Abstract
In the structure of the polymeric title complex, {[Cu(C3H6NO2)(C10H8N2)]ClO4·H2O}n, the carboxylate group of the chelating amino acid is further linked to a neighbouring Cu centre, generating a supramolecular single-stranded chain parallel to [010]. The structure displays intermolecular N—H⋯O and O—H⋯O hydrogen bonding, which consolidates the crystal packing.
Related literature
For related structures, see: Antolini et al. (1983 ▶); Masuda et al. (1991 ▶); Sgarabotto et al. (1999 ▶); Solans et al. (1992 ▶).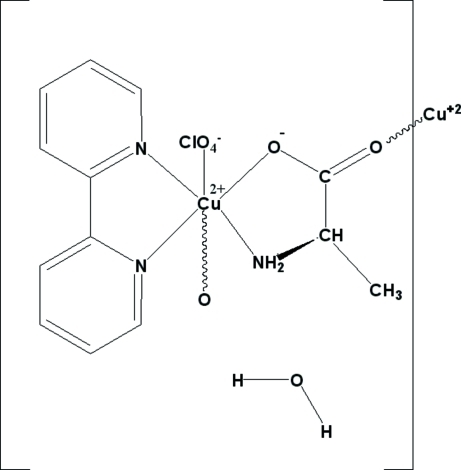
Experimental
Crystal data
[Cu(C3H6NO2)(C10H8N2)]ClO4·H2O
M r = 425.28
Monoclinic,
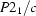
a = 13.1807 (10) Å
b = 8.2656 (6) Å
c = 16.1195 (13) Å
β = 110.606 (2)°
V = 1643.8 (2) Å3
Z = 4
Mo Kα radiation
μ = 1.53 mm−1
T = 220 (2) K
0.60 × 0.30 × 0.30 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1997 ▶) T min = 0.460, T max = 0.656
13671 measured reflections
3939 independent reflections
3611 reflections with I > 2σ(I)
R int = 0.020
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.078
S = 1.11
3939 reflections
290 parameters
All H-atom parameters refined
Δρmax = 0.89 e Å−3
Δρmin = −0.43 e Å−3
Data collection: SMART (Bruker, 1998 ▶); cell refinement: SAINT (Bruker, 1998 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 1999 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808040725/tk2300sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808040725/tk2300Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3—H2N3⋯O7i | 0.74 (4) | 2.60 (4) | 3.293 (4) | 159 (5) |
| N3—H1N3⋯O1ii | 0.83 (4) | 2.48 (4) | 3.225 (3) | 149 (3) |
| N3—H1N3⋯O2ii | 0.83 (4) | 2.91 (4) | 3.225 (3) | 105 (3) |
| N3—H1N3⋯O7ii | 0.83 (4) | 2.70 (5) | 3.059 (3) | 108 (3) |
| N3—H2N3⋯O7ii | 0.74 (4) | 2.69 (5) | 3.059 (3) | 114 (4) |
| O7—H1O7⋯O2ii | 0.79 (4) | 2.08 (4) | 2.857 (3) | 166 (3) |
Symmetry codes: (i) 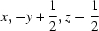 ; (ii)
; (ii) 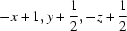 .
.
Acknowledgments
The authors thank Professor Evamarie Hey-Hawkins for cooperation.
supplementary crystallographic information
Comment
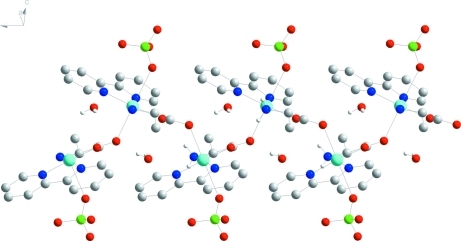
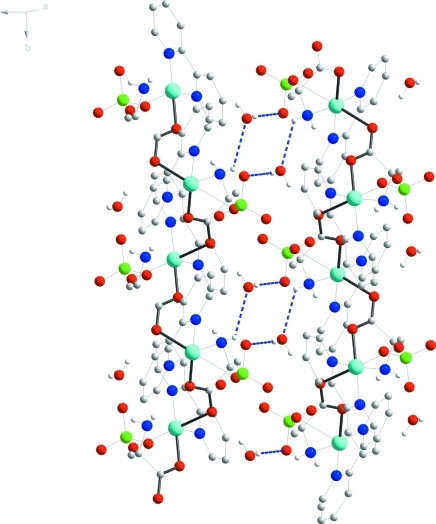
The structure of the title complex, (I) and Fig. 1, is of interest with respect to the stereochemistry of the complexed aminoacid, the coordination geometry of the metal centre and the single-stranded supramolecular assembly created primarly by further coordination of the carboxylate group of the aminoacid, Fig. 2. The secondary association is by crosslinks realised through H-bonds between the created chains, Fig. 3. The supramolecular structure described for (I) is found in other (aminoacidato)(2,2'-bipyridyl)copper(II) complexes, such as in the tryptophanato (Masuda et al., 1991) and aspartato complexes (Antolini et al., 1983). The assembly has also been identified in the proline complex but not described as a supramolecular association (Sgarabotto et al., 1999). For the alaninate complex, see also Solans et al. (1992).
Experimental
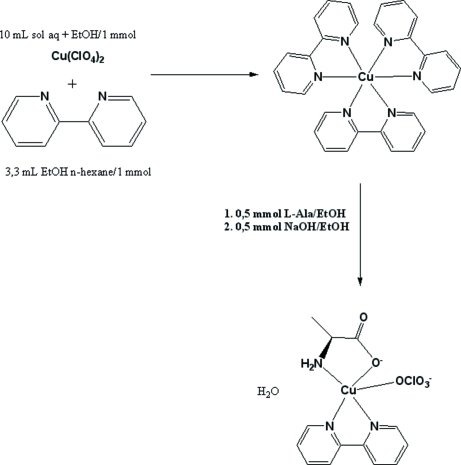
The synthesis of (I) was realized by using an intermediate complex, i.e. tris(2,2'-bipyridyl)copper(II), as shown in Fig. 4. The cation in (I) was prepared according to the following procedure: Two ethanolic solutions, one containing 2,2'-bipyridyl (0.31 g, 2 mmol/5 mL) and another containing Cu(ClO4)2.2H2O (0.6 g, 2 mmol/5 mL) were mixed with stirring. To the resulting suspension of a blue powder, an alkaline solution of L-alanine (0.18 g alanine + 0.08 g NaOH 2 mmol/10 mL water) was added dropwise (see also Scheme 2). The suspension cleared and changed colour to dark-blue. The mixture was heated to 50°C and Na2ClO4 (1 mmol) was added. After 10 mins, the solution was cooled and filtered. The filtrate was allowed to stand at room temperature for several days when dark-blue crystals, suitable for X-ray analysis, separated, collected and washed with a methanolic solution.
Refinement
The H atoms were refined freely: O-H = 0.69 (5) - 0.79 (3) Å, N-H = 0.73 (4) - 0.83 (3) Å, and C-H = 0.89 (3) - 1.16 (4) Å.
Figures
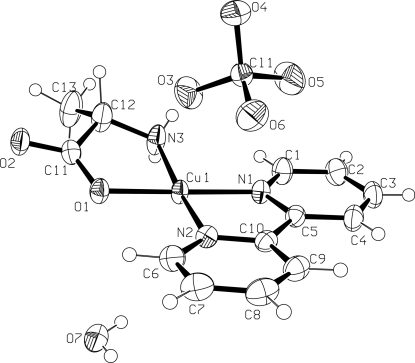
Fig. 1.
The asymmetric unit in (I) showing the crystallographic numbering scheme. Displacement ellipsoids are shown at the 50% probability level.
Fig. 2.
Single strand supramolecular assembly mediated by further coordination of the carboxylato group of the aminoacid. Colour code: cyan = Cu, green = Cl, red = O, blue = N, grey = C. Hydrogen atoms have been omitted for clarity.
Fig. 3.
Supramolecular assembly at the secondary level formed by H-bond formation. The single strand chain is represented with thick bonds whereas the H-bonds are represented by blue dashed lines. For clarity only hydrogens (shown in white) involved in intermolecular associations are represented.
Fig. 4.
The formation of the title compound.
Crystal data
| [Cu(C3H6NO2)(C10H8N2)]ClO4·H2O | F(000) = 868 |
| Mr = 425.28 | Dx = 1.718 Mg m−3 |
| Monoclinic, P21/c | Melting point: 253 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.1807 (10) Å | Cell parameters from 4860 reflections |
| b = 8.2656 (6) Å | θ = 2.7–28.3° |
| c = 16.1195 (13) Å | µ = 1.53 mm−1 |
| β = 110.606 (2)° | T = 220 K |
| V = 1643.8 (2) Å3 | Prism, dark blue |
| Z = 4 | 0.60 × 0.30 × 0.30 mm |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3939 independent reflections |
| Radiation source: fine-focus sealed tube | 3611 reflections with I > 2σ(I) |
| graphite | Rint = 0.020 |
| Detector resolution: 81.92 pixels mm-1 | θmax = 28.3°, θmin = 2.6° |
| φ scans | h = −17→17 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1997) | k = −11→11 |
| Tmin = 0.460, Tmax = 0.656 | l = −20→21 |
| 13671 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.078 | All H-atom parameters refined |
| S = 1.11 | w = 1/[σ2(Fo2) + (0.0317P)2 + 1.4344P] where P = (Fo2 + 2Fc2)/3 |
| 3939 reflections | (Δ/σ)max = 0.001 |
| 290 parameters | Δρmax = 0.89 e Å−3 |
| 0 restraints | Δρmin = −0.43 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.364703 (18) | 0.28840 (3) | 0.167573 (16) | 0.02538 (8) | |
| Cl1 | 0.17905 (4) | 0.19812 (6) | −0.06197 (3) | 0.02952 (11) | |
| O1 | 0.43017 (11) | 0.07522 (17) | 0.20438 (10) | 0.0300 (3) | |
| O2 | 0.55154 (13) | −0.09670 (18) | 0.18782 (10) | 0.0365 (3) | |
| O3 | 0.28355 (13) | 0.1556 (3) | 0.00253 (12) | 0.0505 (4) | |
| O4 | 0.16505 (15) | 0.1197 (2) | −0.14439 (11) | 0.0450 (4) | |
| O5 | 0.17426 (19) | 0.3705 (2) | −0.07423 (13) | 0.0605 (5) | |
| O6 | 0.09645 (14) | 0.1466 (3) | −0.02883 (12) | 0.0510 (4) | |
| N1 | 0.28664 (13) | 0.4972 (2) | 0.12453 (11) | 0.0275 (3) | |
| N2 | 0.22426 (13) | 0.2334 (2) | 0.18192 (11) | 0.0259 (3) | |
| N3 | 0.49264 (17) | 0.3247 (2) | 0.13184 (19) | 0.0391 (5) | |
| C1 | 0.32627 (18) | 0.6292 (3) | 0.09841 (16) | 0.0358 (5) | |
| C2 | 0.2644 (2) | 0.7669 (3) | 0.06765 (17) | 0.0398 (5) | |
| C3 | 0.1579 (2) | 0.7682 (3) | 0.06305 (16) | 0.0382 (5) | |
| C4 | 0.11629 (18) | 0.6331 (3) | 0.09049 (15) | 0.0350 (5) | |
| C5 | 0.18218 (15) | 0.4985 (2) | 0.12098 (12) | 0.0264 (4) | |
| C6 | 0.19879 (18) | 0.0907 (3) | 0.20930 (14) | 0.0330 (4) | |
| C7 | 0.0946 (2) | 0.0558 (3) | 0.20633 (16) | 0.0391 (5) | |
| C8 | 0.01505 (19) | 0.1715 (3) | 0.17510 (16) | 0.0391 (5) | |
| C9 | 0.04044 (17) | 0.3198 (3) | 0.14800 (14) | 0.0340 (4) | |
| C10 | 0.14634 (15) | 0.3474 (2) | 0.15143 (12) | 0.0264 (4) | |
| C11 | 0.50804 (16) | 0.0390 (2) | 0.17798 (13) | 0.0288 (4) | |
| C12 | 0.5466 (2) | 0.1693 (3) | 0.12787 (19) | 0.0429 (5) | |
| C13 | 0.6666 (2) | 0.1814 (4) | 0.1552 (3) | 0.0605 (8) | |
| H2N3 | 0.482 (4) | 0.357 (6) | 0.087 (3) | 0.098 (16)* | |
| H1N3 | 0.536 (3) | 0.384 (5) | 0.170 (3) | 0.083 (13)* | |
| H1 | 0.398 (2) | 0.628 (3) | 0.1013 (17) | 0.042 (7)* | |
| H2 | 0.293 (2) | 0.844 (4) | 0.0488 (19) | 0.048 (8)* | |
| H3 | 0.116 (2) | 0.855 (4) | 0.0419 (17) | 0.044 (7)* | |
| H4 | 0.049 (2) | 0.634 (4) | 0.0873 (19) | 0.054 (8)* | |
| H6 | 0.257 (2) | 0.014 (3) | 0.2313 (16) | 0.037 (6)* | |
| H7 | 0.082 (2) | −0.043 (4) | 0.2244 (18) | 0.045 (7)* | |
| H8 | −0.057 (2) | 0.151 (4) | 0.1713 (18) | 0.052 (8)* | |
| H9 | −0.014 (2) | 0.401 (4) | 0.1242 (18) | 0.046 (7)* | |
| H12 | 0.539 (3) | 0.124 (5) | 0.059 (3) | 0.094 (12)* | |
| H13A | 0.691 (3) | 0.261 (4) | 0.116 (2) | 0.073 (10)* | |
| H13B | 0.699 (3) | 0.072 (5) | 0.145 (2) | 0.077 (11)* | |
| H13C | 0.691 (4) | 0.225 (5) | 0.228 (3) | 0.105 (15)* | |
| O7 | 0.4174 (2) | 0.1391 (3) | 0.41504 (15) | 0.0516 (5) | |
| H1O7 | 0.418 (3) | 0.220 (4) | 0.389 (2) | 0.056 (10)* | |
| H2O7 | 0.367 (4) | 0.142 (7) | 0.419 (3) | 0.110 (19)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.02196 (12) | 0.01912 (12) | 0.03468 (14) | 0.00188 (8) | 0.00948 (9) | 0.00357 (9) |
| Cl1 | 0.0286 (2) | 0.0299 (2) | 0.0284 (2) | 0.00289 (17) | 0.00796 (18) | 0.00106 (18) |
| O1 | 0.0300 (7) | 0.0218 (7) | 0.0391 (8) | 0.0031 (5) | 0.0133 (6) | 0.0050 (6) |
| O2 | 0.0399 (8) | 0.0251 (7) | 0.0431 (8) | 0.0101 (6) | 0.0128 (7) | 0.0037 (6) |
| O3 | 0.0284 (8) | 0.0680 (13) | 0.0458 (9) | 0.0050 (8) | 0.0016 (7) | 0.0056 (9) |
| O4 | 0.0561 (10) | 0.0443 (10) | 0.0355 (8) | 0.0040 (8) | 0.0171 (7) | −0.0061 (7) |
| O5 | 0.0954 (16) | 0.0274 (9) | 0.0515 (11) | 0.0022 (9) | 0.0167 (10) | 0.0023 (8) |
| O6 | 0.0375 (9) | 0.0659 (13) | 0.0562 (11) | 0.0028 (8) | 0.0248 (8) | 0.0028 (9) |
| N1 | 0.0251 (8) | 0.0227 (8) | 0.0333 (8) | 0.0019 (6) | 0.0082 (6) | 0.0011 (6) |
| N2 | 0.0253 (8) | 0.0245 (8) | 0.0276 (8) | −0.0015 (6) | 0.0088 (6) | −0.0017 (6) |
| N3 | 0.0310 (10) | 0.0249 (9) | 0.0666 (15) | 0.0059 (7) | 0.0237 (10) | 0.0115 (10) |
| C1 | 0.0320 (11) | 0.0268 (10) | 0.0480 (12) | 0.0003 (8) | 0.0132 (9) | 0.0051 (9) |
| C2 | 0.0467 (13) | 0.0245 (10) | 0.0470 (13) | 0.0005 (9) | 0.0149 (10) | 0.0051 (9) |
| C3 | 0.0416 (12) | 0.0288 (11) | 0.0378 (11) | 0.0118 (9) | 0.0062 (9) | 0.0029 (9) |
| C4 | 0.0287 (10) | 0.0347 (11) | 0.0372 (11) | 0.0091 (9) | 0.0060 (8) | −0.0004 (9) |
| C5 | 0.0245 (9) | 0.0263 (9) | 0.0257 (9) | 0.0023 (7) | 0.0054 (7) | −0.0027 (7) |
| C6 | 0.0356 (11) | 0.0284 (10) | 0.0353 (10) | −0.0028 (8) | 0.0131 (9) | −0.0010 (8) |
| C7 | 0.0436 (12) | 0.0347 (12) | 0.0442 (12) | −0.0134 (10) | 0.0220 (10) | −0.0047 (10) |
| C8 | 0.0299 (10) | 0.0483 (13) | 0.0425 (12) | −0.0106 (10) | 0.0168 (9) | −0.0093 (10) |
| C9 | 0.0254 (9) | 0.0426 (12) | 0.0337 (10) | −0.0007 (9) | 0.0098 (8) | −0.0057 (9) |
| C10 | 0.0248 (9) | 0.0289 (10) | 0.0245 (8) | −0.0008 (7) | 0.0074 (7) | −0.0049 (7) |
| C11 | 0.0261 (9) | 0.0247 (9) | 0.0309 (9) | 0.0011 (7) | 0.0042 (7) | 0.0014 (8) |
| C12 | 0.0388 (12) | 0.0332 (12) | 0.0623 (15) | 0.0089 (9) | 0.0249 (11) | 0.0115 (11) |
| C13 | 0.0427 (14) | 0.0452 (15) | 0.105 (3) | 0.0125 (12) | 0.0408 (16) | 0.0190 (16) |
| O7 | 0.0602 (13) | 0.0449 (11) | 0.0585 (12) | 0.0192 (9) | 0.0318 (10) | 0.0107 (9) |
Geometric parameters (Å, °)
| Cu1—O1 | 1.9598 (14) | C2—H2 | 0.85 (3) |
| Cu1—N3 | 1.987 (2) | C3—C4 | 1.384 (4) |
| Cu1—N2 | 1.9970 (16) | C3—H3 | 0.90 (3) |
| Cu1—N1 | 2.0043 (17) | C4—C5 | 1.390 (3) |
| Cu1—O2i | 2.3965 (16) | C4—H4 | 0.86 (3) |
| Cl1—O4 | 1.4307 (16) | C5—C10 | 1.479 (3) |
| Cl1—O6 | 1.4358 (18) | C6—C7 | 1.387 (3) |
| Cl1—O5 | 1.4365 (19) | C6—H6 | 0.96 (3) |
| Cl1—O3 | 1.4469 (16) | C7—C8 | 1.377 (4) |
| O1—C11 | 1.277 (2) | C7—H7 | 0.90 (3) |
| O2—C11 | 1.244 (2) | C8—C9 | 1.381 (3) |
| O2—Cu1ii | 2.3965 (16) | C8—H8 | 0.95 (3) |
| N1—C1 | 1.340 (3) | C9—C10 | 1.396 (3) |
| N1—C5 | 1.358 (2) | C9—H9 | 0.96 (3) |
| N2—C6 | 1.343 (3) | C11—C12 | 1.536 (3) |
| N2—C10 | 1.353 (3) | C12—C13 | 1.488 (4) |
| N3—C12 | 1.481 (3) | C12—H12 | 1.15 (4) |
| N3—H2N3 | 0.74 (4) | C13—H13A | 1.04 (4) |
| N3—H1N3 | 0.83 (4) | C13—H13B | 1.04 (4) |
| C1—C2 | 1.386 (3) | C13—H13C | 1.16 (5) |
| C1—H1 | 0.92 (3) | O7—H1O7 | 0.79 (4) |
| C2—C3 | 1.380 (4) | O7—H2O7 | 0.69 (5) |
| O1—Cu1—N3 | 83.99 (7) | C4—C3—H3 | 120.4 (18) |
| O1—Cu1—N2 | 95.03 (6) | C3—C4—C5 | 119.4 (2) |
| N3—Cu1—N2 | 169.62 (10) | C3—C4—H4 | 119 (2) |
| O1—Cu1—N1 | 175.41 (6) | C5—C4—H4 | 122 (2) |
| N3—Cu1—N1 | 98.87 (7) | N1—C5—C4 | 121.28 (19) |
| N2—Cu1—N1 | 81.51 (7) | N1—C5—C10 | 114.65 (16) |
| O1—Cu1—O2i | 93.37 (6) | C4—C5—C10 | 124.07 (19) |
| N3—Cu1—O2i | 94.28 (9) | N2—C6—C7 | 122.0 (2) |
| N2—Cu1—O2i | 96.09 (6) | N2—C6—H6 | 116.2 (16) |
| N1—Cu1—O2i | 90.00 (6) | C7—C6—H6 | 121.8 (16) |
| O4—Cl1—O6 | 110.13 (11) | C8—C7—C6 | 119.0 (2) |
| O4—Cl1—O5 | 109.66 (11) | C8—C7—H7 | 123.1 (18) |
| O6—Cl1—O5 | 110.07 (13) | C6—C7—H7 | 117.9 (18) |
| O4—Cl1—O3 | 109.65 (11) | C7—C8—C9 | 119.6 (2) |
| O6—Cl1—O3 | 108.38 (11) | C7—C8—H8 | 121.0 (19) |
| O5—Cl1—O3 | 108.93 (13) | C9—C8—H8 | 119.4 (19) |
| C11—O1—Cu1 | 115.46 (12) | C8—C9—C10 | 118.9 (2) |
| C11—O2—Cu1ii | 121.01 (14) | C8—C9—H9 | 120.8 (17) |
| C1—N1—C5 | 118.82 (17) | C10—C9—H9 | 120.3 (17) |
| C1—N1—Cu1 | 126.92 (14) | N2—C10—C9 | 121.42 (19) |
| C5—N1—Cu1 | 114.25 (13) | N2—C10—C5 | 114.75 (16) |
| C6—N2—C10 | 119.06 (18) | C9—C10—C5 | 123.82 (19) |
| C6—N2—Cu1 | 125.95 (14) | O2—C11—O1 | 123.87 (19) |
| C10—N2—Cu1 | 114.56 (13) | O2—C11—C12 | 118.42 (19) |
| C12—N3—Cu1 | 110.57 (14) | O1—C11—C12 | 117.66 (18) |
| C12—N3—H2N3 | 101 (4) | N3—C12—C13 | 113.9 (2) |
| Cu1—N3—H2N3 | 117 (3) | N3—C12—C11 | 109.41 (19) |
| C12—N3—H1N3 | 109 (3) | C13—C12—C11 | 113.9 (2) |
| Cu1—N3—H1N3 | 108 (3) | N3—C12—H12 | 116 (2) |
| H2N3—N3—H1N3 | 111 (4) | C13—C12—H12 | 91.9 (19) |
| N1—C1—C2 | 122.4 (2) | C11—C12—H12 | 111 (2) |
| N1—C1—H1 | 118.5 (18) | C12—C13—H13A | 113 (2) |
| C2—C1—H1 | 119.1 (18) | C12—C13—H13B | 111 (2) |
| C3—C2—C1 | 119.0 (2) | H13A—C13—H13B | 102 (3) |
| C3—C2—H2 | 123 (2) | C12—C13—H13C | 102 (2) |
| C1—C2—H2 | 118 (2) | H13A—C13—H13C | 113 (3) |
| C2—C3—C4 | 119.1 (2) | H13B—C13—H13C | 117 (3) |
| C2—C3—H3 | 120.5 (18) | H1O7—O7—H2O7 | 102 (5) |
Symmetry codes: (i) −x+1, y+1/2, −z+1/2; (ii) −x+1, y−1/2, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3—H2N3···O7iii | 0.74 (4) | 2.60 (4) | 3.293 (4) | 159 (5) |
| N3—H1N3···O1i | 0.83 (4) | 2.48 (4) | 3.225 (3) | 149 (3) |
| N3—H1N3···O2i | 0.83 (4) | 2.91 (4) | 3.225 (3) | 105 (3) |
| N3—H1N3···O7i | 0.83 (4) | 2.70 (5) | 3.059 (3) | 108 (3) |
| N3—H2N3···O7i | 0.74 (4) | 2.69 (5) | 3.059 (3) | 114 (4) |
| O7—H1O7···O2i | 0.79 (4) | 2.08 (4) | 2.857 (3) | 166 (3) |
Symmetry codes: (iii) x, −y+1/2, z−1/2; (i) −x+1, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2300).
References
- Antolini, L., Marcotrigiano, G., Menabue, L. & Pellacani, G. C. (1983). Inorg. Chem.22, 141–145.
- Brandenburg, K. (1999). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (1998). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Masuda, H., Sugimori, T., Odani, A. & Yamauchi, O. (1991). Inorg. Chim. Acta, 180, 73–79.
- Sgarabotto, P., Bisceglie, P., Pelosi, G. & Adbel-Rahman, L. (1999). Polyhedron, 18, 2505–2510.
- Sheldrick, G. M. (1997). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Solans, X., Ruíz-Ramírez, L., Martínez, A., Gasque, L. & Moreno-Esparza, R. (1992). Acta Cryst. C48, 1785–1788.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808040725/tk2300sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808040725/tk2300Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report