Abstract
The asymmetric unit of the title compound, (C23H33N2)[Li(C32H16N8)]·0.5C3H6O·H2O, consists of two symmetry-unrelated lithium phthalocyanine (LiPc−) half-anions, centered at (1,0,0) and (0, ,0), respectively, the bis(adamantyl)imidazolium cation (BAI+), occupying a general site, an acetone molecule, disordered about the inversion centre at (0,
,0), respectively, the bis(adamantyl)imidazolium cation (BAI+), occupying a general site, an acetone molecule, disordered about the inversion centre at (0,  ,
,  ) and a water molecule at a general site. The LiPc− anions pack in a stepped pattern enclosing the bis(adamantyl)imidazolium cation. Attractions between the anion and cation are mediated by a water molecule which forms O—H⋯N hydrogen bonds. In addition, two C—H⋯O interactions are seen.
) and a water molecule at a general site. The LiPc− anions pack in a stepped pattern enclosing the bis(adamantyl)imidazolium cation. Attractions between the anion and cation are mediated by a water molecule which forms O—H⋯N hydrogen bonds. In addition, two C—H⋯O interactions are seen.
Related literature
Similar compounds utilizing nitrogen-based cations have been reported by Homborg & Kalz (1978a
▶,b
▶). For related structures see: Grossie et al. (2006 ▶).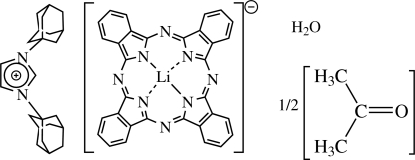
Experimental
Crystal data
(C23H33N2)[Li(C32H16N8)]·0.5C3H6O·H2O
M r = 904.04
Monoclinic,

a = 15.799 (3) Å
b = 17.165 (4) Å
c = 17.831 (4) Å
β = 108.374 (3)°
V = 4588.9 (16) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 173 (2) K
0.44 × 0.39 × 0.20 mm
Data collection
Bruker Smart APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2003 ▶) T min = 0.901, T max = 0.985
54676 measured reflections
14917 independent reflections
11038 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.129
S = 1.03
14917 reflections
649 parameters
8 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.37 e Å−3
Δρmin = −0.30 e Å−3
Data collection: SMART (Bruker, 2002 ▶); cell refinement: SAINT-Plus (Bruker, 2003 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶), ORTEP-3 for Windows, (Farrugia, 1997 ▶), OSCAIL, (McArdle, 1995 ▶); software used to prepare material for publication: enCIFer (Allen et al., 2004 ▶) and publCIF (Westrip, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks I, New_Global_Publ_Block. DOI: 10.1107/S1600536808041135/sj2558sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808041135/sj2558Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯N6 | 0.861 (18) | 2.405 (17) | 3.1618 (17) | 147.1 (18) |
| O1—H1B⋯N3i | 0.889 (18) | 2.025 (19) | 2.8911 (17) | 164.3 (16) |
| C33—H33⋯O1 | 0.95 | 2.18 | 3.127 (2) | 171 |
| C37—H37A⋯O1 | 0.99 | 2.52 | 3.493 (2) | 166 |
Symmetry code: (i)  .
.
Acknowledgments
The authors acknowledge the diffractometer time granted by A. Hunter, Youngstown State University.
supplementary crystallographic information
Comment
The asymmetric unit of the title compound consists of two symmetry-unrelated halves of lithium phthalocyanine anions (LiPc-), centered at 1,0,0 and 0, 1/2,0, respectively; the bis(adamantyl)imidazolium cation (BAI+), occupying a general site, an acetone molecule, at 0, 1/2,0.5, and a water molecule at a general site, Fig 1. In addition, the unit cell packing provides interesting detail of the arrangement of molecules within the crystal structure, as seen in Figure 2. Similar compounds utilizing nitrogen-based cations have been reported by Homborg & Kalz (1978a, 1978b).
Although they may appear to be in Figure 2, the symmetry-related LiPc- anions are not parallel, as seen in the angle (2.39°) between their mean planes. Additionally, their intermolecular distances are quite large (10.10 Å as measured between mesonitrogens) in comparison to those seen in Li2PC (3.06–3.38 Å)(Grossie, et al., 2006) The large spacing between LiPc- molecules is easily attributed to the bulky adamantyl substituents of the BAI+ molecules, in which one cation appears to be enclosed within four LiPc- anions forming ionic pockets. This is shown in Fig. 2, which presents the organized but unique packing of molecules. Rows of symmetry-related anions along the b axis are offset from each other, stacking in a stair-step manner. These molecules appear to be nearly orthogonal to the columnar anions, in which alternating columns have slightly different orientations. It is necessary, though, to view the packing from all angles to get a true understanding of ion arrangements.
Probably the most intriguing information obtained was the role of solvent molecules within the crystal structure. It was seen in the crystal structure of Li2PC that acetone and water ligated to lithium, forming dimers that were found in between LiPc- pairs. In the current structure it can be seen that water molecules are crucial to the crystallization of the complex ions. Here, it is noticed that water forms O—H···N and C—H···O hydrogen bonds, Table 1, with the two symmetry-unrelated LiPc- anions and one BAI+ cation, acting as an intermediate to the three ions. Interatomic distances between hydrogen atoms and isoindoline and meso nitrogen of individual LiPc- ions were found to be 2.405 (17) Å and 2.025 (19) Å, respectively. The distance between the oxygen atom of water and the hydrogen on the 2- position of the BAI+ cation was calculated to be about 2.18 Å.
Experimental
The 1,3-bis(1-adamantyl)imidazolium tetrafluoroborate (0.884 g) was purified by dissolving it in 70 ml of acetone and filtering the insoluble impurities. The solution was evaporated to dryness to give 0.843 g (1.98 mmol) of the pure salt, which was redissolved in 10 ml of acetone and added to a solution of 0.991 g (1.98 mmol) of dilithium phthalocyanine in 100 ml of acetone. The solution was evaporated to approximately 20–30 ml under reduced pressure to the point of crystallization, sealed and crystallized at 5°C for 72 h. The resulting solid was redissolved in 125 ml of hot acetone with stirring (some undissolved solid remained). The volume was reduced and crystallized at 5°C. The dry product isolated by filtration gave 0.893 g (55.3%) of purple crystals. m.p. 349–351°C. Anal. Calc. for C55H49LiN10 (856.99): C, 77.08; H, 5.76; N, 16.34. Found: C, 76.89; H, 5.90; N, 15.94.
Refinement
Hydrogen atoms of the water molecule and of the methyl group of the acetone molecule were located in a difference Fourier map and refined with appropriate distance restraints. All other H-atoms were positioned geometrically and refined using a riding model with d(C-H) = 0.95Å, Uiso=1.2Ueq (C) for aromatic 1.00Å, Uiso = 1.2Ueq (C) for CH and 0.99Å, Uiso = 1.2Ueq (C) for CH2 atoms
Figures
Fig. 1.
The asymmetric unit of I. Labelled atoms are related to unlabelled atoms by the symmetry codes –x+2, -y, -z for the Li1 anion and -x, -y+1, -z for the Li2 anion. Hydrogen atoms are not shown.
Fig. 2.
Unit cell packing diagram, viewed along the b axis. Acetone molecules have been omitted for clarity.
Crystal data
| (C23H33N2)[Li(C32H16N8)]·0.5C3H6O·H2O | F(000) = 1912 |
| Mr = 904.04 | Dx = 1.309 Mg m−3 |
| Monoclinic, P21/n | Melting point: 622 K |
| Hall symbol: -P 2yn | Mo Kα radiation, λ = 0.71073 Å |
| a = 15.799 (3) Å | Cell parameters from 64888 reflections |
| b = 17.165 (4) Å | θ = 2.4–30.1° |
| c = 17.831 (4) Å | µ = 0.08 mm−1 |
| β = 108.374 (3)° | T = 173 K |
| V = 4588.9 (16) Å3 | Block, violet |
| Z = 4 | 0.44 × 0.39 × 0.20 mm |
Data collection
| Bruker Smart APEXII CCD diffractometer | 14917 independent reflections |
| Radiation source: fine-focus sealed tube | 11038 reflections with I > 2σ(I) |
| graphite | Rint = 0.039 |
| ω scans | θmax = 31.7°, θmin = 1.7° |
| Absorption correction: multi-scan (SADABS; Bruker, 2003) | h = −22→23 |
| Tmin = 0.901, Tmax = 0.985 | k = −25→24 |
| 54676 measured reflections | l = −26→26 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.052 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.129 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0482P)2 + 2.081P] where P = (Fo2 + 2Fc2)/3 |
| 14917 reflections | (Δ/σ)max = 0.001 |
| 649 parameters | Δρmax = 0.37 e Å−3 |
| 8 restraints | Δρmin = −0.29 e Å−3 |
Special details
| Experimental. 1H NMR (300 MHz, DMSO-d6) δ = 9.35–9.25 (m, 8H, Ar—H), 9.06 (s, 1H, Ar—H), 8.12–8.02 (m, 8H, Ar—H), 8.01 (d, 2H, 3 J = 1.1 Hz, Ar—H), 2.24–2.09 (m, 18H, Al—H), 1.82–1.62 (m, 12H, Al—H); 13 C-NMR (75 MHz, DMSO-d6) δ = 154.14, 140.05, 131.23, 127.51, 119.31, 59.35, 41.46, 34.81, 28.83; IR (KBr) cm-1 = 3053 (Ar—H), 2912 (C—H), 1604 (C—C), 1583 (C—N), 1485 (C—N), 1092 (C—C), 1055 (C—N); UV/Vis (DMSO) λmax nm (log ε) = 665 (5.25), 636 (4.47), 601 (4.48), 380 (4.55), 327 (4.55), 255 (4.63); |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Li1 | 1.0000 | 0.0000 | 0.0000 | 0.0221 (7) | |
| N1 | 0.84511 (7) | 0.01780 (6) | 0.09200 (6) | 0.0183 (2) | |
| N2 | 0.94090 (7) | 0.08811 (6) | 0.03257 (6) | 0.0173 (2) | |
| N3 | 1.01005 (7) | 0.19613 (6) | −0.01323 (6) | 0.0182 (2) | |
| N4 | 1.06695 (7) | 0.07380 (6) | −0.04398 (6) | 0.0178 (2) | |
| C1 | 0.87993 (8) | 0.08236 (7) | 0.07194 (7) | 0.0167 (2) | |
| C2 | 0.85314 (8) | 0.15984 (7) | 0.08970 (7) | 0.0173 (2) | |
| C3 | 0.79734 (9) | 0.18458 (8) | 0.13159 (8) | 0.0213 (3) | |
| H3 | 0.7654 | 0.1483 | 0.1526 | 0.026* | |
| C4 | 0.78994 (10) | 0.26408 (8) | 0.14163 (8) | 0.0240 (3) | |
| H4 | 0.7524 | 0.2828 | 0.1701 | 0.029* | |
| C5 | 0.83698 (10) | 0.31696 (8) | 0.11036 (9) | 0.0256 (3) | |
| H5 | 0.8310 | 0.3711 | 0.1183 | 0.031* | |
| C6 | 0.89232 (9) | 0.29251 (8) | 0.06800 (8) | 0.0222 (3) | |
| H6 | 0.9237 | 0.3290 | 0.0466 | 0.027* | |
| C7 | 0.90028 (9) | 0.21275 (7) | 0.05796 (7) | 0.0179 (2) | |
| C8 | 0.95462 (8) | 0.16503 (7) | 0.02226 (7) | 0.0173 (2) | |
| C9 | 1.06334 (8) | 0.15280 (7) | −0.04204 (7) | 0.0172 (2) | |
| C10 | 1.12886 (8) | 0.18684 (7) | −0.07575 (7) | 0.0174 (2) | |
| C11 | 1.15259 (9) | 0.26268 (8) | −0.08836 (8) | 0.0201 (2) | |
| H11 | 1.1230 | 0.3062 | −0.0750 | 0.024* | |
| C12 | 1.22089 (9) | 0.27270 (8) | −0.12104 (8) | 0.0221 (3) | |
| H12 | 1.2382 | 0.3240 | −0.1301 | 0.026* | |
| C13 | 1.26472 (9) | 0.20899 (8) | −0.14097 (8) | 0.0228 (3) | |
| H13 | 1.3115 | 0.2177 | −0.1629 | 0.027* | |
| C14 | 1.24094 (9) | 0.13324 (8) | −0.12920 (8) | 0.0213 (3) | |
| H14 | 1.2704 | 0.0898 | −0.1428 | 0.026* | |
| C15 | 1.17233 (9) | 0.12325 (7) | −0.09671 (8) | 0.0181 (2) | |
| C16 | 1.13064 (9) | 0.05364 (7) | −0.07705 (7) | 0.0177 (2) | |
| Li2 | 0.0000 | 0.5000 | 0.0000 | 0.0223 (7) | |
| N5 | −0.00519 (7) | 0.50482 (6) | 0.18826 (6) | 0.0193 (2) | |
| N6 | 0.08896 (7) | 0.49153 (7) | 0.10472 (6) | 0.0192 (2) | |
| N7 | 0.22215 (7) | 0.47223 (6) | 0.06714 (7) | 0.0195 (2) | |
| N8 | 0.09266 (7) | 0.48310 (7) | −0.04998 (6) | 0.0187 (2) | |
| C17 | 0.07282 (9) | 0.49409 (7) | 0.17554 (7) | 0.0183 (2) | |
| C18 | 0.15585 (9) | 0.48247 (7) | 0.24040 (8) | 0.0187 (2) | |
| C19 | 0.17516 (10) | 0.47967 (8) | 0.32180 (8) | 0.0229 (3) | |
| H19 | 0.1297 | 0.4863 | 0.3457 | 0.027* | |
| C20 | 0.26314 (10) | 0.46682 (9) | 0.36727 (8) | 0.0279 (3) | |
| H20 | 0.2782 | 0.4649 | 0.4232 | 0.033* | |
| C21 | 0.32991 (10) | 0.45674 (9) | 0.33197 (9) | 0.0286 (3) | |
| H21 | 0.3895 | 0.4475 | 0.3643 | 0.034* | |
| C22 | 0.31062 (9) | 0.45991 (8) | 0.25046 (8) | 0.0237 (3) | |
| H22 | 0.3561 | 0.4534 | 0.2266 | 0.028* | |
| C23 | 0.22251 (9) | 0.47299 (8) | 0.20492 (8) | 0.0196 (2) | |
| C24 | 0.17785 (9) | 0.47881 (7) | 0.11946 (8) | 0.0187 (2) | |
| C25 | 0.18188 (9) | 0.47596 (7) | −0.01094 (8) | 0.0183 (2) | |
| C26 | 0.23144 (9) | 0.47344 (7) | −0.06777 (8) | 0.0183 (2) | |
| C27 | 0.32210 (9) | 0.47045 (8) | −0.05927 (8) | 0.0213 (3) | |
| H27 | 0.3657 | 0.4665 | −0.0086 | 0.026* | |
| C28 | 0.34626 (10) | 0.47343 (8) | −0.12769 (9) | 0.0251 (3) | |
| H28 | 0.4077 | 0.4719 | −0.1235 | 0.030* | |
| C29 | 0.28251 (10) | 0.47858 (9) | −0.20269 (9) | 0.0255 (3) | |
| H29 | 0.3013 | 0.4799 | −0.2483 | 0.031* | |
| C30 | 0.19217 (10) | 0.48184 (8) | −0.21123 (8) | 0.0224 (3) | |
| H30 | 0.1487 | 0.4857 | −0.2619 | 0.027* | |
| C31 | 0.16757 (9) | 0.47920 (7) | −0.14276 (8) | 0.0185 (2) | |
| C32 | 0.08121 (9) | 0.48562 (7) | −0.12921 (8) | 0.0179 (2) | |
| N9 | 0.21347 (8) | 0.75837 (7) | 0.32337 (7) | 0.0206 (2) | |
| N10 | 0.31358 (8) | 0.73900 (7) | 0.26556 (7) | 0.0210 (2) | |
| C33 | 0.22743 (9) | 0.73102 (8) | 0.25812 (8) | 0.0212 (3) | |
| H33 | 0.1832 | 0.7094 | 0.2137 | 0.025* | |
| C34 | 0.29366 (9) | 0.78481 (9) | 0.37394 (8) | 0.0238 (3) | |
| H34 | 0.3033 | 0.8071 | 0.4247 | 0.029* | |
| C35 | 0.35583 (9) | 0.77306 (9) | 0.33786 (8) | 0.0245 (3) | |
| H35 | 0.4173 | 0.7859 | 0.3584 | 0.029* | |
| C36 | 0.12688 (9) | 0.75260 (8) | 0.33990 (8) | 0.0191 (2) | |
| C37 | 0.05180 (9) | 0.78827 (8) | 0.27195 (8) | 0.0205 (3) | |
| H37A | 0.0471 | 0.7607 | 0.2220 | 0.025* | |
| H37B | 0.0649 | 0.8438 | 0.2654 | 0.025* | |
| C38 | −0.03623 (9) | 0.78110 (8) | 0.29063 (8) | 0.0222 (3) | |
| H38 | −0.0855 | 0.8046 | 0.2466 | 0.027* | |
| C39 | −0.02848 (10) | 0.82412 (9) | 0.36785 (9) | 0.0258 (3) | |
| H39A | −0.0155 | 0.8799 | 0.3624 | 0.031* | |
| H39B | −0.0856 | 0.8204 | 0.3796 | 0.031* | |
| C40 | 0.04642 (10) | 0.78776 (9) | 0.43521 (8) | 0.0262 (3) | |
| H40 | 0.0512 | 0.8156 | 0.4856 | 0.031* | |
| C41 | 0.13491 (9) | 0.79498 (9) | 0.41737 (8) | 0.0245 (3) | |
| H41A | 0.1491 | 0.8506 | 0.4127 | 0.029* | |
| H41B | 0.1837 | 0.7717 | 0.4610 | 0.029* | |
| C42 | −0.05610 (10) | 0.69499 (9) | 0.29889 (9) | 0.0252 (3) | |
| H42A | −0.0612 | 0.6673 | 0.2489 | 0.030* | |
| H42B | −0.1135 | 0.6896 | 0.3098 | 0.030* | |
| C43 | 0.01881 (10) | 0.65890 (8) | 0.36645 (9) | 0.0263 (3) | |
| H43 | 0.0055 | 0.6026 | 0.3719 | 0.032* | |
| C44 | 0.02643 (11) | 0.70154 (10) | 0.44379 (9) | 0.0300 (3) | |
| H44A | −0.0301 | 0.6963 | 0.4563 | 0.036* | |
| H44B | 0.0748 | 0.6781 | 0.4876 | 0.036* | |
| C45 | 0.10709 (10) | 0.66639 (8) | 0.34806 (9) | 0.0242 (3) | |
| H45A | 0.1560 | 0.6426 | 0.3912 | 0.029* | |
| H45B | 0.1027 | 0.6386 | 0.2983 | 0.029* | |
| C46 | 0.35926 (9) | 0.71388 (8) | 0.20826 (7) | 0.0188 (2) | |
| C47 | 0.29214 (9) | 0.67521 (8) | 0.13680 (8) | 0.0218 (3) | |
| H47A | 0.2441 | 0.7124 | 0.1105 | 0.026* | |
| H47B | 0.2648 | 0.6295 | 0.1540 | 0.026* | |
| C48 | 0.34101 (9) | 0.64948 (9) | 0.07899 (8) | 0.0240 (3) | |
| H48 | 0.2977 | 0.6241 | 0.0319 | 0.029* | |
| C49 | 0.38222 (11) | 0.72049 (10) | 0.05245 (9) | 0.0301 (3) | |
| H49A | 0.3349 | 0.7583 | 0.0262 | 0.036* | |
| H49B | 0.4125 | 0.7044 | 0.0140 | 0.036* | |
| C50 | 0.44974 (11) | 0.75862 (9) | 0.12455 (9) | 0.0294 (3) | |
| H50 | 0.4773 | 0.8048 | 0.1071 | 0.035* | |
| C51 | 0.52255 (10) | 0.70054 (10) | 0.16613 (9) | 0.0302 (3) | |
| H51A | 0.5655 | 0.7255 | 0.2128 | 0.036* | |
| H51B | 0.5553 | 0.6842 | 0.1297 | 0.036* | |
| C52 | 0.48106 (9) | 0.62939 (9) | 0.19228 (8) | 0.0243 (3) | |
| H52 | 0.5289 | 0.5912 | 0.2189 | 0.029* | |
| C53 | 0.43204 (9) | 0.65519 (8) | 0.24972 (8) | 0.0221 (3) | |
| H53A | 0.4747 | 0.6795 | 0.2970 | 0.027* | |
| H53B | 0.4049 | 0.6094 | 0.2671 | 0.027* | |
| C54 | 0.41484 (9) | 0.59165 (9) | 0.11957 (9) | 0.0254 (3) | |
| H54A | 0.4463 | 0.5752 | 0.0822 | 0.030* | |
| H54B | 0.3884 | 0.5448 | 0.1357 | 0.030* | |
| C55 | 0.40064 (10) | 0.78504 (8) | 0.18204 (9) | 0.0266 (3) | |
| H55A | 0.4430 | 0.8107 | 0.2286 | 0.032* | |
| H55B | 0.3534 | 0.8230 | 0.1557 | 0.032* | |
| O1 | 0.06786 (8) | 0.67405 (6) | 0.11486 (7) | 0.0297 (2) | |
| H1A | 0.0548 (13) | 0.6284 (10) | 0.0945 (12) | 0.045* | |
| H1B | 0.0393 (13) | 0.7070 (11) | 0.0768 (10) | 0.045* | |
| O2 | −0.0403 (2) | 0.44760 (18) | 0.39840 (18) | 0.0555 (7) | 0.50 |
| C56 | −0.0162 (3) | 0.4789 (2) | 0.4612 (2) | 0.0451 (9) | 0.50 |
| C57 | 0.0801 (2) | 0.47921 (16) | 0.50835 (16) | 0.0646 (7) | |
| H57A | 0.1107 (14) | 0.4355 (14) | 0.4973 (17) | 0.097* | |
| H57B | 0.0942 (15) | 0.4617 (18) | 0.5603 (12) | 0.097* | |
| H57C | 0.1131 (14) | 0.5205 (14) | 0.4976 (16) | 0.097* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Li1 | 0.0250 (17) | 0.0152 (14) | 0.0268 (17) | −0.0003 (12) | 0.0094 (14) | 0.0006 (12) |
| N1 | 0.0193 (5) | 0.0163 (5) | 0.0190 (5) | −0.0001 (4) | 0.0058 (4) | 0.0004 (4) |
| N2 | 0.0191 (5) | 0.0153 (5) | 0.0171 (5) | −0.0011 (4) | 0.0052 (4) | 0.0003 (4) |
| N3 | 0.0199 (5) | 0.0168 (5) | 0.0177 (5) | 0.0000 (4) | 0.0056 (4) | 0.0007 (4) |
| N4 | 0.0191 (5) | 0.0151 (5) | 0.0194 (5) | −0.0001 (4) | 0.0064 (4) | 0.0010 (4) |
| C1 | 0.0175 (6) | 0.0162 (5) | 0.0154 (5) | 0.0004 (4) | 0.0038 (4) | −0.0011 (4) |
| C2 | 0.0184 (6) | 0.0161 (5) | 0.0162 (6) | 0.0010 (4) | 0.0036 (5) | −0.0009 (4) |
| C3 | 0.0215 (6) | 0.0214 (6) | 0.0214 (6) | 0.0017 (5) | 0.0073 (5) | 0.0006 (5) |
| C4 | 0.0251 (7) | 0.0235 (6) | 0.0239 (7) | 0.0061 (5) | 0.0085 (5) | −0.0022 (5) |
| C5 | 0.0304 (7) | 0.0180 (6) | 0.0272 (7) | 0.0051 (5) | 0.0075 (6) | −0.0026 (5) |
| C6 | 0.0248 (7) | 0.0162 (6) | 0.0245 (7) | 0.0011 (5) | 0.0060 (5) | −0.0001 (5) |
| C7 | 0.0191 (6) | 0.0163 (5) | 0.0168 (6) | 0.0019 (4) | 0.0035 (5) | −0.0001 (4) |
| C8 | 0.0182 (6) | 0.0156 (5) | 0.0165 (6) | 0.0004 (4) | 0.0033 (5) | −0.0001 (4) |
| C9 | 0.0183 (6) | 0.0152 (5) | 0.0166 (6) | −0.0016 (4) | 0.0034 (5) | 0.0001 (4) |
| C10 | 0.0185 (6) | 0.0168 (5) | 0.0156 (5) | −0.0022 (4) | 0.0039 (4) | 0.0005 (4) |
| C11 | 0.0231 (6) | 0.0170 (6) | 0.0189 (6) | −0.0031 (5) | 0.0049 (5) | −0.0008 (5) |
| C12 | 0.0242 (7) | 0.0197 (6) | 0.0213 (6) | −0.0064 (5) | 0.0056 (5) | 0.0008 (5) |
| C13 | 0.0215 (6) | 0.0247 (6) | 0.0231 (6) | −0.0061 (5) | 0.0083 (5) | 0.0003 (5) |
| C14 | 0.0210 (6) | 0.0210 (6) | 0.0225 (6) | −0.0013 (5) | 0.0075 (5) | −0.0006 (5) |
| C15 | 0.0192 (6) | 0.0172 (5) | 0.0175 (6) | −0.0016 (5) | 0.0054 (5) | 0.0010 (4) |
| C16 | 0.0187 (6) | 0.0165 (5) | 0.0171 (6) | −0.0011 (4) | 0.0046 (5) | 0.0001 (4) |
| Li2 | 0.0212 (16) | 0.0281 (17) | 0.0162 (15) | 0.0012 (13) | 0.0040 (12) | −0.0005 (13) |
| N5 | 0.0207 (5) | 0.0195 (5) | 0.0172 (5) | 0.0008 (4) | 0.0052 (4) | 0.0001 (4) |
| N6 | 0.0185 (5) | 0.0233 (5) | 0.0155 (5) | 0.0016 (4) | 0.0047 (4) | −0.0006 (4) |
| N7 | 0.0193 (5) | 0.0208 (5) | 0.0176 (5) | 0.0020 (4) | 0.0046 (4) | −0.0005 (4) |
| N8 | 0.0179 (5) | 0.0215 (5) | 0.0160 (5) | 0.0010 (4) | 0.0043 (4) | 0.0004 (4) |
| C17 | 0.0192 (6) | 0.0184 (6) | 0.0164 (6) | 0.0002 (5) | 0.0042 (5) | −0.0002 (5) |
| C18 | 0.0202 (6) | 0.0168 (5) | 0.0175 (6) | 0.0008 (5) | 0.0036 (5) | 0.0003 (5) |
| C19 | 0.0276 (7) | 0.0225 (6) | 0.0169 (6) | 0.0025 (5) | 0.0047 (5) | −0.0004 (5) |
| C20 | 0.0320 (8) | 0.0305 (7) | 0.0163 (6) | 0.0040 (6) | 0.0004 (6) | 0.0013 (5) |
| C21 | 0.0241 (7) | 0.0334 (8) | 0.0218 (7) | 0.0057 (6) | −0.0022 (5) | 0.0019 (6) |
| C22 | 0.0205 (6) | 0.0263 (7) | 0.0217 (7) | 0.0042 (5) | 0.0028 (5) | 0.0007 (5) |
| C23 | 0.0213 (6) | 0.0183 (6) | 0.0171 (6) | 0.0021 (5) | 0.0027 (5) | −0.0007 (5) |
| C24 | 0.0184 (6) | 0.0190 (6) | 0.0174 (6) | 0.0009 (5) | 0.0038 (5) | −0.0001 (5) |
| C25 | 0.0180 (6) | 0.0181 (6) | 0.0185 (6) | 0.0016 (5) | 0.0052 (5) | −0.0010 (5) |
| C26 | 0.0204 (6) | 0.0155 (5) | 0.0194 (6) | 0.0007 (5) | 0.0068 (5) | −0.0015 (4) |
| C27 | 0.0199 (6) | 0.0213 (6) | 0.0224 (6) | 0.0007 (5) | 0.0063 (5) | −0.0025 (5) |
| C28 | 0.0218 (7) | 0.0266 (7) | 0.0296 (7) | 0.0014 (5) | 0.0122 (6) | −0.0024 (6) |
| C29 | 0.0284 (7) | 0.0276 (7) | 0.0248 (7) | 0.0010 (6) | 0.0143 (6) | −0.0002 (5) |
| C30 | 0.0262 (7) | 0.0221 (6) | 0.0201 (6) | 0.0012 (5) | 0.0089 (5) | −0.0007 (5) |
| C31 | 0.0205 (6) | 0.0154 (5) | 0.0203 (6) | 0.0012 (5) | 0.0074 (5) | −0.0010 (5) |
| C32 | 0.0198 (6) | 0.0169 (5) | 0.0175 (6) | 0.0002 (5) | 0.0065 (5) | −0.0010 (4) |
| N9 | 0.0190 (5) | 0.0252 (6) | 0.0181 (5) | 0.0013 (4) | 0.0064 (4) | −0.0031 (4) |
| N10 | 0.0189 (5) | 0.0261 (6) | 0.0180 (5) | 0.0018 (4) | 0.0059 (4) | −0.0028 (4) |
| C33 | 0.0185 (6) | 0.0275 (7) | 0.0173 (6) | 0.0018 (5) | 0.0054 (5) | −0.0022 (5) |
| C34 | 0.0203 (6) | 0.0303 (7) | 0.0196 (6) | 0.0003 (5) | 0.0048 (5) | −0.0059 (5) |
| C35 | 0.0198 (6) | 0.0318 (7) | 0.0206 (6) | −0.0002 (5) | 0.0047 (5) | −0.0063 (5) |
| C36 | 0.0190 (6) | 0.0223 (6) | 0.0175 (6) | 0.0013 (5) | 0.0078 (5) | −0.0012 (5) |
| C37 | 0.0211 (6) | 0.0226 (6) | 0.0187 (6) | −0.0001 (5) | 0.0073 (5) | 0.0011 (5) |
| C38 | 0.0200 (6) | 0.0246 (6) | 0.0223 (6) | 0.0011 (5) | 0.0071 (5) | 0.0009 (5) |
| C39 | 0.0246 (7) | 0.0260 (7) | 0.0305 (7) | 0.0011 (5) | 0.0140 (6) | −0.0044 (6) |
| C40 | 0.0267 (7) | 0.0349 (8) | 0.0202 (6) | −0.0023 (6) | 0.0120 (5) | −0.0080 (6) |
| C41 | 0.0225 (7) | 0.0336 (7) | 0.0189 (6) | −0.0012 (6) | 0.0085 (5) | −0.0069 (5) |
| C42 | 0.0247 (7) | 0.0276 (7) | 0.0256 (7) | −0.0065 (5) | 0.0113 (6) | −0.0046 (5) |
| C43 | 0.0315 (8) | 0.0223 (6) | 0.0286 (7) | −0.0010 (6) | 0.0146 (6) | 0.0030 (5) |
| C44 | 0.0327 (8) | 0.0385 (8) | 0.0226 (7) | 0.0008 (6) | 0.0142 (6) | 0.0050 (6) |
| C45 | 0.0283 (7) | 0.0219 (6) | 0.0243 (7) | 0.0036 (5) | 0.0108 (6) | 0.0020 (5) |
| C46 | 0.0176 (6) | 0.0239 (6) | 0.0161 (6) | 0.0005 (5) | 0.0072 (5) | −0.0011 (5) |
| C47 | 0.0164 (6) | 0.0301 (7) | 0.0189 (6) | 0.0002 (5) | 0.0057 (5) | −0.0037 (5) |
| C48 | 0.0199 (6) | 0.0342 (7) | 0.0177 (6) | −0.0007 (5) | 0.0057 (5) | −0.0044 (5) |
| C49 | 0.0334 (8) | 0.0388 (8) | 0.0206 (7) | 0.0006 (6) | 0.0121 (6) | 0.0029 (6) |
| C50 | 0.0348 (8) | 0.0307 (7) | 0.0284 (7) | −0.0084 (6) | 0.0180 (6) | −0.0012 (6) |
| C51 | 0.0200 (7) | 0.0452 (9) | 0.0278 (7) | −0.0074 (6) | 0.0112 (6) | −0.0105 (7) |
| C52 | 0.0182 (6) | 0.0320 (7) | 0.0226 (7) | 0.0049 (5) | 0.0061 (5) | −0.0035 (5) |
| C53 | 0.0194 (6) | 0.0274 (7) | 0.0194 (6) | 0.0027 (5) | 0.0058 (5) | −0.0003 (5) |
| C54 | 0.0227 (7) | 0.0293 (7) | 0.0254 (7) | 0.0001 (5) | 0.0092 (5) | −0.0068 (6) |
| C55 | 0.0313 (8) | 0.0233 (7) | 0.0271 (7) | −0.0022 (6) | 0.0121 (6) | −0.0006 (5) |
| O1 | 0.0350 (6) | 0.0229 (5) | 0.0269 (6) | 0.0015 (4) | 0.0035 (5) | 0.0014 (4) |
| O2 | 0.071 (2) | 0.0482 (17) | 0.0459 (17) | −0.0024 (15) | 0.0170 (15) | 0.0017 (13) |
| C56 | 0.065 (3) | 0.0350 (18) | 0.0354 (19) | −0.0166 (17) | 0.0160 (18) | 0.0093 (15) |
| C57 | 0.0824 (19) | 0.0574 (15) | 0.0572 (14) | −0.0113 (13) | 0.0265 (14) | 0.0118 (12) |
Geometric parameters (Å, °)
| Li1—N2 | 1.9592 (11) | N9—C33 | 1.3358 (17) |
| Li1—N2i | 1.9592 (11) | N9—C34 | 1.3798 (18) |
| Li1—N4 | 1.9650 (11) | N9—C36 | 1.4906 (17) |
| Li1—N4i | 1.9650 (11) | N10—C33 | 1.3323 (17) |
| N1—C1 | 1.3352 (16) | N10—C35 | 1.3808 (17) |
| N1—C16i | 1.3365 (16) | N10—C46 | 1.4885 (16) |
| N2—C8 | 1.3597 (16) | C33—H33 | 0.9500 |
| N2—C1 | 1.3625 (16) | C34—C35 | 1.3481 (19) |
| N3—C9 | 1.3411 (16) | C34—H34 | 0.9500 |
| N3—C8 | 1.3424 (16) | C35—H35 | 0.9500 |
| N4—C9 | 1.3581 (16) | C36—C45 | 1.529 (2) |
| N4—C16 | 1.3617 (16) | C36—C41 | 1.5307 (18) |
| C1—C2 | 1.4603 (17) | C36—C37 | 1.5309 (19) |
| C2—C3 | 1.3895 (18) | C37—C38 | 1.5337 (19) |
| C2—C7 | 1.4020 (18) | C37—H37A | 0.9900 |
| C3—C4 | 1.3859 (19) | C37—H37B | 0.9900 |
| C3—H3 | 0.9500 | C38—C42 | 1.528 (2) |
| C4—C5 | 1.396 (2) | C38—C39 | 1.533 (2) |
| C4—H4 | 0.9500 | C38—H38 | 1.0000 |
| C5—C6 | 1.388 (2) | C39—C40 | 1.528 (2) |
| C5—H5 | 0.9500 | C39—H39A | 0.9900 |
| C6—C7 | 1.3914 (18) | C39—H39B | 0.9900 |
| C6—H6 | 0.9500 | C40—C44 | 1.531 (2) |
| C7—C8 | 1.4688 (18) | C40—C41 | 1.5335 (19) |
| C9—C10 | 1.4720 (18) | C40—H40 | 1.0000 |
| C10—C11 | 1.3923 (18) | C41—H41A | 0.9900 |
| C10—C15 | 1.4016 (18) | C41—H41B | 0.9900 |
| C11—C12 | 1.3892 (19) | C42—C43 | 1.529 (2) |
| C11—H11 | 0.9500 | C42—H42A | 0.9900 |
| C12—C13 | 1.399 (2) | C42—H42B | 0.9900 |
| C12—H12 | 0.9500 | C43—C44 | 1.532 (2) |
| C13—C14 | 1.3875 (19) | C43—C45 | 1.535 (2) |
| C13—H13 | 0.9500 | C43—H43 | 1.0000 |
| C14—C15 | 1.3913 (18) | C44—H44A | 0.9900 |
| C14—H14 | 0.9500 | C44—H44B | 0.9900 |
| C15—C16 | 1.4593 (18) | C45—H45A | 0.9900 |
| C16—N1i | 1.3365 (16) | C45—H45B | 0.9900 |
| Li2—N6 | 1.9567 (11) | C46—C55 | 1.5267 (19) |
| Li2—N6ii | 1.9567 (11) | C46—C47 | 1.5287 (18) |
| Li2—N8 | 1.9606 (11) | C46—C53 | 1.5322 (19) |
| Li2—N8ii | 1.9606 (11) | C47—C48 | 1.5347 (19) |
| N5—C17 | 1.3343 (17) | C47—H47A | 0.9900 |
| N5—C32ii | 1.3345 (17) | C47—H47B | 0.9900 |
| N6—C24 | 1.3625 (17) | C48—C49 | 1.526 (2) |
| N6—C17 | 1.3656 (17) | C48—C54 | 1.529 (2) |
| N7—C24 | 1.3357 (17) | C48—H48 | 1.0000 |
| N7—C25 | 1.3374 (17) | C49—C50 | 1.534 (2) |
| N8—C25 | 1.3667 (17) | C49—H49A | 0.9900 |
| N8—C32 | 1.3672 (17) | C49—H49B | 0.9900 |
| C17—C18 | 1.4634 (18) | C50—C51 | 1.526 (2) |
| C18—C19 | 1.3867 (18) | C50—C55 | 1.536 (2) |
| C18—C23 | 1.3984 (19) | C50—H50 | 1.0000 |
| C19—C20 | 1.388 (2) | C51—C52 | 1.527 (2) |
| C19—H19 | 0.9500 | C51—H51A | 0.9900 |
| C20—C21 | 1.399 (2) | C51—H51B | 0.9900 |
| C20—H20 | 0.9500 | C52—C54 | 1.530 (2) |
| C21—C22 | 1.389 (2) | C52—C53 | 1.5315 (19) |
| C21—H21 | 0.9500 | C52—H52 | 1.0000 |
| C22—C23 | 1.3910 (19) | C53—H53A | 0.9900 |
| C22—H22 | 0.9500 | C53—H53B | 0.9900 |
| C23—C24 | 1.4671 (18) | C54—H54A | 0.9900 |
| C25—C26 | 1.4636 (18) | C54—H54B | 0.9900 |
| C26—C27 | 1.3930 (19) | C55—H55A | 0.9900 |
| C26—C31 | 1.4019 (19) | C55—H55B | 0.9900 |
| C27—C28 | 1.3894 (19) | O1—H1A | 0.862 (15) |
| C27—H27 | 0.9500 | O1—H1B | 0.890 (15) |
| C28—C29 | 1.400 (2) | O2—C56 | 1.191 (5) |
| C28—H28 | 0.9500 | C56—C57iii | 1.476 (5) |
| C29—C30 | 1.388 (2) | C56—C57 | 1.487 (5) |
| C29—H29 | 0.9500 | C56—C56iii | 1.503 (8) |
| C30—C31 | 1.3939 (18) | C57—C56iii | 1.476 (5) |
| C30—H30 | 0.9500 | C57—H57A | 0.948 (18) |
| C31—C32 | 1.4628 (18) | C57—H57B | 0.931 (18) |
| C32—N5ii | 1.3345 (17) | C57—H57C | 0.935 (18) |
| N2—Li1—N2i | 180.00 (8) | N10—C35—H35 | 126.3 |
| N2—Li1—N4 | 89.28 (5) | N9—C36—C45 | 108.13 (11) |
| N2i—Li1—N4 | 90.72 (5) | N9—C36—C41 | 109.07 (11) |
| N2—Li1—N4i | 90.72 (5) | C45—C36—C41 | 109.59 (11) |
| N2i—Li1—N4i | 89.28 (5) | N9—C36—C37 | 110.33 (10) |
| N4—Li1—N4i | 180.00 (8) | C45—C36—C37 | 109.37 (11) |
| C1—N1—C16i | 122.68 (11) | C41—C36—C37 | 110.32 (11) |
| C8—N2—C1 | 107.98 (10) | C36—C37—C38 | 109.00 (11) |
| C8—N2—Li1 | 126.75 (9) | C36—C37—H37A | 109.9 |
| C1—N2—Li1 | 125.25 (8) | C38—C37—H37A | 109.9 |
| C9—N3—C8 | 122.79 (11) | C36—C37—H37B | 109.9 |
| C9—N4—C16 | 107.90 (11) | C38—C37—H37B | 109.9 |
| C9—N4—Li1 | 126.97 (9) | H37A—C37—H37B | 108.3 |
| C16—N4—Li1 | 124.98 (9) | C42—C38—C39 | 109.88 (12) |
| N1—C1—N2 | 128.03 (11) | C42—C38—C37 | 109.06 (11) |
| N1—C1—C2 | 121.71 (11) | C39—C38—C37 | 109.59 (11) |
| N2—C1—C2 | 110.25 (11) | C42—C38—H38 | 109.4 |
| C3—C2—C7 | 121.79 (12) | C39—C38—H38 | 109.4 |
| C3—C2—C1 | 132.17 (12) | C37—C38—H38 | 109.4 |
| C7—C2—C1 | 105.98 (11) | C40—C39—C38 | 109.39 (11) |
| C4—C3—C2 | 117.65 (13) | C40—C39—H39A | 109.8 |
| C4—C3—H3 | 121.2 | C38—C39—H39A | 109.8 |
| C2—C3—H3 | 121.2 | C40—C39—H39B | 109.8 |
| C3—C4—C5 | 120.76 (13) | C38—C39—H39B | 109.8 |
| C3—C4—H4 | 119.6 | H39A—C39—H39B | 108.2 |
| C5—C4—H4 | 119.6 | C39—C40—C44 | 109.89 (12) |
| C6—C5—C4 | 121.77 (13) | C39—C40—C41 | 109.54 (12) |
| C6—C5—H5 | 119.1 | C44—C40—C41 | 109.21 (12) |
| C4—C5—H5 | 119.1 | C39—C40—H40 | 109.4 |
| C5—C6—C7 | 117.74 (13) | C44—C40—H40 | 109.4 |
| C5—C6—H6 | 121.1 | C41—C40—H40 | 109.4 |
| C7—C6—H6 | 121.1 | C36—C41—C40 | 109.10 (11) |
| C6—C7—C2 | 120.29 (12) | C36—C41—H41A | 109.9 |
| C6—C7—C8 | 133.90 (12) | C40—C41—H41A | 109.9 |
| C2—C7—C8 | 105.72 (11) | C36—C41—H41B | 109.9 |
| N3—C8—N2 | 127.26 (12) | C40—C41—H41B | 109.9 |
| N3—C8—C7 | 122.66 (11) | H41A—C41—H41B | 108.3 |
| N2—C8—C7 | 110.07 (11) | C38—C42—C43 | 109.65 (12) |
| N3—C9—N4 | 126.87 (12) | C38—C42—H42A | 109.7 |
| N3—C9—C10 | 122.93 (11) | C43—C42—H42A | 109.7 |
| N4—C9—C10 | 110.20 (11) | C38—C42—H42B | 109.7 |
| C11—C10—C15 | 120.37 (12) | C43—C42—H42B | 109.7 |
| C11—C10—C9 | 134.17 (12) | H42A—C42—H42B | 108.2 |
| C15—C10—C9 | 105.46 (11) | C42—C43—C44 | 109.72 (12) |
| C12—C11—C10 | 117.89 (12) | C42—C43—C45 | 109.12 (11) |
| C12—C11—H11 | 121.1 | C44—C43—C45 | 109.47 (12) |
| C10—C11—H11 | 121.1 | C42—C43—H43 | 109.5 |
| C11—C12—C13 | 121.46 (12) | C44—C43—H43 | 109.5 |
| C11—C12—H12 | 119.3 | C45—C43—H43 | 109.5 |
| C13—C12—H12 | 119.3 | C40—C44—C43 | 109.39 (12) |
| C14—C13—C12 | 120.97 (13) | C40—C44—H44A | 109.8 |
| C14—C13—H13 | 119.5 | C43—C44—H44A | 109.8 |
| C12—C13—H13 | 119.5 | C40—C44—H44B | 109.8 |
| C13—C14—C15 | 117.54 (13) | C43—C44—H44B | 109.8 |
| C13—C14—H14 | 121.2 | H44A—C44—H44B | 108.2 |
| C15—C14—H14 | 121.2 | C36—C45—C43 | 109.16 (11) |
| C14—C15—C10 | 121.77 (12) | C36—C45—H45A | 109.8 |
| C14—C15—C16 | 132.13 (12) | C43—C45—H45A | 109.8 |
| C10—C15—C16 | 106.10 (11) | C36—C45—H45B | 109.8 |
| N1i—C16—N4 | 128.09 (12) | C43—C45—H45B | 109.8 |
| N1i—C16—C15 | 121.59 (12) | H45A—C45—H45B | 108.3 |
| N4—C16—C15 | 110.31 (11) | N10—C46—C55 | 109.00 (11) |
| N6—Li2—N6ii | 180.00 (7) | N10—C46—C47 | 109.81 (10) |
| N6—Li2—N8 | 90.47 (5) | C55—C46—C47 | 110.10 (11) |
| N6ii—Li2—N8 | 89.53 (5) | N10—C46—C53 | 108.16 (10) |
| N6—Li2—N8ii | 89.53 (5) | C55—C46—C53 | 110.19 (11) |
| N6ii—Li2—N8ii | 90.47 (5) | C47—C46—C53 | 109.55 (11) |
| N8—Li2—N8ii | 180.00 (3) | C46—C47—C48 | 108.73 (11) |
| C17—N5—C32ii | 122.18 (11) | C46—C47—H47A | 109.9 |
| C24—N6—C17 | 107.97 (11) | C48—C47—H47A | 109.9 |
| C24—N6—Li2 | 125.63 (9) | C46—C47—H47B | 109.9 |
| C17—N6—Li2 | 126.35 (9) | C48—C47—H47B | 109.9 |
| C24—N7—C25 | 122.74 (12) | H47A—C47—H47B | 108.3 |
| C25—N8—C32 | 107.97 (11) | C49—C48—C54 | 109.21 (12) |
| C25—N8—Li2 | 125.43 (9) | C49—C48—C47 | 109.43 (12) |
| C32—N8—Li2 | 126.19 (9) | C54—C48—C47 | 109.83 (11) |
| N5—C17—N6 | 127.84 (12) | C49—C48—H48 | 109.5 |
| N5—C17—C18 | 122.04 (12) | C54—C48—H48 | 109.5 |
| N6—C17—C18 | 110.13 (11) | C47—C48—H48 | 109.5 |
| C19—C18—C23 | 121.39 (12) | C48—C49—C50 | 109.44 (12) |
| C19—C18—C17 | 132.72 (13) | C48—C49—H49A | 109.8 |
| C23—C18—C17 | 105.89 (11) | C50—C49—H49A | 109.8 |
| C18—C19—C20 | 117.78 (13) | C48—C49—H49B | 109.8 |
| C18—C19—H19 | 121.1 | C50—C49—H49B | 109.8 |
| C20—C19—H19 | 121.1 | H49A—C49—H49B | 108.2 |
| C19—C20—C21 | 120.99 (13) | C51—C50—C49 | 110.30 (13) |
| C19—C20—H20 | 119.5 | C51—C50—C55 | 109.30 (12) |
| C21—C20—H20 | 119.5 | C49—C50—C55 | 108.73 (13) |
| C22—C21—C20 | 121.20 (13) | C51—C50—H50 | 109.5 |
| C22—C21—H21 | 119.4 | C49—C50—H50 | 109.5 |
| C20—C21—H21 | 119.4 | C55—C50—H50 | 109.5 |
| C21—C22—C23 | 117.79 (13) | C50—C51—C52 | 109.91 (12) |
| C21—C22—H22 | 121.1 | C50—C51—H51A | 109.7 |
| C23—C22—H22 | 121.1 | C52—C51—H51A | 109.7 |
| C22—C23—C18 | 120.83 (12) | C50—C51—H51B | 109.7 |
| C22—C23—C24 | 133.03 (13) | C52—C51—H51B | 109.7 |
| C18—C23—C24 | 106.13 (11) | H51A—C51—H51B | 108.2 |
| N7—C24—N6 | 127.88 (12) | C51—C52—C54 | 108.96 (12) |
| N7—C24—C23 | 122.24 (12) | C51—C52—C53 | 109.23 (12) |
| N6—C24—C23 | 109.88 (11) | C54—C52—C53 | 109.53 (11) |
| N7—C25—N8 | 127.62 (12) | C51—C52—H52 | 109.7 |
| N7—C25—C26 | 122.45 (12) | C54—C52—H52 | 109.7 |
| N8—C25—C26 | 109.93 (11) | C53—C52—H52 | 109.7 |
| C27—C26—C31 | 120.84 (12) | C52—C53—C46 | 109.16 (11) |
| C27—C26—C25 | 132.99 (12) | C52—C53—H53A | 109.8 |
| C31—C26—C25 | 106.09 (11) | C46—C53—H53A | 109.8 |
| C28—C27—C26 | 117.44 (13) | C52—C53—H53B | 109.8 |
| C28—C27—H27 | 121.3 | C46—C53—H53B | 109.8 |
| C26—C27—H27 | 121.3 | H53A—C53—H53B | 108.3 |
| C27—C28—C29 | 121.79 (13) | C48—C54—C52 | 110.00 (12) |
| C27—C28—H28 | 119.1 | C48—C54—H54A | 109.7 |
| C29—C28—H28 | 119.1 | C52—C54—H54A | 109.7 |
| C30—C29—C28 | 120.83 (13) | C48—C54—H54B | 109.7 |
| C30—C29—H29 | 119.6 | C52—C54—H54B | 109.7 |
| C28—C29—H29 | 119.6 | H54A—C54—H54B | 108.2 |
| C29—C30—C31 | 117.62 (13) | C46—C55—C50 | 108.86 (12) |
| C29—C30—H30 | 121.2 | C46—C55—H55A | 109.9 |
| C31—C30—H30 | 121.2 | C50—C55—H55A | 109.9 |
| C30—C31—C26 | 121.48 (12) | C46—C55—H55B | 109.9 |
| C30—C31—C32 | 132.41 (13) | C50—C55—H55B | 109.9 |
| C26—C31—C32 | 106.03 (11) | H55A—C55—H55B | 108.3 |
| N5ii—C32—N8 | 127.75 (12) | H1A—O1—H1B | 105.0 (18) |
| N5ii—C32—C31 | 122.23 (12) | O2—C56—C57iii | 120.8 (4) |
| N8—C32—C31 | 109.99 (11) | O2—C56—C57 | 120.0 (4) |
| C33—N9—C34 | 108.36 (11) | C57iii—C56—C57 | 119.1 (3) |
| C33—N9—C36 | 124.24 (11) | O2—C56—C56iii | 177.4 (5) |
| C34—N9—C36 | 127.12 (11) | C57iii—C56—C56iii | 59.9 (3) |
| C33—N10—C35 | 108.33 (11) | C57—C56—C56iii | 59.2 (3) |
| C33—N10—C46 | 127.08 (11) | C56iii—C57—C56 | 60.9 (3) |
| C35—N10—C46 | 124.55 (11) | C56iii—C57—H57A | 156.5 (19) |
| N10—C33—N9 | 108.73 (12) | C56—C57—H57A | 112.2 (15) |
| N10—C33—H33 | 125.6 | C56iii—C57—H57B | 76.6 (18) |
| N9—C33—H33 | 125.6 | C56—C57—H57B | 116.3 (15) |
| C35—C34—N9 | 107.22 (12) | H57A—C57—H57B | 88 (2) |
| C35—C34—H34 | 126.4 | C56iii—C57—H57C | 101.4 (18) |
| N9—C34—H34 | 126.4 | C56—C57—H57C | 115.1 (15) |
| C34—C35—N10 | 107.36 (12) | H57A—C57—H57C | 102 (3) |
| C34—C35—H35 | 126.3 | H57B—C57—H57C | 118 (3) |
| N4—Li1—N2—C8 | −0.79 (11) | C24—N7—C25—N8 | −3.2 (2) |
| N4i—Li1—N2—C8 | 179.21 (11) | C24—N7—C25—C26 | 176.20 (12) |
| N4—Li1—N2—C1 | −178.85 (10) | C32—N8—C25—N7 | 179.25 (13) |
| N4i—Li1—N2—C1 | 1.15 (10) | Li2—N8—C25—N7 | 6.3 (2) |
| N2—Li1—N4—C9 | 1.91 (11) | C32—N8—C25—C26 | −0.18 (14) |
| N2i—Li1—N4—C9 | −178.09 (11) | Li2—N8—C25—C26 | −173.14 (8) |
| N2—Li1—N4—C16 | 176.80 (11) | N7—C25—C26—C27 | −3.0 (2) |
| N2i—Li1—N4—C16 | −3.20 (11) | N8—C25—C26—C27 | 176.45 (14) |
| C16i—N1—C1—N2 | 2.2 (2) | N7—C25—C26—C31 | −179.63 (12) |
| C16i—N1—C1—C2 | −179.29 (12) | N8—C25—C26—C31 | −0.17 (14) |
| C8—N2—C1—N1 | 177.65 (12) | C31—C26—C27—C28 | 0.03 (19) |
| Li1—N2—C1—N1 | −3.99 (19) | C25—C26—C27—C28 | −176.19 (13) |
| C8—N2—C1—C2 | −1.00 (14) | C26—C27—C28—C29 | −0.5 (2) |
| Li1—N2—C1—C2 | 177.36 (8) | C27—C28—C29—C30 | 0.8 (2) |
| N1—C1—C2—C3 | 5.0 (2) | C28—C29—C30—C31 | −0.5 (2) |
| N2—C1—C2—C3 | −176.21 (13) | C29—C30—C31—C26 | 0.0 (2) |
| N1—C1—C2—C7 | −177.94 (12) | C29—C30—C31—C32 | 176.01 (14) |
| N2—C1—C2—C7 | 0.80 (14) | C27—C26—C31—C30 | 0.24 (19) |
| C7—C2—C3—C4 | −0.38 (19) | C25—C26—C31—C30 | 177.36 (12) |
| C1—C2—C3—C4 | 176.23 (13) | C27—C26—C31—C32 | −176.71 (12) |
| C2—C3—C4—C5 | 0.2 (2) | C25—C26—C31—C32 | 0.41 (13) |
| C3—C4—C5—C6 | 0.3 (2) | C25—N8—C32—N5ii | −177.29 (13) |
| C4—C5—C6—C7 | −0.5 (2) | Li2—N8—C32—N5ii | −4.40 (19) |
| C5—C6—C7—C2 | 0.3 (2) | C25—N8—C32—C31 | 0.45 (14) |
| C5—C6—C7—C8 | −175.63 (14) | Li2—N8—C32—C31 | 173.34 (8) |
| C3—C2—C7—C6 | 0.2 (2) | C30—C31—C32—N5ii | 0.9 (2) |
| C1—C2—C7—C6 | −177.23 (12) | C26—C31—C32—N5ii | 177.34 (12) |
| C3—C2—C7—C8 | 177.10 (12) | C30—C31—C32—N8 | −177.02 (14) |
| C1—C2—C7—C8 | −0.29 (13) | C26—C31—C32—N8 | −0.55 (14) |
| C9—N3—C8—N2 | −2.3 (2) | C35—N10—C33—N9 | −0.38 (16) |
| C9—N3—C8—C7 | 176.25 (12) | C46—N10—C33—N9 | 177.78 (12) |
| C1—N2—C8—N3 | 179.48 (12) | C34—N9—C33—N10 | 0.13 (16) |
| Li1—N2—C8—N3 | 1.15 (19) | C36—N9—C33—N10 | −174.13 (12) |
| C1—N2—C8—C7 | 0.81 (14) | C33—N9—C34—C35 | 0.17 (17) |
| Li1—N2—C8—C7 | −177.52 (8) | C36—N9—C34—C35 | 174.22 (13) |
| C6—C7—C8—N3 | −2.7 (2) | N9—C34—C35—N10 | −0.40 (17) |
| C2—C7—C8—N3 | −179.05 (12) | C33—N10—C35—C34 | 0.48 (17) |
| C6—C7—C8—N2 | 176.03 (14) | C46—N10—C35—C34 | −177.73 (13) |
| C2—C7—C8—N2 | −0.30 (14) | C33—N9—C36—C45 | 64.57 (16) |
| C8—N3—C9—N4 | 3.6 (2) | C34—N9—C36—C45 | −108.59 (15) |
| C8—N3—C9—C10 | −175.40 (12) | C33—N9—C36—C41 | −176.32 (13) |
| C16—N4—C9—N3 | −179.22 (12) | C34—N9—C36—C41 | 10.52 (19) |
| Li1—N4—C9—N3 | −3.61 (19) | C33—N9—C36—C37 | −55.00 (17) |
| C16—N4—C9—C10 | −0.14 (14) | C34—N9—C36—C37 | 131.84 (14) |
| Li1—N4—C9—C10 | 175.46 (8) | N9—C36—C37—C38 | 179.77 (11) |
| N3—C9—C10—C11 | −1.2 (2) | C45—C36—C37—C38 | 60.96 (14) |
| N4—C9—C10—C11 | 179.65 (14) | C41—C36—C37—C38 | −59.65 (14) |
| N3—C9—C10—C15 | 178.30 (12) | C36—C37—C38—C42 | −60.66 (14) |
| N4—C9—C10—C15 | −0.82 (14) | C36—C37—C38—C39 | 59.67 (14) |
| C15—C10—C11—C12 | −0.87 (19) | C42—C38—C39—C40 | 59.37 (15) |
| C9—C10—C11—C12 | 178.61 (13) | C37—C38—C39—C40 | −60.45 (15) |
| C10—C11—C12—C13 | 0.1 (2) | C38—C39—C40—C44 | −59.55 (15) |
| C11—C12—C13—C14 | 0.4 (2) | C38—C39—C40—C41 | 60.44 (15) |
| C12—C13—C14—C15 | −0.2 (2) | N9—C36—C41—C40 | −178.95 (11) |
| C13—C14—C15—C10 | −0.6 (2) | C45—C36—C41—C40 | −60.75 (15) |
| C13—C14—C15—C16 | 179.63 (13) | C37—C36—C41—C40 | 59.72 (15) |
| C11—C10—C15—C14 | 1.1 (2) | C39—C40—C41—C36 | −59.79 (15) |
| C9—C10—C15—C14 | −178.49 (12) | C44—C40—C41—C36 | 60.61 (15) |
| C11—C10—C15—C16 | −179.02 (12) | C39—C38—C42—C43 | −59.47 (15) |
| C9—C10—C15—C16 | 1.36 (13) | C37—C38—C42—C43 | 60.67 (14) |
| C9—N4—C16—N1i | −178.37 (13) | C38—C42—C43—C44 | 59.57 (15) |
| Li1—N4—C16—N1i | 5.92 (19) | C38—C42—C43—C45 | −60.37 (15) |
| C9—N4—C16—C15 | 1.03 (14) | C39—C40—C44—C43 | 59.74 (15) |
| Li1—N4—C16—C15 | −174.68 (8) | C41—C40—C44—C43 | −60.45 (16) |
| C14—C15—C16—N1i | −2.3 (2) | C42—C43—C44—C40 | −59.58 (16) |
| C10—C15—C16—N1i | 177.90 (12) | C45—C43—C44—C40 | 60.15 (16) |
| C14—C15—C16—N4 | 178.29 (14) | N9—C36—C45—C43 | 179.11 (11) |
| C10—C15—C16—N4 | −1.54 (14) | C41—C36—C45—C43 | 60.33 (15) |
| N8—Li2—N6—C24 | 0.27 (11) | C37—C36—C45—C43 | −60.72 (14) |
| N8ii—Li2—N6—C24 | −179.73 (11) | C42—C43—C45—C36 | 60.18 (15) |
| N8—Li2—N6—C17 | −176.88 (11) | C44—C43—C45—C36 | −59.92 (15) |
| N8ii—Li2—N6—C17 | 3.12 (11) | C33—N10—C46—C55 | 120.09 (15) |
| N6—Li2—N8—C25 | −4.10 (11) | C35—N10—C46—C55 | −62.03 (17) |
| N6ii—Li2—N8—C25 | 175.90 (11) | C33—N10—C46—C47 | −0.60 (19) |
| N6—Li2—N8—C32 | −175.80 (11) | C35—N10—C46—C47 | 177.28 (13) |
| N6ii—Li2—N8—C32 | 4.20 (11) | C33—N10—C46—C53 | −120.10 (14) |
| C32ii—N5—C17—N6 | 0.5 (2) | C35—N10—C46—C53 | 57.77 (17) |
| C32ii—N5—C17—C18 | −179.08 (12) | N10—C46—C47—C48 | −179.67 (11) |
| C24—N6—C17—N5 | −179.52 (13) | C55—C46—C47—C48 | 60.31 (15) |
| Li2—N6—C17—N5 | −2.0 (2) | C53—C46—C47—C48 | −61.02 (14) |
| C24—N6—C17—C18 | 0.07 (15) | C46—C47—C48—C49 | −59.97 (15) |
| Li2—N6—C17—C18 | 177.63 (9) | C46—C47—C48—C54 | 59.90 (15) |
| N5—C17—C18—C19 | 0.0 (2) | C54—C48—C49—C50 | −59.32 (16) |
| N6—C17—C18—C19 | −179.67 (14) | C47—C48—C49—C50 | 60.93 (16) |
| N5—C17—C18—C23 | 179.73 (12) | C48—C49—C50—C51 | 58.81 (16) |
| N6—C17—C18—C23 | 0.11 (15) | C48—C49—C50—C55 | −61.02 (16) |
| C23—C18—C19—C20 | −0.2 (2) | C49—C50—C51—C52 | −58.90 (15) |
| C17—C18—C19—C20 | 179.54 (14) | C55—C50—C51—C52 | 60.58 (15) |
| C18—C19—C20—C21 | −0.3 (2) | C50—C51—C52—C54 | 59.22 (15) |
| C19—C20—C21—C22 | 0.6 (2) | C50—C51—C52—C53 | −60.39 (15) |
| C20—C21—C22—C23 | −0.4 (2) | C51—C52—C53—C46 | 59.54 (15) |
| C21—C22—C23—C18 | −0.1 (2) | C54—C52—C53—C46 | −59.72 (15) |
| C21—C22—C23—C24 | −179.03 (14) | N10—C46—C53—C52 | −179.12 (11) |
| C19—C18—C23—C22 | 0.4 (2) | C55—C46—C53—C52 | −60.06 (14) |
| C17—C18—C23—C22 | −179.42 (12) | C47—C46—C53—C52 | 61.21 (14) |
| C19—C18—C23—C24 | 179.58 (12) | C49—C48—C54—C52 | 60.81 (15) |
| C17—C18—C23—C24 | −0.23 (14) | C47—C48—C54—C52 | −59.20 (15) |
| C25—N7—C24—N6 | −1.5 (2) | C51—C52—C54—C48 | −60.47 (15) |
| C25—N7—C24—C23 | 178.69 (12) | C53—C52—C54—C48 | 58.95 (16) |
| C17—N6—C24—N7 | 179.97 (13) | N10—C46—C55—C50 | 178.57 (11) |
| Li2—N6—C24—N7 | 2.4 (2) | C47—C46—C55—C50 | −60.91 (15) |
| C17—N6—C24—C23 | −0.22 (15) | C53—C46—C55—C50 | 60.03 (15) |
| Li2—N6—C24—C23 | −177.80 (9) | C51—C50—C55—C46 | −59.87 (16) |
| C22—C23—C24—N7 | −0.8 (2) | C49—C50—C55—C46 | 60.58 (16) |
| C18—C23—C24—N7 | −179.89 (12) | O2—C56—C57—C56iii | 177.2 (5) |
| C22—C23—C24—N6 | 179.34 (14) | C57iii—C56—C57—C56iii | 0.000 (1) |
| C18—C23—C24—N6 | 0.28 (15) |
Symmetry codes: (i) −x+2, −y, −z; (ii) −x, −y+1, −z; (iii) −x, −y+1, −z+1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···N6 | 0.86 (2) | 2.40 (2) | 3.1618 (17) | 147 (2) |
| O1—H1B···N3iv | 0.89 (2) | 2.03 (2) | 2.8911 (17) | 164 (2) |
| C33—H33···O1 | 0.95 | 2.18 | 3.127 (2) | 171 |
| C37—H37A···O1 | 0.99 | 2.52 | 3.493 (2) | 166 |
Symmetry codes: (iv) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2558).
References
- Allen, F. H., Johnson, O., Shields, G. P., Smith, B. R. & Towler, M. (2004). J. Appl. Cryst.37, 335–338.
- Bruker (2002). SMART Bruker AXS Inc., Madison (WI), USA.
- Bruker (2003). SAINT-Plus and SADABS Bruker AXS Inc., Madison (WI), USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Grossie, D. A., Feld, W. A., Scanlon, L., Sandi, G. & Wawrzak, Z. (2006). Acta Cryst. E62, m827–m829.
- Homborg, H. & Kalz, W. (1978a). Z. Naturforsch. Teil B, 33, 968–975.
- Homborg, H. & Kalz, W. (1978b). Z. Naturforsch. Teil B, 33, 1067–1071.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- McArdle, P. (1995). J. Appl. Cryst.28, 65. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2009). publCIF In preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, New_Global_Publ_Block. DOI: 10.1107/S1600536808041135/sj2558sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808041135/sj2558Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




