitude of influx of inflammatory cells into the injured cortex
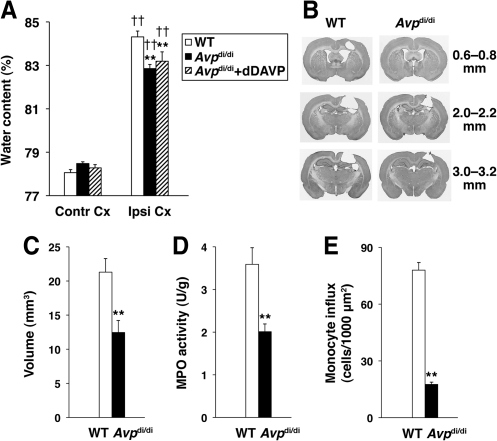
In this study, the homozygous AVP-deficient Brattleboro rats (Avpdi/di) were compared with their parental Long-Evans strain. The extent of edema in the cerebral cortex adjacent to the post-traumatic lesion, measured as an increase in brain water content at 24 h post-TBI, was smaller by 30% in Avpdi/di than in wild-type rats (Fig. 1A). Since fluid balance in the Brattleboro rats is altered compared to wild-type animals (DePasquale et al., 1989), we investigated whether this factor would have an effect on the formation of edema in AVP-deficient rats. To correct their fluid balance, Avpdi/di rats were implanted subcutaneously with osmotic minipumps to deliver dDAVP, an AVP analog selective for AVPR2 in the kidney, at 50 ng/kg/h, starting 6 days prior to injury. Similar results were obtained in this experimental group and the Avpdi/di rats, that were not treated with dDAVP (Fig. 1A), suggesting that altered fluid balance, as observed in Avpdi/di rats, does not have an effect on the formation of post-traumatic brain edema in these animals. In addition to decreased formation of edema, we found that the loss of neural tissue, which predominantly involved the ipsilateral cerebral cortex, was reduced by 42% in Avpdi/di versus wild-type rats (Figs. 1B and C).
FIG. 1.
A comparison of arginine vasopressin (AVP)-deficient Brattleboro rats (Avpdi/di) with their parental Long-Evans strain. The controlled cortical impact (CCI) model of traumatic brain injury (TBI) was used. (A) Changes in water content in the injured cortex adjacent to the post-traumatic lesion measured at 24 h post-TBI (n = 13–15 per group). To correct their fluid balance, the Brattleboro rats were infused with dDAVP, an AVP analog selective for AVPR2 in the kidney, at 50 ng/kg/h, starting 6 days prior to injury. Note that similar results were obtained in this experimental group when compared to Avpdi/di rats that were not treated with dDAVP, suggesting that altered fluid balance, as observed in Avpdi/di rats, does not have an effect on the formation of post-traumatic brain edema in these animals (WT, wild-type; Contr Cx, contralateral cortex; Ipsl Cx, ipsilateral cortex). (B) Coronal brain sections cut at various distances from the bregma. Note that the injury in the CCI model primarily involves the ipsilateral cerebral cortex (WT, wild-type). (C) The extent of post-traumatic loss of neural tissue in Avpdi/di versus wild-type (WT) rats (n = 7 per group). (D) The magnitude of neutrophil influx into the ipsilateral cortex at 24 h post-TBI in Avpdi/di versus wild-type (WT) rats as assessed by the myeloperoxidase (MPO) activity assay (n = 7 per group). The MPO activity in the contralateral cortex was undetectable. (E) The magnitude of monocyte influx into the ipsilateral cortex at 24 h post-TBI in Avpdi/di versus wild-type (WT) rats (n = 4 per group). Monocytes were stained with anti-ED1 antibody on coronal brain sections. These inflammatory cells were not detected in the contralateral cortex (**p < 0.01 for Avpdi/di versus wild-type rats; ††p < 0.01 for the ipsilateral versus the contralateral cortex).
Both neutrophils and monocytes have previously been shown to play an important role in exacerbating edema and augmenting the loss of neural tissue in the injured brain (Chen et al., 2003; Dimitrijevic et al., 2007; Neumann et al., 2008; Schoettle et al., 1990). Therefore, we hypothesized that the decreased formation of post-traumatic edema and reduced loss of neural tissue observed in Avpdi/di versus wild-type rats is related to a smaller magnitude of post-traumatic influx of inflammatory cells in the AVP-deficient rats compared to wild-type animals. As expected, the magnitude of neutrophil influx into the injured cortex, as assessed by measuring the activity of MPO at 24 h post-TBI, was found to be 44% greater in wild-type versus Avpdi/di rats (Fig. 1D). Similarly, the magnitude of influx of monocytes into the traumatized cortex at 24 h post-TBI was 77% greater in wild-type rats than in AVP-deficient animals (Fig. 1E).
Vasopressin deficiency is associated with reduced cortical synthesis of neutrophil and monocyte chemoattractants in response to injury
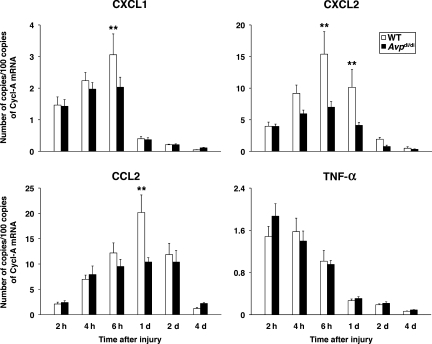
We have previously shown that the synthesis of CXC chemokines in the injured cortex is rapidly increased after the impact, peaking at 6 h post-TBI (Szmydynger-Chodobska et al., 2009). By comparison, the expression of CXC chemokines in the contralateral cortex was low and did not change in response to injury. In the present study we examined changes in expression of two neutrophil chemoattractants, CXCL1 (also known as cytokine-induced neutrophil chemoattractant-1 or CINC-1), and CXCL2 (also known as macrophage inflammatory protein-2 or MIP-2). The cerebral cortex adjacent to the post-traumatic lesion was analyzed. Consistent with the greater magnitude of neutrophil influx observed in wild-type versus AVP-deficient rats, we found that at 6–24 h post-TBI, the levels of mRNA for CXCL1 and CXCL2 in the ipsilateral cortex were significantly higher in wild-type than in Avpdi/di rats (Fig. 2). Similarly, we found that a greater magnitude of monocyte influx was seen in wild-type than in Avpdi/di rats, and that this was associated with a significantly higher level of expression of CCL2 (also known as monocyte chemoattractant protein-1 or MCP-1) in the ipsilateral cortex of wild-type rats than in AVP-deficient animals at 24 h post-TBI (Fig. 2). Overall, the levels of cortical expression of CXC and CC chemokines were higher by 34–55% in wild-type than in Avpdi/di rats.
FIG. 2.
Post-traumatic synthesis of proinflammatory mediators in the ipsilateral cortex of wild-type (WT) versus Avpdi/di rats as analyzed by real-time reverse-transcriptase polymerase chain reaction (n = 6 per rat strain per time point). The numbers of copies of transcripts for each gene relative to the message for cyclophilin A are shown. Note that differences in the cortical expression of the chemokines CXCL1, CXCL2, and CCL2 between wild-type and arginine vasopressin (AVP)-deficient rats were observed at 6–24 h post-TBI. The levels of expression of these chemokines in the contralateral cortex were low, and did not change in response to injury. Note that no differences in cortical expression of TNF-α were found between wild-type and Avpdi/di rats (**p < 0.01 for wild-type versus Avpdi/di rats; TBI, traumatic brain injury; Cycl-A, cyclophilin A).
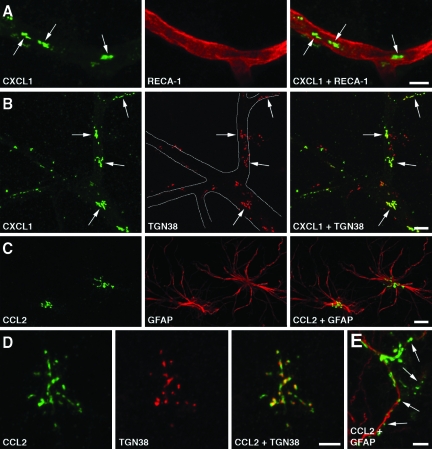
In the injured brain, CXC and CC chemokines are synthesized by the cerebrovascular endothelium and astrocytes
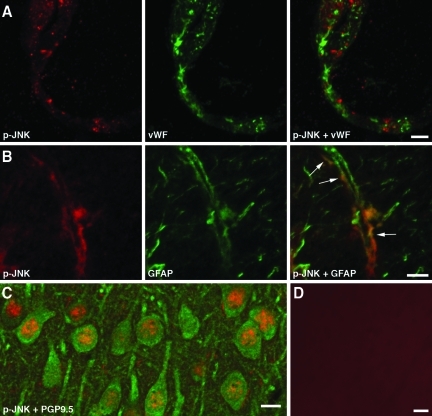
Immunohistochemical analysis of the injured cortex of wild-type rats at 6 h post-TBI showed that the immunoreactive products for CXCL1, CXCL2, and CCL2 were predominantly associated with cortical blood vessels (Fig. 3). Both mid-size vessels and cortical microvessels were found to be immunopositive for the CXC and CC chemokines. This distinct pattern of immunopositive staining for CXC and CC chemokines was not associated with perivascular macrophages (as assessed by double immunostaining with anti-ED2 monoclonal antibody), or pericytes (as assessed by double immunostaining with monoclonal antibody to NG2 chondroitin sulfate proteoglycan), and did not co-localize with α-smooth muscle actin (data not shown), suggesting that these chemokines are produced by the cerebrovascular endothelium. The chemokine-immunoreactive product co-localized with the Golgi complex, as demonstrated by double immunostaining with monoclonal antibody to the Golgi marker, TGN38 (Fig. 3B). The localization of CXC and CC chemokines to the Golgi complex is consistent with the increased synthesis of these proteins and their secretion after injury. CCL2, but not CXCL1 or CXCL2, was found to also be highly expressed in cortical astrocytes (Fig. 3C). In both, brain endothelium and astrocytes, the CCL2-immunoreactive product co-localized with the Golgi complex (Fig. 3D). These observations suggest that the higher levels of cortical expression of CXC and CC chemokines found in wild-type versus Avpdi/di rats reflect the increased chemokine synthesis seen in the cerebrovascular endothelium and astrocytes of wild-type animals. At 24 h post-TBI, the astrocytes continued to express CCL2, but the CCL2-immunopositive product was predominantly localized extracellularly, along the astrocyte processes (Fig. 3E). Since chemokines have the ability to bind to heparan sulfates (Kuschert et al., 1999), it is possible that the CCL2-immunopositive staining observed at 1 day post-TBI reflected the binding of this protein to heparan sulfate proteoglycans expressed on the surface of astrocytic cells and/or within the extracellular matrix. Unlike astrocytes, cortical microvessels no longer expressed CXC or CC chemokines at 1 day after TBI. Consistent with the previous mRNA analysis (Szmydynger-Chodobska et al., 2009), no CXCL1-, CXCL2-, or CCL2-immunopositive staining was observed in the contralateral cortex (data not shown).
FIG. 3.
Immunohistochemical analysis of injured brains from Long-Evans rats. Confocal microscopy images are shown. (A) The immunoreactive product for CXCL1 (arrows) associated with microvessels in the cerebral cortex ipsilateral to injury at 6 h post-TBI. Double immunostaining with an antibody to RECA-1, a marker for vascular endothelium, is shown. Similar results were obtained for CXCL2 and CCL2. (B) Double immunostaining with an antibody to the Golgi marker, TGN38, demonstrates that the CXCL1-immunoreactive product in cortical microvessels (outlined) is predominantly associated with the Golgi complex (arrows). (C) The ipsilateral cortex at 6 h post-TBI. Expression of CCL2 in astrocytes is shown by double immunostaining with an antibody to the astrocyte marker GFAP. (D) CCL2 expressed in cortical astrocytes at 6 h post-TBI co-localizes with the Golgi complex. (E) Astrocytic expression of CCL2 at 24 h post-TBI. Note that at this time point after injury, the CCL2-immunopositive product is predominantly localized extracellularly along the astrocyte processes (arrows). This pattern of CCL2-immunopositive staining likely reflects the binding of this secreted protein to heparan sulfate proteoglycans expressed on the surface of astrocytic cells and/or within the extracellular matrix (scale bars = 10 μm in A–C, 5 μm in D and E; GFAP, glial fibrillary acidic protein; TBI, traumatic brain injury; TGN38, trans-Golgi network protein 38).
Vasopressin amplifies the TNF-α-dependent production of CXC and CC chemokines in astrocyte and brain endothelial cell cultures
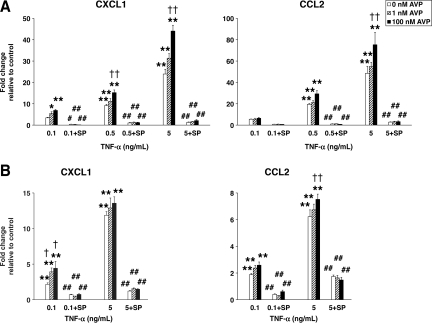
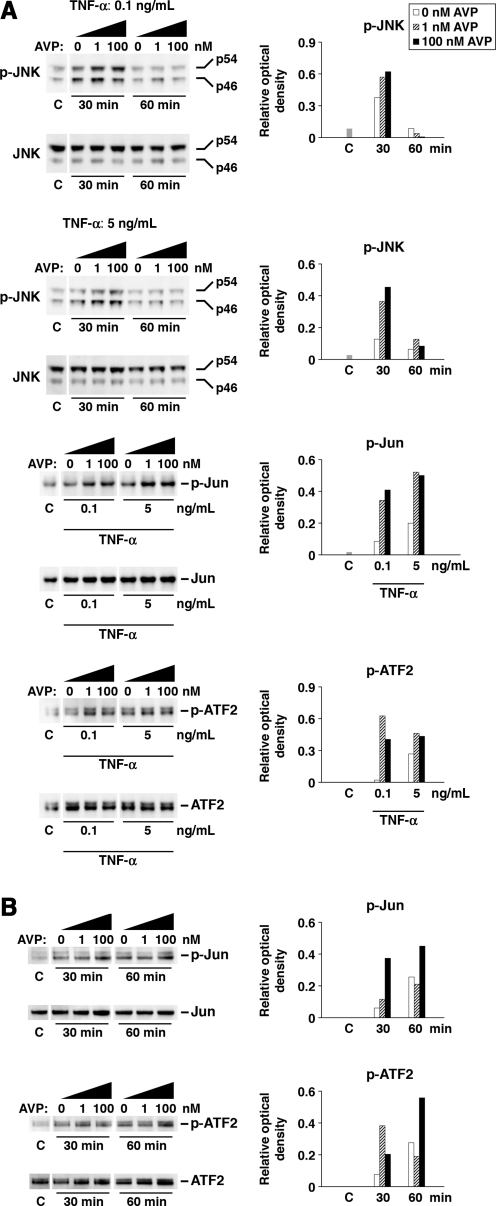
We next investigated the ability of AVP to stimulate chemokine synthesis in astrocyte and brain endothelial cell cultures. In the astrocytic cell line, SV-NRA, AVP at 10–7 M produced only a moderate increase in CXCL1 and CCL2 expression (3.3- and 2.3-fold, respectively; p < 0.01). Therefore, we tested the hypothesis that AVP acts synergistically with TNF-α to augment the TNF-α-dependent induction of chemokine synthesis. This cytokine is a well-known inducer of chemokine synthesis in a variety of cells, and the levels of its post-traumatic expression in the injured cortex of wild-type and Avpdi/di rats were similar (Fig. 2). A significantly higher increase in CXCL1 and CCL2 synthesis was seen after the SV-NRA cells were exposed to a combination of TNF-α and AVP, compared to the increase in chemokine synthesis seen in these cells in response to TNF-α alone (Fig. 4A). Similarly, AVP augmented the TNF-α-dependent production of CXC and CC chemokines in the brain endothelial cell line, SV-RBEC (Fig. 4B), although in these cells, AVP alone did not induce chemokine synthesis. These in vitro data are consistent with the results obtained in a rat model of TBI.
FIG. 4.
The synergistic interactions between TNF-α and AVP in astrocyte and brain endothelial cell cultures as assessed by real-time reverse-transcriptase polymerase chain reaction analysis. (A) The synthesis of CXC and CC chemokines in an astrocytic cell line, SV-NRA (n = 4–6 per group). The cells were incubated for 1 h with either TNF-α alone, or with a combination of TNF-α and AVP. A selective inhibitor of JNK activity, SP600125 (SP), was used at a concentration of 50 μM. Note that SP600125 abolished the induction of the chemokine synthesis observed in response to TNF-α alone, or to a combination of TNF-α and AVP. AVP (100 nM) alone produced only a moderate increase in CXCL1 and CCL2 expression (3.3- and 2.3-fold, respectively; p < 0.01), which was completely eliminated by SP600125 (p < 0.01). (B) The synthesis of CXC and CC chemokines in the brain endothelial cell line, SV-RBEC (n = 4–6 per group). The protocols used were similar to those used in the experiments on SV-NRA cells. In contrast to the astrocytic cell line, in SV-RBEC cells AVP alone did not induce chemokine synthesis (*p < 0.05, **p < 0.01 for treatment versus controls; †p < 0.05, ††p < 0.01 for TNF-α + AVP versus TNF-α alone; #p < 0.05, ##p < 0.01 for incubation with SP600125 versus incubation without SP600125; AVP, arginine vasopressin; TNF-α, tumor necrosis factor-α; JNK, c-Jun N-terminal kinase).
The synergistic interactions between TNF-α and AVP are mediated by JNK
The JNK signaling cascade is one of the major signal transduction pathways activated by TNF-α, and this signaling cascade plays an important role in regulating the expression of CXC and CC chemokines (Hanazawa et al., 1993; Kim et al., 2003; Wang et al., 2006). Accordingly, we investigated whether the synergistic interactions between TNF-α and AVP are mediated by JNK. A selective inhibitor of JNK activity, SP600125, was found to completely eliminate the AVP-dependent induction of CXCL1 and CCL2 synthesis in the SV-NRA cells (p < 0.01). In addition, in both astrocyte and endothelial cell cultures, SP600125 abolished the induction of chemokine synthesis seen in response to TNF-α alone, or to a combination of TNF-α and AVP (Fig. 4A and B).
The results obtained with the use of a pharmacological approach were confirmed by the Western blot analysis. This latter analysis demonstrated the higher level of activation (phosphorylation) of JNK in the SV-NRA cells seen in response to a combination of TNF-α and AVP, compared to that seen in response to TNF-α alone (Fig. 5A). We then investigated whether AVP also augments the activation (phosphorylation) of the transcription factors c-Jun and ATF2, the two major targets for JNK. c-Jun and ATF2, in combination with other transcription factors, form activating protein 1 (AP-1), a sequence-specific transcriptional activator that regulates the expression of a number of genes, including those encoding CXC and CC chemokines. In both the SV-NRA and SV-RBEC cells, c-Jun and ATF2 were activated to a greater degree in response to a combination of TNF-α and AVP than in response to TNF-α alone (Fig. 5A and B).
FIG. 5.
The synergistic interactions between TNF-α and AVP in astrocyte and brain endothelial cell cultures as assessed by Western blot analysis. The ratios of optical density of bands for phosphorylated proteins over the optical density of bands for the total proteins are shown. (A) The activation of the JNK signaling cascade in SV-NRA cells. The cells were exposed to either TNF-α alone or a combination of TNF-α and AVP. The activation of the transcription factors, c-Jun and ATF2, was analyzed at 1 h after the exposure to TNF-α or TNF-α and AVP. For each lane, 30 μg of total protein was loaded. The representative immunoblots are shown based on three independent experiments. (B) The activation of the JNK signaling cascade in SV-RBEC cells. Similar to the astrocytic cells, the brain endothelial cells were exposed to either TNF-α alone or a combination of TNF-α and AVP. TNF-α was used at a concentration of 5 ng/mL. For each lane, 15 μg of total protein was loaded. The representative immunoblots are shown based on three independent experiments (AVP, arginine vasopressin; TNF-α, tumor necrosis factor-α; JNK, c-Jun N-terminal kinase; C, control).
c-Jun N-terminal kinase is activated in response to injury, and the magnitude of its activation is regulated by AVP
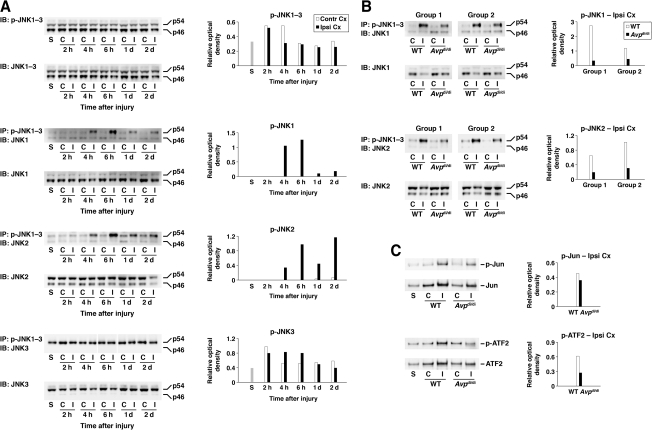
Three genes, JNK1, JNK2, and JNK3, encode the JNK proteins, and alternative splicing yields 10 isoforms of JNK with molecular weights of 46 and 54 kDa (p46 and p54, respectively; Waetzig and Herdegen, 2005). Using a combination of immunoprecipitation and Western blotting, we found that in wild-type rats the injury resulted in significant activation of JNK1 and JNK2 (predominantly the p54 isoforms) in the ipsilateral cortex starting at 4 h post-TBI (Fig. 6A). The level of activation of JNK1 peaked at 6 h after impact, but it had decreased rather rapidly by 1 and 2 days post-TBI. In comparison, the activity of JNK2 was maintained at a relatively high level at 1 and 2 days after TBI. The activity of JNK1 and JNK2 in the contralateral cortex and in the cortices of sham-injured animals was low. In contrast to JNK1 and JNK2, the activity of JNK3 in the contralateral and ipsilateral cortex, and in the cortices of sham-injured animals was high. In addition, the impact did not appear to have any major effect on activity of JNK3 in the ipsilateral cortex. When an antibody that recognizes the phosphorylated forms of all three JNKs was used on immunoblots, no difference was seen in the level of JNK activation between the ipsilateral and contralateral cortices, suggesting that JNK3 is the predominant JNK protein expressed in the brain. Consistent with this idea, the immunohistochemical analysis of brain tissue with anti-p-JNK antibody demonstrated high levels of JNK activation in cortical neurons, not only in the injured rats, but also in sham-injured and intact animals (Fig. 7C). Using the same antibody, we also found that at 6 h post-TBI, there was a substantial increase in JNK activity in cortical microvessels and astrocytes ipsilateral to injury (Fig. 7A and B). Such activation was not observed in the contralateral cortex or in the cortex from sham-injured rats (data not shown). These data are consistent with observation that after injury, CXC and CC chemokines are predominantly expressed in cortical microvessels and astrocytes (Fig. 3A–E).
FIG. 6.
The activation of the JNK signaling cascade in the injured cortex and the role of AVP in this activation. In these optical density graphs are shown the ratios of optical density of bands for phosphorylated proteins over the optical density of bands for the total proteins. (A) Immunoprecipitation/Western blot analysis in Long-Evans rats. Initially, anti-p-JNK antibody that recognizes all three JNK proteins was used. For each lane, 20 μg of total protein was loaded. Note that with this antibody no differences were seen between the level of activation of JNK in the ipsilateral and contralateral cortices. Immunoprecipitation (120 μg of total protein) with anti-pJNK1–3 antibody, followed by immunoblotting with JNK1-, JNK2-, and JNK3-specific antibodies demonstrated significant activation of JNK1 and JNK2 in the ipsilateral cortex in response to injury. Note that JNK3 is constitutively activated in the cerebral cortex, and that the impact does not appear to have any major effect on activity of this kinase. Similar results were obtained in a separate group of animals. (B) Differences in the magnitude of activation of JNK1 and JNK2 between wild-type and AVP-deficient rats. Cortical samples collected at 6 h post-TBI (a time point at which a significant increase in the level of activation of JNK1 and JNK2 was observed) were analyzed in two separate groups of animals. (C) Shown are differences in the magnitude of activation of the transcription factors, c-Jun and ATF2, between wild-type and Avpdi/di rats assessed at 6 h post-TBI. In these experiments, nuclear fractions isolated from cortical tissue were analyzed. For each lane, 5 μg of total protein was loaded. Note that the injury not only resulted in a significant increase in the level of activation of c-Jun and ATF2, but it also increased their expression. Similar results were obtained in a separate group of animals (C and Contra Cx, contralateral cortex; I and Ipsi Cx, ipsilateral cortex; S, sham; WT, wild-type; AVP, arginine vasopressin; JNK, c-Jun N-terminal kinase; IP, immunoprecipitation; IB, immunoblotting).
FIG. 7.
Immunohistochemical analysis of the injured brains from Long-Evans rats. Confocal microscopy images are shown. (A) Image of the ipsilateral cortex at 6 h post-TBI, showing localization of activated (phosphorylated) JNK to a cortical microvessel. An anti-von Willebrand factor (vWF) antibody was used for double staining with p-JNK. (B) Shown here is expression of p-JNK in astrocyte processes (arrows), associated with a cortical microvessel at 6 h post-TBI. (C) There was a high level of expression of p-JNK in cortical neurons in a sham-injured rat. Double immunostaining with an antibody to neuronal marker, PGP9.5, is shown. (D) Image showing negative control staining for p-JNK. In these experiments, the brain sections were incubated with anti-p-JNK antibody that had been pre-absorbed overnight with the immunogenic peptide at 100 μg/mL (scale bar = 5 μm in A and B, 10 μm in C, 20 μm in D; TBI, traumatic brain injury; JNK, c-Jun N-terminal kinase).
Trauma not only resulted in increased activity of JNK1 and JNK2 in the injured cortex of wild-type animals, but we also observed differences in the magnitude of their post-traumatic activation between Avpdi/di and wild-type rats (Fig. 6B). In addition, higher levels of activation of c-Jun and ATF2 in the injured cortex were found in wild-type versus Avpdi/di rats (Fig. 6C). These findings are in accord with the results from the in vitro studies, and provide further evidence for the AVP-mediated activation of the JNK signaling pathway.
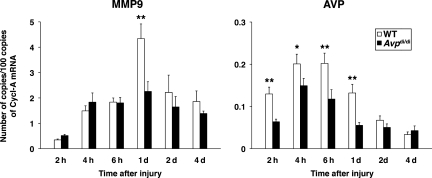
We also examined whether there is a difference in post-traumatic expression of matrix metalloproteinase 9 (MMP9) between Avpdi/di and wild-type rats. The expression of the Mmp9 gene is known to be regulated by JNK/AP-1 (Sato and Seiki, 1993), and MMP9 has previously been shown to be synthesized by the cerebrovascular endothelium and astrocytes (Rosenberg et al., 2001). The level of MMP9 mRNA assessed in the ipsilateral cortex at 24 h post-TBI was found to be higher by 48% in wild-type rats than in AVP-deficient animals (Fig. 8). In addition, we found a much larger increase in mRNA for AVP in the injured cortex of wild-type than in Avpdi/di rats at multiple time points after injury (Fig. 8). This was consistent with the AP-1-dependent regulation of expression of the Avp gene (Yoshida et al., 2006), and suggests that AVP may act to augment its own synthesis in the injured cortex.
FIG. 8.
Post-traumatic synthesis of MMP9 and AVP in the ipsilateral cortex of wild-type versus Avpdi/di rats as analyzed by real-time reverse-transcriptase polymerase chain reaction (n = 6 per rat strain per time point). The numbers of copies of transcripts for each gene relative to the message for cyclophilin A are shown. A much larger increase was seen in the message for AVP in the injured cortex of wild-type than in Avpdi/di rats, which is consistent with the AP-1-dependent regulation of expression of the Avp gene (*p < 0.05, **p < 0.01 for wild-type versus Avpdi/di rats; Cycl-A, cyclophilin A; WT, wild-type; MMP9, matrix metalloproteinase 9; AVP, arginine vasopressin).
Discussion
A number of animal studies have demonstrated that in various forms of brain injury, such as cerebral ischemia, intracerebral hemorrhage, and TBI, AVP is a significant factor involved in disruption of the BBB, the formation of cerebral edema, and post-ischemic or post-traumatic loss of neural tissue (Bemana and Nagao, 1999; Dickinson and Betz, 1992; Rosenberg et al., 1992; Shuaib et al., 2002; Trabold et al., 2008; Vakili et al., 2005). These consistent findings in various forms of brain injury suggest that there are common mechanisms underlying the actions of AVP on the injured brain. In the present study, a rodent model of AVP deficiency, the Brattleboro rat, which has a single nucleotide deletion in the Avp gene (Schmale and Richter, 1984), was employed. Our data on the AVP-dependent formation of edema and post-traumatic loss of neural tissue are similar to recently reported results obtained in a mouse model of TBI, in which an AVPR1A/AVPR1B antagonist was used (Trabold et al., 2008). These similar results, produced by applying a pharmacological approach in wild-type animals, and by employing a genetic model of AVP deficiency, provide further evidence for the pathophysiological actions of AVP on the injured brain.
The cellular and molecular mechanisms underlying the increased formation of edema and augmented loss of neural tissue seen in brain-injured wild-type versus Avpdi/di rats are not fully understood. It is possible that the increased influx of neutrophils and monocytes, which is seen more in the traumatized cortex of wild-type than in Avpdi/di animals, plays a mediatory role in the pathophysiological actions of AVP. Indeed, neutrophils have previously been reported to exacerbate the formation of post-traumatic brain edema (Schoettle et al., 1990), and more recent studies have shown that these inflammatory cells are highly toxic to vulnerable neurons (Neumann et al., 2008). Similarly, invading monocytes have been demonstrated to significantly contribute to the formation of edema and loss of neural tissue seen in rodent models of ischemic brain injury (Chen et al., 2003; Dimitrijevic et al., 2007). Consistent with these findings, treatments directed at countering this neutrophil and monocyte influx into the injured brain have shown beneficial therapeutic effects (Beech et al., 2001; Yamasaki et al., 1997).
The greater magnitude of the post-traumatic influx of inflammatory cells seen in wild-type than in Avpdi/di rats correlated well with the higher levels of cortical synthesis of neutrophil (CXCL1 and CXCL2) and monocyte (CCL2) chemoattractants seen in wild-type rats compared to AVP-deficient animals. Overexpression of CXC or CC chemokines in rodent brain has been shown to augment the recruitment of inflammatory cells, with resulting disruption of the BBB, and exacerbation of ischemic brain injury (Bell et al., 1996; Chen et al., 2003). It is important to note that CCL2 not only acts as a strong chemoattractant for monocytes, but it also has the ability to increase the permeability of the BBB (Stamatovic et al., 2005). We have demonstrated that after injury, CXC and CC chemokines are predominantly produced by brain endothelium and astrocytes (Figs. 3A–E). These latter findings are in accord with our previous observations, that neurotrauma results in a significant increase in expression of AVPR1A on cerebrovascular endothelium and astrocytes (Szmydynger-Chodobska et al., 2004), and suggest that these two types of parenchymal cells are the primary targets for AVP in the injured brain.
In addition to augmented production of the CXC and CC chemokines, we have shown a higher level of cortical expression of MMP9 in brain-injured wild-type rats than in AVP-deficient animals. Similar to chemokines, MMP9 has been found to be expressed in brain endothelium and astrocytes after ischemic brain injury (Rosenberg et al., 2001). This suggests that MMP9 may also play a mediatory role in the pathophysiological actions of AVP on the injured brain. This idea is supported by studies done in Mmp9–/– mice, which demonstrated that MMP9 significantly contributes to the loss of neural tissue in a rodent model of TBI (Wang et al., 2000). This metalloproteinase is also well known for its ability to disrupt the integrity of the BBB, by attacking the basal lamina of the endothelial cells (Cunningham et al., 2005).
The results obtained in the in vivo model of TBI are supported by in vitro studies of astrocyte and endothelial cell cultures, where AVP was found to amplify the TNF-α-dependent synthesis of CXC and CC chemokines. TNF-α is well known for its ability to induce the synthesis of CXC and CC chemokines, and the pathophysiological role of this cytokine in brain injury, particularly during the acute stage soon after the insult, has been well documented (Shohami et al., 1999). A previous study in a rat model of TBI has shown that the cortical levels of biologically active TNF-α are elevated at 3−8 h post-TBI (Taupin et al., 1993), and we have recently demonstrated that the synthesis of AVP in the injured cortex is significantly increased between 4 and 24 h post-TBI (Pascale et al., 2006). The differences in the levels of cortical expression of CXC and CC chemokines and MMP9 between wild-type and Avpdi/di rats were seen between 6 and 24 h after injury, which coincided with increased production of TNF-α and AVP in the traumatized cortex.
The synergistic interactions between TNF-α and AVP that lead to the increased production of CXC and CC chemokines seen more in wild-type than in Avpdi/di rats are mediated by JNK. There are several lines of evidence to support this hypothesis. First, JNK/AP-1 plays an important role in regulating the expression of the genes Cxcl1, Cxcl2, and Ccl2 (Hanazawa et al., 1993; Kim et al., 2003; Wang et al., 2006). Second, in addition to augmented production of chemokines, we found a much larger increase in the message for MMP9 and AVP in the injured cortices of wild-type rats compared to AVP-deficient animals. This observation is consistent with the AP-1-dependent regulation of expression of the Mmp9 and Avp genes (Sato and Seiki, 1993; Yoshida et al., 2006), and suggests that AVP may also act to augment its own synthesis in the injured cortex. Third, the levels of activation of JNK1 and JNK2 and their major targets, the transcription factors c-Jun and ATF2, were higher in the injured cortex of wild-type than in Avpdi/di rats. Fourth, the exposure of astrocytic or endothelial cells to a combination of TNF-α and AVP resulted in a greater magnitude of activation of JNK, c-Jun, and ATF2, compared to the level of activation of this signaling cascade seen in response to TNF-α alone. Fifth, a selective inhibitor of JNK, SP600125, abolished the production of chemokines induced by TNF-α or a combination of TNF-α and AVP.
The mechanisms by which AVP activates JNK, and consequently amplifies the cytokine-dependent production of proinflammatory mediators, are presently unclear. It is possible that these actions of AVP are mediated by the α-subunit of Gq/11 (heterotrimeric guanine nucleotide-binding regulatory protein), which transduces signal from AVPR1A, and involves the activation of protein kinase C and the Src family kinases (Nagao et al., 1998).
Brain injury resulted in a significant increase in the activity of JNK1 and JNK2 in the traumatized cortex starting at 4 h post-TBI, which coincided with a post-traumatic increase in cortical synthesis of AVP (Pascale et al., 2006). These findings are in accord with those of a previous study in a similar model of TBI, which demonstrated the increased DNA binding activity of AP-1 in response to the insult (Yang et al., 1994). The activity of JNK1 and JNK2 in the cortex of sham-injured rats and in the cortex contralateral to injury was barely detectable. In contrast, JNK3 was constitutively activated in both the contralateral and ipsilateral cortices, and the level of its activation remained largely unchanged over time after injury. The immunohistochemical analysis suggested that the constitutively-activated JNK3 is predominantly expressed in neurons. At the same time, the post-traumatic induction of expression of p-JNK in brain microvessels and astrocytes was seen. This latter observation is consistent with the idea that the cerebrovascular endothelium and astrocytes are the primary target cells for AVP in the injured brain.
In summary, we have shown that in a rat model of TBI, the increased influx of inflammatory cells in wild-type versus Avpdi/di rats is associated with the higher levels of cortical synthesis of CXC and CC chemokines found in wild-type versus AVP-deficient rats. In the injured cortex, CXC and CC chemokines are predominantly produced by the cerebrovascular endothelium and astrocytes, and the in vitro experiments in astrocyte and brain endothelial cell cultures have demonstrated that AVP acts synergistically with TNF-α to increase the TNF-α-dependent production of these chemokines. Finally, we have shown that these actions of AVP were mediated by the JNK signaling pathway.
Acknowledgments
We thank Dr. Danica Stanimirovic (Institute for Biological Sciences, National Research Council, Ottawa, Canada) for providing the SV-RBEC and SV-NRA cell lines. We also thank Ms. Julie Sarri for her technical assistance and Ms. Virginia Hovanesian for her help in acquiring and processing confocal microscopy images. This work was supported by grant NS49479 from the National Institutes of Health (to A.C.), and by funds from the Department of Emergency Medicine at Alpert Medical School of Brown University.
Author Disclosure Statement
No competing financial interests exist.
References
- Barreca T. Gandolfo C. Corsini G. Del Sette M. Cataldi A. Rolandi E. Franceschini R. Evaluation of the secretory pattern of plasma arginine vasopressin in stroke patients. Cerebrovasc. Dis. 2001;11:113–118. doi: 10.1159/000047622. [DOI] [PubMed] [Google Scholar]
- Beech J.S. Reckless J. Mosedale D.E. Grainger D.J. Williams S.C. Menon D.K. Neuroprotection in ischemia-reperfusion injury: an antiinflammatory approach using a novel broad-spectrum chemokine inhibitor. J. Cereb. Blood Flow Metab. 2001;21:683–689. doi: 10.1097/00004647-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Bell M.D. Taub D.D. Kunkel S.J. Strieter R.M. Foley R. Gauldie J. Perry V.H. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur. J. Neurosci. 1996;8:1803–1811. doi: 10.1111/j.1460-9568.1996.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Bemana I. Nagao S. Treatment of brain edema with a nonpeptide arginine vasopressin V1 receptor antagonist OPC-21268 in rats. Neurosurgery. 1999;44:148–154. doi: 10.1097/00006123-199901000-00091. [DOI] [PubMed] [Google Scholar]
- Chang Y. Chen T.Y. Chen C.H. Crain B.J. Toung T.J. Bhardwaj A. Plasma arginine-vasopressin following experimental stroke: effect of osmotherapy. J. Appl. Physiol. 2006;100:1445–1451. doi: 10.1152/japplphysiol.00763.2005. [DOI] [PubMed] [Google Scholar]
- Chen Y. Hallenbeck J.M. Ruetzler C. Bol D. Thomas K. Berman N.E. Vogel S.N. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J. Cereb. Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Cunningham L.A. Wetzel M. Rosenberg G.A. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- DePasquale M. Patlak C.S. Cserr H.F. Brain ion and volume regulation during acute hypernatremia in Brattleboro rats. Am. J. Physiol. 1989;256:F1059–F1066. doi: 10.1152/ajprenal.1989.256.6.F1059. [DOI] [PubMed] [Google Scholar]
- Dickinson L.D. Betz A.L. Attenuated development of ischemic brain edema in vasopressin-deficient rats. J. Cereb. Blood Flow Metab. 1992;12:681–690. doi: 10.1038/jcbfm.1992.93. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic O.B. Stamatovic S.M. Keep R.F. Andjelkovic A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Garberg P. Ball M. Borg N. Cecchelli R. Fenart L. Hurst R.D. Lindmark T. Mabondzo A. Nilsson J.E. Raub T.J. Stanimirovic D. Terasaki T. Öberg J.O. Österberg T. In vitro models for the blood-brain barrier. Toxicol. In Vitro. 2005;19:299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Hanazawa S. Takeshita A. Amano S. Semba T. Nirazuka T. Katoh H. Kitano S. Tumor necrosis factor-α induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J. Biol. Chem. 1993;268:9526–9532. [PubMed] [Google Scholar]
- Hirasawa A. Hashimoto K. Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur. J. Pharmacol. 1994;267:71–75. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Kim D.S. Han J.H. Kwon H.J. NF-κB and c-Jun-dependent regulation of macrophage inflammatory protein-2 gene expression in response to lipopolysaccharide in RAW 264.7 cells. Mol. Immunol. 2003;40:633–643. doi: 10.1016/j.molimm.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Kuschert G.S. Coulin F. Power C.A. Proudfoot A.E. Hubbard R.E. Hoogewerf A.J. Wells T.N. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- Liu X. Jin Y. Zheng H. Chen G. Tan B. Wu B. Arginine vasopressin gene expression in supraoptic nucleus and paraventricular nucleus of hypothalamus following cerebral ischemia and reperfusion. Chin. Med. Sci. J. 2000;15:157–161. [PubMed] [Google Scholar]
- Longhi L. Perego C. Ortolano F. Zanier E.R. Bianchi P. Stocchetti N. McIntosh T.K. De Simoni M.G. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit. Care Med. 2009;37:659–665. doi: 10.1097/CCM.0b013e318195998a. [DOI] [PubMed] [Google Scholar]
- Mather H.M. Ang V. Jenkins J.S. Vasopressin in plasma and CSF of patients with subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry. 1981;44:216–219. doi: 10.1136/jnnp.44.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M. Yamauchi J. Kaziro Y. Itoh H. Involvement of protein kinase C and Src family tyrosine kinase in Gαq/11-induced activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J. Biol. Chem. 1998;273:22892–22898. doi: 10.1074/jbc.273.36.22892. [DOI] [PubMed] [Google Scholar]
- Neumann J. Sauerzweig S. Rönicke R. Gunzer F. Dinkel K. Ullrich O. Gunzer M. Reymann K.G. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J. Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski N.L. Lolait S.J. Young W.S., III Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Pascale C.L. Szmydynger-Chodobska J. Sarri J.E. Chodobski A. Traumatic brain injury results in a concomitant increase in neocortical expression of vasopressin and its V1A receptor. J. Physiol. Pharmacol. 2006;57(Suppl. 11):161–167. [PubMed] [Google Scholar]
- Rosenberg G.A. Cunningham L.A. Wallace J. Alexander S. Estrada E.Y. Grossetete M. Razhagi A. Miller K. Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. Scremin O. Estrada E. Kyner W.T. Arginine vasopressin V1-antagonist and atrial natriuretic peptide reduce hemorrhagic brain edema in rats. Stroke. 1992;23:1767–1773. doi: 10.1161/01.str.23.12.1767. [DOI] [PubMed] [Google Scholar]
- Sato H. Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- Schmale H. Richter D. Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature. 1984;308:705–709. doi: 10.1038/308705a0. [DOI] [PubMed] [Google Scholar]
- Schoettle R.J. Kochanek P.M. Magargee M.J. Uhl M.W. Nemoto E.M. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J. Neurotrauma. 1990;7:207–217. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- Shohami E. Ginis I. Hallenbeck J.M. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Shuaib A. Xu Wang C. Yang T. Noor R. Effects of nonpeptide V1 vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke. 2002;33:3033–3037. doi: 10.1161/01.str.0000039405.31526.06. [DOI] [PubMed] [Google Scholar]
- Sørensen P.S. Gjerris F. Hammer M. Cerebrospinal fluid vasopressin in neurological and psychiatric disorders. J. Neurol. Neurosurg. Psychiatry. 1985;48:50–57. doi: 10.1136/jnnp.48.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic S.M. Shakui P. Keep R.F. Moore B.B. Kunkel S.L. Van Rooijen N. Andjelkovic A.V. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J. Chung I. Koźniewska E. Tran B. Harrington F.J. Duncan J.A. Chodobski A. Increased expression of vasopressin V1a receptors after traumatic brain injury. J. Neurotrauma. 2004;21:1090–1102. doi: 10.1089/0897715041651033. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J. Strazielle N. Zink B.J. Ghersi-Egea J.F. Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:1503–1516. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin V. Toulmond S. Serrano A. Benavides J. Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J. Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Trabold R. Krieg S. Scholler K. Plesnila N. Role of vasopressin V1a and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J. Neurotrauma. 2008;25:1459–1465. doi: 10.1089/neu.2008.0597. [DOI] [PubMed] [Google Scholar]
- Vakili A. Kataoka H. Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005;25:1012–1019. doi: 10.1038/sj.jcbfm.9600097. [DOI] [PubMed] [Google Scholar]
- Waetzig V. Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol. Sci. 2005;26:455–461. doi: 10.1016/j.tips.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Wang X. Jung J. Asahi M. Chwang W. Russo L. Moskowitz M.A. Dixon C.E. Fini M.E. Lo E.H. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J. Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Luo W. Stricker R. Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J. Neurochem. 2006;98:1046–1060. doi: 10.1111/j.1471-4159.2006.03950.x. [DOI] [PubMed] [Google Scholar]
- Xu M. Su W. Huang W.D. Lu Y.Q. Xu Q.P. Chen Z.J. Effect of AVP on brain edema following traumatic brain injury. Chin. J. Traumatol. 2007;10:90–93. [PubMed] [Google Scholar]
- Yamasaki Y. Matsuo Y. Zagorski J. Matsuura N. Onodera H. Itoyama Y. Kogure K. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997;759:103–111. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- Yang K. Mu X.S. Xue J.J. Whitson J. Salminen A. Dixon C.E. Liu P.K. Hayes R.L. Increased expression of c-fos mRNA and AP-1 transcription factors after cortical impact injury in rats. Brain Res. 1994;664:141–147. doi: 10.1016/0006-8993(94)91964-x. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Iwasaki Y. Asai M. Takayasu S. Taguchi T. Itoi K. Hashimoto K. Oiso Y. Identification of a functional AP1 element in the rat vasopressin gene promoter. Endocrinology. 2006;147:2850–2863. doi: 10.1210/en.2005-1222. [DOI] [PubMed] [Google Scholar]
- Young L.J. Toloczko D. Insel T.R. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J. Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]