Abstract
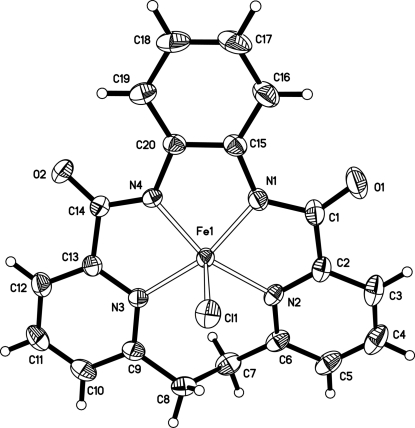
In the title compound, [Fe(C20H14N4O2)Cl], the FeIII ion is in a distorted square-pyramidal environment, with two pyridine and two deprotonated amide N atoms in the basal plane and the Cl ion in the apical position. The FeIII ion is displaced from the basal plane of the square- pyramid towards the apical Cl atom by 0.2942 (4) Å. The molecules are linked into a three-dimensional network by C—H⋯Cl and C—H⋯O hydrogen bonds.
Related literature
For general background, see: Liu et al. (2006 ▶); Yang et al. (2007 ▶); Momenteau & Reed (1994 ▶). For related structures, see: Rath et al. (2004 ▶); Xu et al. (2007 ▶).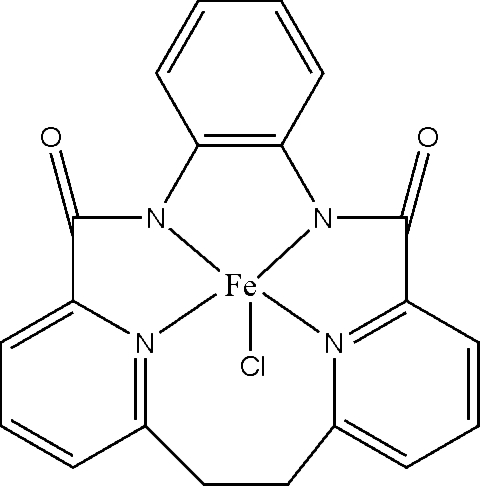
Experimental
Crystal data
[Fe(C20H14N4O2)Cl]
M r = 433.65
Monoclinic,
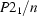
a = 11.8532 (2) Å
b = 8.2028 (1) Å
c = 19.3507 (3) Å
β = 106.889 (1)°
V = 1800.31 (5) Å3
Z = 4
Mo Kα radiation
μ = 1.01 mm−1
T = 296 (2) K
0.44 × 0.16 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS, Sheldrick, 1996 ▶) T min = 0.778, T max = 1.000 (expected range = 0.703–0.904)
24687 measured reflections
4142 independent reflections
3415 reflections with I > 2σ(I)
R int = 0.041
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.126
S = 1.01
4142 reflections
253 parameters
H-atom parameters constrained
Δρmax = 0.62 e Å−3
Δρmin = −0.43 e Å−3
Data collection: SMART (Bruker, 1997 ▶); cell refinement: SAINT (Bruker, 1997 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808041354/ci2736sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808041354/ci2736Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Fe1—N1 | 1.871 (2) |
| Fe1—N4 | 1.889 (2) |
| Fe1—N3 | 2.016 (2) |
| Fe1—N2 | 2.032 (2) |
| Fe1—Cl1 | 2.3080 (8) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3A⋯Cl1i | 0.93 | 2.80 | 3.595 (4) | 144 |
| C10—H10A⋯Cl1ii | 0.93 | 2.71 | 3.617 (3) | 165 |
| C11—H11A⋯O1iii | 0.93 | 2.46 | 3.290 (5) | 149 |
Symmetry codes: (i) 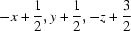 ; (ii)
; (ii) 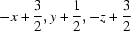 ; (iii)
; (iii) 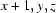 .
.
Acknowledgments
The project was sponsored by the Foundation of Yibin University.
supplementary crystallographic information
Comment
The chemistry of macrocyclic complexes has attracted the interest of both inorganic and bioinorganic chemists in recent years. Iron(III) complexes are involved in numerous biological redox reactions performed by metalloenzymes (Momenteau et al., 1994). As part of our studies on catalysis by N4 non-porphyrin complexes (Liu et al., 2006; Yang et al., 2007), we report here the crystal structure of a iron(III) complex with 1,2-[bis(6'-pyridine-2'-carboxamido)-ethane]benzene.
As shown in Fig.1, the complex has a five-coordinate structure with two pyridine and two deprotonated amide N atoms in the basal plane while the Cl ion is bonded to the FeIII center in the apical position. The geometry around the FeIII ion is approximately square-pyramidal. The Fe—N(amide) distances are shorter than the Fe—N(pyridine) distances (Table 1), both of which are shorter than the Fe—N distances found in the non-ring related Fe—N4 complexes such as [NEt4][Fe(bbpc)Cl2][H2bbpc is N,N'-(4,5-dichloro-o-phenylene)bis(4-tertbutylpyridine-2-carboxamide)] (Xu et al., 2007). The Fe—Cl distance of 2.3080 (8) Å is slightly shorter than that observed in [Fe(bbpc)Cl2](Et4N) (2.3299 (9) Å and 2.3880 (9) Å), while it is longer than that in [FeCl(meso-NH2-octaethylporphyrin)] (2.2596 (8) Å, Sankar et al., 2004).
In the crystalline state, the molecules are linked into a three-dimensional network by C—H···Cl and C—H···O hydrogen bonds (Table 2).
Experimental
1,2-[Bis(6'-pyridine-2'carboxamido)-ethane]benzene (132 mg, 0.38 mmol) and sodium acetate (80 mg, 0.76 mmol) were added to a stirred solution of FeCl3.6H2O (244 mg, 0.9 mmol) in CH3OH (20 ml). The colour of the mixture turned green almost immediately. The mixture was refluxed for 3 h and dark green microcrystals appeared. They were collected by filtration, washed with methanol, and air-dried. (123 mg, yield 75%). Single crystals suitable for X-ray diffraction were grown via diffusion of Et2O into a DMF solution of the complex. Selected IR data (KBr, cm-1):ν=1629 (C=O), 1602 (C—N), 1572, 1346, 1287, 1142, 1083, 1081, 762. MS (FAB): 398.3([Fe(bpeb)]+).
Refinement
All H atoms were positioned geometrically and refined as riding, with C-H = 0.93 Å (aromatic) or 0.97 Å (methylene) and Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 30% probability level.
Crystal data
| [Fe(C20H14N4O2)Cl] | F(000) = 884 |
| Mr = 433.65 | Dx = 1.600 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 9989 reflections |
| a = 11.8532 (2) Å | θ = 2.2–27.4° |
| b = 8.2028 (1) Å | µ = 1.01 mm−1 |
| c = 19.3507 (3) Å | T = 296 K |
| β = 106.889 (1)° | Plate, black |
| V = 1800.31 (5) Å3 | 0.44 × 0.16 × 0.10 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 4142 independent reflections |
| Radiation source: fine-focus sealed tube | 3415 reflections with I > 2σ(I) |
| graphite | Rint = 0.041 |
| φ and ω scans | θmax = 27.5°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS, Sheldrick, 1996) | h = −15→15 |
| Tmin = 0.778, Tmax = 1.000 | k = −10→10 |
| 24687 measured reflections | l = −25→25 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.126 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0642P)2 + 2.4383P] where P = (Fo2 + 2Fc2)/3 |
| 4142 reflections | (Δ/σ)max = 0.001 |
| 253 parameters | Δρmax = 0.62 e Å−3 |
| 0 restraints | Δρmin = −0.43 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F^2^ against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F^2^, conventional R-factors R are based on F, with F set to zero for negative F^2^. The threshold expression of F^2^ > σ(F^2^) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F^2^ are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Fe1 | 0.45008 (3) | 0.56516 (5) | 0.62759 (2) | 0.02844 (13) | |
| Cl1 | 0.49959 (7) | 0.48979 (10) | 0.74759 (4) | 0.0423 (2) | |
| O1 | 0.1136 (2) | 0.6292 (4) | 0.61432 (18) | 0.0676 (8) | |
| O2 | 0.5587 (2) | 0.2335 (3) | 0.51016 (14) | 0.0529 (6) | |
| N1 | 0.2870 (2) | 0.5301 (3) | 0.59827 (14) | 0.0352 (5) | |
| N2 | 0.4003 (2) | 0.7937 (3) | 0.64688 (12) | 0.0321 (5) | |
| N3 | 0.6194 (2) | 0.5763 (3) | 0.62630 (12) | 0.0298 (5) | |
| N4 | 0.44925 (19) | 0.3762 (3) | 0.57162 (12) | 0.0305 (5) | |
| C1 | 0.2181 (3) | 0.6411 (4) | 0.61888 (17) | 0.0399 (7) | |
| C2 | 0.2867 (3) | 0.7921 (4) | 0.64770 (16) | 0.0373 (6) | |
| C3 | 0.2337 (3) | 0.9212 (4) | 0.67153 (19) | 0.0509 (9) | |
| H3A | 0.1555 | 0.9156 | 0.6719 | 0.061* | |
| C4 | 0.3014 (4) | 1.0606 (5) | 0.6950 (2) | 0.0564 (10) | |
| H4A | 0.2696 | 1.1495 | 0.7127 | 0.068* | |
| C5 | 0.4145 (4) | 1.0649 (4) | 0.69173 (18) | 0.0483 (8) | |
| H5A | 0.4594 | 1.1585 | 0.7062 | 0.058* | |
| C6 | 0.4643 (3) | 0.9306 (4) | 0.66690 (16) | 0.0377 (7) | |
| C7 | 0.5853 (3) | 0.9393 (4) | 0.65875 (18) | 0.0443 (7) | |
| H7A | 0.6159 | 1.0483 | 0.6716 | 0.053* | |
| H7B | 0.5800 | 0.9222 | 0.6083 | 0.053* | |
| C8 | 0.6751 (3) | 0.8146 (4) | 0.70492 (17) | 0.0418 (7) | |
| H8A | 0.7468 | 0.8722 | 0.7301 | 0.050* | |
| H8B | 0.6425 | 0.7683 | 0.7411 | 0.050* | |
| C9 | 0.7061 (2) | 0.6787 (4) | 0.66208 (15) | 0.0352 (6) | |
| C10 | 0.8207 (3) | 0.6559 (4) | 0.65877 (18) | 0.0453 (8) | |
| H10A | 0.8794 | 0.7287 | 0.6824 | 0.054* | |
| C11 | 0.8485 (3) | 0.5277 (5) | 0.62112 (19) | 0.0479 (8) | |
| H11A | 0.9255 | 0.5124 | 0.6195 | 0.057* | |
| C12 | 0.7599 (3) | 0.4217 (4) | 0.58564 (17) | 0.0413 (7) | |
| H12A | 0.7760 | 0.3331 | 0.5600 | 0.050* | |
| C13 | 0.6471 (2) | 0.4504 (3) | 0.58911 (15) | 0.0314 (6) | |
| C14 | 0.5471 (2) | 0.3397 (4) | 0.55255 (15) | 0.0333 (6) | |
| C15 | 0.2484 (2) | 0.3809 (4) | 0.56301 (16) | 0.0348 (6) | |
| C16 | 0.1338 (3) | 0.3196 (4) | 0.5414 (2) | 0.0499 (8) | |
| H16A | 0.0724 | 0.3773 | 0.5509 | 0.060* | |
| C17 | 0.1128 (3) | 0.1721 (5) | 0.5056 (2) | 0.0590 (10) | |
| H17A | 0.0364 | 0.1311 | 0.4905 | 0.071* | |
| C18 | 0.2030 (3) | 0.0846 (4) | 0.4919 (2) | 0.0547 (9) | |
| H18A | 0.1870 | −0.0153 | 0.4683 | 0.066* | |
| C19 | 0.3178 (3) | 0.1438 (4) | 0.51280 (16) | 0.0414 (7) | |
| H19A | 0.3789 | 0.0840 | 0.5041 | 0.050* | |
| C20 | 0.3393 (2) | 0.2947 (3) | 0.54712 (15) | 0.0328 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Fe1 | 0.0262 (2) | 0.0225 (2) | 0.0375 (2) | −0.00026 (14) | 0.01073 (15) | −0.00375 (15) |
| Cl1 | 0.0483 (4) | 0.0381 (4) | 0.0427 (4) | 0.0044 (3) | 0.0169 (3) | 0.0083 (3) |
| O1 | 0.0352 (13) | 0.0650 (18) | 0.109 (2) | 0.0042 (12) | 0.0307 (14) | −0.0117 (17) |
| O2 | 0.0472 (13) | 0.0475 (14) | 0.0671 (15) | 0.0005 (11) | 0.0215 (11) | −0.0268 (12) |
| N1 | 0.0278 (11) | 0.0299 (13) | 0.0484 (14) | 0.0000 (9) | 0.0119 (10) | −0.0014 (10) |
| N2 | 0.0410 (13) | 0.0221 (11) | 0.0345 (12) | 0.0034 (10) | 0.0132 (10) | −0.0013 (9) |
| N3 | 0.0287 (11) | 0.0274 (12) | 0.0334 (11) | −0.0026 (9) | 0.0091 (9) | −0.0020 (9) |
| N4 | 0.0301 (11) | 0.0237 (11) | 0.0370 (12) | −0.0025 (9) | 0.0088 (9) | −0.0049 (9) |
| C1 | 0.0337 (15) | 0.0377 (17) | 0.0515 (17) | 0.0065 (12) | 0.0175 (13) | 0.0020 (14) |
| C2 | 0.0424 (16) | 0.0333 (15) | 0.0396 (15) | 0.0099 (12) | 0.0173 (12) | 0.0021 (12) |
| C3 | 0.058 (2) | 0.047 (2) | 0.055 (2) | 0.0213 (17) | 0.0282 (17) | 0.0042 (16) |
| C4 | 0.083 (3) | 0.0406 (19) | 0.0494 (19) | 0.0225 (19) | 0.0254 (18) | −0.0016 (15) |
| C5 | 0.076 (2) | 0.0272 (15) | 0.0401 (16) | 0.0053 (15) | 0.0136 (16) | −0.0027 (13) |
| C6 | 0.0547 (18) | 0.0249 (14) | 0.0325 (14) | 0.0017 (13) | 0.0108 (12) | 0.0010 (11) |
| C7 | 0.0553 (19) | 0.0264 (15) | 0.0517 (18) | −0.0103 (13) | 0.0166 (15) | −0.0024 (13) |
| C8 | 0.0421 (16) | 0.0388 (17) | 0.0411 (16) | −0.0095 (13) | 0.0069 (13) | −0.0082 (13) |
| C9 | 0.0322 (14) | 0.0346 (15) | 0.0365 (14) | −0.0063 (12) | 0.0066 (11) | −0.0002 (12) |
| C10 | 0.0314 (15) | 0.051 (2) | 0.0500 (18) | −0.0110 (14) | 0.0058 (13) | 0.0020 (15) |
| C11 | 0.0270 (14) | 0.063 (2) | 0.0540 (19) | 0.0007 (14) | 0.0126 (13) | 0.0059 (17) |
| C12 | 0.0330 (15) | 0.0472 (19) | 0.0462 (17) | 0.0054 (13) | 0.0154 (13) | 0.0001 (14) |
| C13 | 0.0311 (13) | 0.0311 (14) | 0.0321 (13) | 0.0019 (11) | 0.0092 (10) | 0.0020 (11) |
| C14 | 0.0339 (14) | 0.0296 (14) | 0.0360 (14) | 0.0024 (11) | 0.0094 (11) | −0.0030 (11) |
| C15 | 0.0318 (14) | 0.0287 (14) | 0.0423 (15) | −0.0045 (11) | 0.0084 (11) | 0.0036 (12) |
| C16 | 0.0325 (16) | 0.0450 (19) | 0.070 (2) | −0.0058 (14) | 0.0115 (15) | 0.0041 (17) |
| C17 | 0.0431 (19) | 0.047 (2) | 0.079 (3) | −0.0221 (16) | 0.0051 (17) | 0.0039 (19) |
| C18 | 0.063 (2) | 0.0346 (18) | 0.059 (2) | −0.0211 (16) | 0.0073 (17) | −0.0051 (15) |
| C19 | 0.0493 (18) | 0.0317 (16) | 0.0420 (16) | −0.0082 (13) | 0.0114 (13) | −0.0036 (13) |
| C20 | 0.0326 (14) | 0.0281 (14) | 0.0360 (14) | −0.0047 (11) | 0.0073 (11) | 0.0012 (11) |
Geometric parameters (Å, °)
| Fe1—N1 | 1.871 (2) | C7—C8 | 1.557 (5) |
| Fe1—N4 | 1.889 (2) | C7—H7A | 0.97 |
| Fe1—N3 | 2.016 (2) | C7—H7B | 0.97 |
| Fe1—N2 | 2.032 (2) | C8—C9 | 1.497 (4) |
| Fe1—Cl1 | 2.3080 (8) | C8—H8A | 0.97 |
| O1—C1 | 1.220 (4) | C8—H8B | 0.97 |
| O2—C14 | 1.231 (3) | C9—C10 | 1.392 (4) |
| N1—C1 | 1.358 (4) | C10—C11 | 1.373 (5) |
| N1—C15 | 1.412 (4) | C10—H10A | 0.93 |
| N2—C6 | 1.348 (4) | C11—C12 | 1.383 (5) |
| N2—C2 | 1.350 (4) | C11—H11A | 0.93 |
| N3—C9 | 1.352 (4) | C12—C13 | 1.378 (4) |
| N3—C13 | 1.353 (4) | C12—H12A | 0.93 |
| N4—C14 | 1.349 (4) | C13—C14 | 1.498 (4) |
| N4—C20 | 1.418 (3) | C15—C16 | 1.394 (4) |
| C1—C2 | 1.498 (5) | C15—C20 | 1.396 (4) |
| C2—C3 | 1.378 (4) | C16—C17 | 1.380 (5) |
| C3—C4 | 1.395 (6) | C16—H16A | 0.93 |
| C3—H3A | 0.93 | C17—C18 | 1.376 (6) |
| C4—C5 | 1.361 (6) | C17—H17A | 0.93 |
| C4—H4A | 0.93 | C18—C19 | 1.390 (5) |
| C5—C6 | 1.399 (4) | C18—H18A | 0.93 |
| C5—H5A | 0.93 | C19—C20 | 1.392 (4) |
| C6—C7 | 1.490 (5) | C19—H19A | 0.93 |
| N1—Fe1—N4 | 82.38 (10) | C8—C7—H7B | 108.5 |
| N1—Fe1—N3 | 161.28 (10) | H7A—C7—H7B | 107.5 |
| N4—Fe1—N3 | 82.51 (9) | C9—C8—C7 | 114.1 (3) |
| N1—Fe1—N2 | 82.39 (10) | C9—C8—H8A | 108.7 |
| N4—Fe1—N2 | 155.32 (10) | C7—C8—H8A | 108.7 |
| N3—Fe1—N2 | 107.69 (10) | C9—C8—H8B | 108.7 |
| N1—Fe1—Cl1 | 101.67 (8) | C7—C8—H8B | 108.7 |
| N4—Fe1—Cl1 | 108.32 (8) | H8A—C8—H8B | 107.6 |
| N3—Fe1—Cl1 | 93.53 (7) | N3—C9—C10 | 120.1 (3) |
| N2—Fe1—Cl1 | 93.71 (7) | N3—C9—C8 | 118.3 (3) |
| C1—N1—C15 | 125.8 (3) | C10—C9—C8 | 121.7 (3) |
| C1—N1—Fe1 | 117.7 (2) | C11—C10—C9 | 121.0 (3) |
| C15—N1—Fe1 | 116.19 (19) | C11—C10—H10A | 119.5 |
| C6—N2—C2 | 119.0 (3) | C9—C10—H10A | 119.5 |
| C6—N2—Fe1 | 130.7 (2) | C10—C11—C12 | 118.7 (3) |
| C2—N2—Fe1 | 109.69 (19) | C10—C11—H11A | 120.6 |
| C9—N3—C13 | 118.7 (2) | C12—C11—H11A | 120.6 |
| C9—N3—Fe1 | 129.3 (2) | C13—C12—C11 | 118.4 (3) |
| C13—N3—Fe1 | 111.65 (18) | C13—C12—H12A | 120.8 |
| C14—N4—C20 | 125.7 (2) | C11—C12—H12A | 120.8 |
| C14—N4—Fe1 | 118.49 (18) | N3—C13—C12 | 123.1 (3) |
| C20—N4—Fe1 | 115.45 (18) | N3—C13—C14 | 115.6 (2) |
| O1—C1—N1 | 127.6 (3) | C12—C13—C14 | 121.3 (3) |
| O1—C1—C2 | 121.5 (3) | O2—C14—N4 | 127.6 (3) |
| N1—C1—C2 | 110.8 (2) | O2—C14—C13 | 121.2 (3) |
| N2—C2—C3 | 123.4 (3) | N4—C14—C13 | 111.2 (2) |
| N2—C2—C1 | 116.0 (2) | C16—C15—C20 | 119.9 (3) |
| C3—C2—C1 | 120.6 (3) | C16—C15—N1 | 127.5 (3) |
| C2—C3—C4 | 117.6 (3) | C20—C15—N1 | 112.5 (2) |
| C2—C3—H3A | 121.2 | C17—C16—C15 | 119.0 (3) |
| C4—C3—H3A | 121.2 | C17—C16—H16A | 120.5 |
| C5—C4—C3 | 119.1 (3) | C15—C16—H16A | 120.5 |
| C5—C4—H4A | 120.4 | C18—C17—C16 | 121.1 (3) |
| C3—C4—H4A | 120.4 | C18—C17—H17A | 119.4 |
| C4—C5—C6 | 121.0 (3) | C16—C17—H17A | 119.4 |
| C4—C5—H5A | 119.5 | C17—C18—C19 | 120.8 (3) |
| C6—C5—H5A | 119.5 | C17—C18—H18A | 119.6 |
| N2—C6—C5 | 119.8 (3) | C19—C18—H18A | 119.6 |
| N2—C6—C7 | 119.2 (3) | C18—C19—C20 | 118.5 (3) |
| C5—C6—C7 | 120.9 (3) | C18—C19—H19A | 120.7 |
| C6—C7—C8 | 115.3 (3) | C20—C19—H19A | 120.7 |
| C6—C7—H7A | 108.5 | C19—C20—C15 | 120.6 (3) |
| C8—C7—H7A | 108.5 | C19—C20—N4 | 127.0 (3) |
| C6—C7—H7B | 108.5 | C15—C20—N4 | 112.4 (2) |
| N4—Fe1—N1—C1 | −176.9 (2) | C2—N2—C6—C7 | −173.6 (3) |
| N3—Fe1—N1—C1 | −140.5 (3) | Fe1—N2—C6—C7 | 16.6 (4) |
| N2—Fe1—N1—C1 | −16.3 (2) | C4—C5—C6—N2 | −1.0 (5) |
| Cl1—Fe1—N1—C1 | 75.9 (2) | C4—C5—C6—C7 | 175.9 (3) |
| N4—Fe1—N1—C15 | 9.6 (2) | N2—C6—C7—C8 | −63.1 (4) |
| N3—Fe1—N1—C15 | 46.0 (4) | C5—C6—C7—C8 | 119.9 (3) |
| N2—Fe1—N1—C15 | 170.1 (2) | C6—C7—C8—C9 | 107.4 (3) |
| Cl1—Fe1—N1—C15 | −97.6 (2) | C13—N3—C9—C10 | 1.6 (4) |
| N1—Fe1—N2—C6 | −173.7 (3) | Fe1—N3—C9—C10 | 174.0 (2) |
| N4—Fe1—N2—C6 | −121.4 (3) | C13—N3—C9—C8 | −178.2 (3) |
| N3—Fe1—N2—C6 | −9.9 (3) | Fe1—N3—C9—C8 | −5.7 (4) |
| Cl1—Fe1—N2—C6 | 85.0 (2) | C7—C8—C9—N3 | −62.5 (4) |
| N1—Fe1—N2—C2 | 15.76 (19) | C7—C8—C9—C10 | 117.7 (3) |
| N4—Fe1—N2—C2 | 68.1 (3) | N3—C9—C10—C11 | −1.8 (5) |
| N3—Fe1—N2—C2 | 179.57 (18) | C8—C9—C10—C11 | 177.9 (3) |
| Cl1—Fe1—N2—C2 | −85.53 (19) | C9—C10—C11—C12 | 0.7 (5) |
| N1—Fe1—N3—C9 | 150.2 (3) | C10—C11—C12—C13 | 0.6 (5) |
| N4—Fe1—N3—C9 | −173.5 (3) | C9—N3—C13—C12 | −0.3 (4) |
| N2—Fe1—N3—C9 | 29.6 (3) | Fe1—N3—C13—C12 | −174.0 (2) |
| Cl1—Fe1—N3—C9 | −65.4 (2) | C9—N3—C13—C14 | 178.4 (2) |
| N1—Fe1—N3—C13 | −37.0 (4) | Fe1—N3—C13—C14 | 4.7 (3) |
| N4—Fe1—N3—C13 | −0.60 (19) | C11—C12—C13—N3 | −0.8 (5) |
| N2—Fe1—N3—C13 | −157.53 (18) | C11—C12—C13—C14 | −179.4 (3) |
| Cl1—Fe1—N3—C13 | 107.44 (18) | C20—N4—C14—O2 | 1.6 (5) |
| N1—Fe1—N4—C14 | 164.8 (2) | Fe1—N4—C14—O2 | −171.3 (3) |
| N3—Fe1—N4—C14 | −4.1 (2) | C20—N4—C14—C13 | −179.7 (2) |
| N2—Fe1—N4—C14 | 112.5 (3) | Fe1—N4—C14—C13 | 7.5 (3) |
| Cl1—Fe1—N4—C14 | −95.4 (2) | N3—C13—C14—O2 | 171.0 (3) |
| N1—Fe1—N4—C20 | −8.8 (2) | C12—C13—C14—O2 | −10.3 (4) |
| N3—Fe1—N4—C20 | −177.7 (2) | N3—C13—C14—N4 | −7.8 (4) |
| N2—Fe1—N4—C20 | −61.1 (3) | C12—C13—C14—N4 | 170.9 (3) |
| Cl1—Fe1—N4—C20 | 91.03 (19) | C1—N1—C15—C16 | 1.3 (5) |
| C15—N1—C1—O1 | 4.1 (6) | Fe1—N1—C15—C16 | 174.3 (3) |
| Fe1—N1—C1—O1 | −168.8 (3) | C1—N1—C15—C20 | 178.5 (3) |
| C15—N1—C1—C2 | −174.1 (3) | Fe1—N1—C15—C20 | −8.6 (3) |
| Fe1—N1—C1—C2 | 13.1 (3) | C20—C15—C16—C17 | 1.3 (5) |
| C6—N2—C2—C3 | −3.4 (4) | N1—C15—C16—C17 | 178.3 (3) |
| Fe1—N2—C2—C3 | 168.4 (3) | C15—C16—C17—C18 | 0.7 (6) |
| C6—N2—C2—C1 | 175.0 (3) | C16—C17—C18—C19 | −1.0 (6) |
| Fe1—N2—C2—C1 | −13.2 (3) | C17—C18—C19—C20 | −0.8 (5) |
| O1—C1—C2—N2 | −176.9 (3) | C18—C19—C20—C15 | 2.8 (5) |
| N1—C1—C2—N2 | 1.3 (4) | C18—C19—C20—N4 | −179.2 (3) |
| O1—C1—C2—C3 | 1.5 (5) | C16—C15—C20—C19 | −3.1 (5) |
| N1—C1—C2—C3 | 179.8 (3) | N1—C15—C20—C19 | 179.5 (3) |
| N2—C2—C3—C4 | 0.8 (5) | C16—C15—C20—N4 | 178.7 (3) |
| C1—C2—C3—C4 | −177.5 (3) | N1—C15—C20—N4 | 1.3 (4) |
| C2—C3—C4—C5 | 1.6 (5) | C14—N4—C20—C19 | 15.3 (5) |
| C3—C4—C5—C6 | −1.5 (5) | Fe1—N4—C20—C19 | −171.7 (2) |
| C2—N2—C6—C5 | 3.4 (4) | C14—N4—C20—C15 | −166.7 (3) |
| Fe1—N2—C6—C5 | −166.4 (2) | Fe1—N4—C20—C15 | 6.4 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3A···Cl1i | 0.93 | 2.80 | 3.595 (4) | 144 |
| C10—H10A···Cl1ii | 0.93 | 2.71 | 3.617 (3) | 165 |
| C11—H11A···O1iii | 0.93 | 2.46 | 3.290 (5) | 149 |
Symmetry codes: (i) −x+1/2, y+1/2, −z+3/2; (ii) −x+3/2, y+1/2, −z+3/2; (iii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI2736).
References
- Bruker (1997). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Liu, H. H., Wang, Y., Shu, Y. J., Zhou, X. G., Wu, J. & Yan, S. Y. (2006). J. Mol. Catal. A Chem.246, 49–52.
- Momenteau, M. & Reed, C. A. (1994). Chem. Rev.94, 659–698.
- Rath, S. P., Kalish, H., Latos-Grazyński, L. Olmstead, M. M. & Balch, A. L. (2004). J. Am. Chem. Soc.126, 646–654. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Xu, X.-B., Yang, L., Qu, Y.-Y., Li, L.-X. & Zhou, X.-G. (2007). Acta Cryst. E63, m1790.
- Yang, L., Wei, R. L., Li, R., Zhou, X. G. & Zuo, J. L. (2007). J. Mol. Catal. A Chem.266, 284–289.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808041354/ci2736sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808041354/ci2736Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report