Abstract
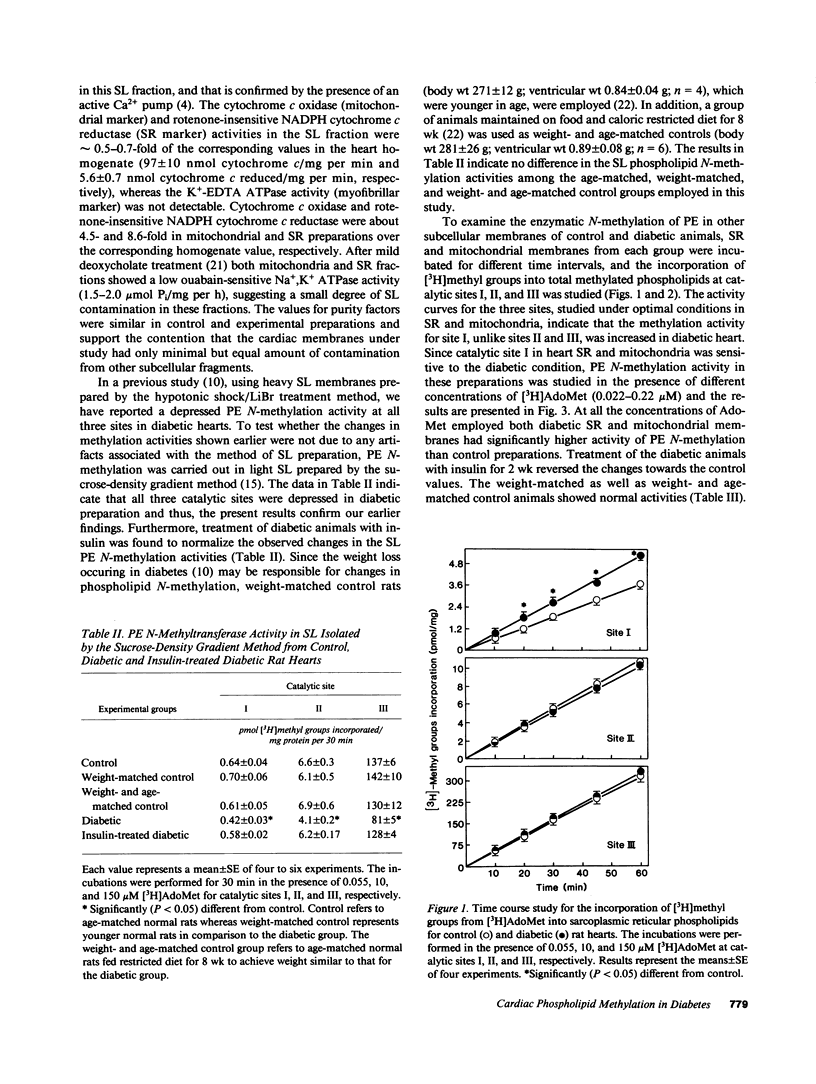
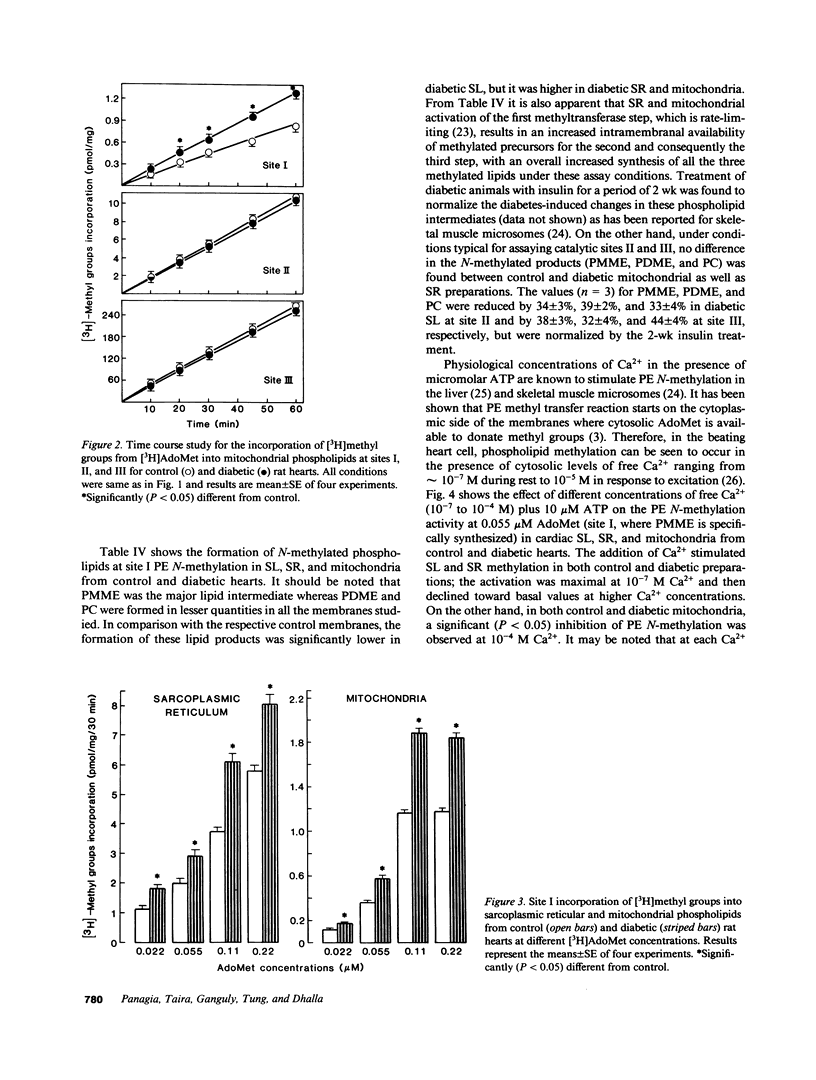
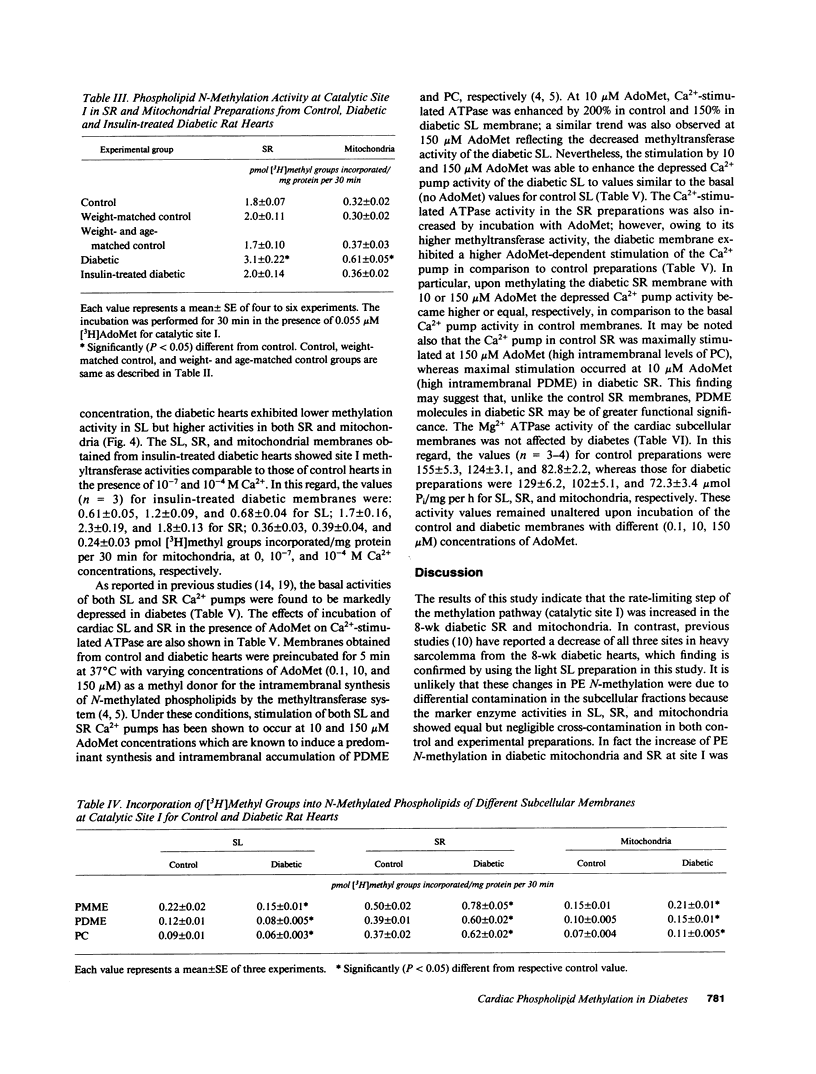
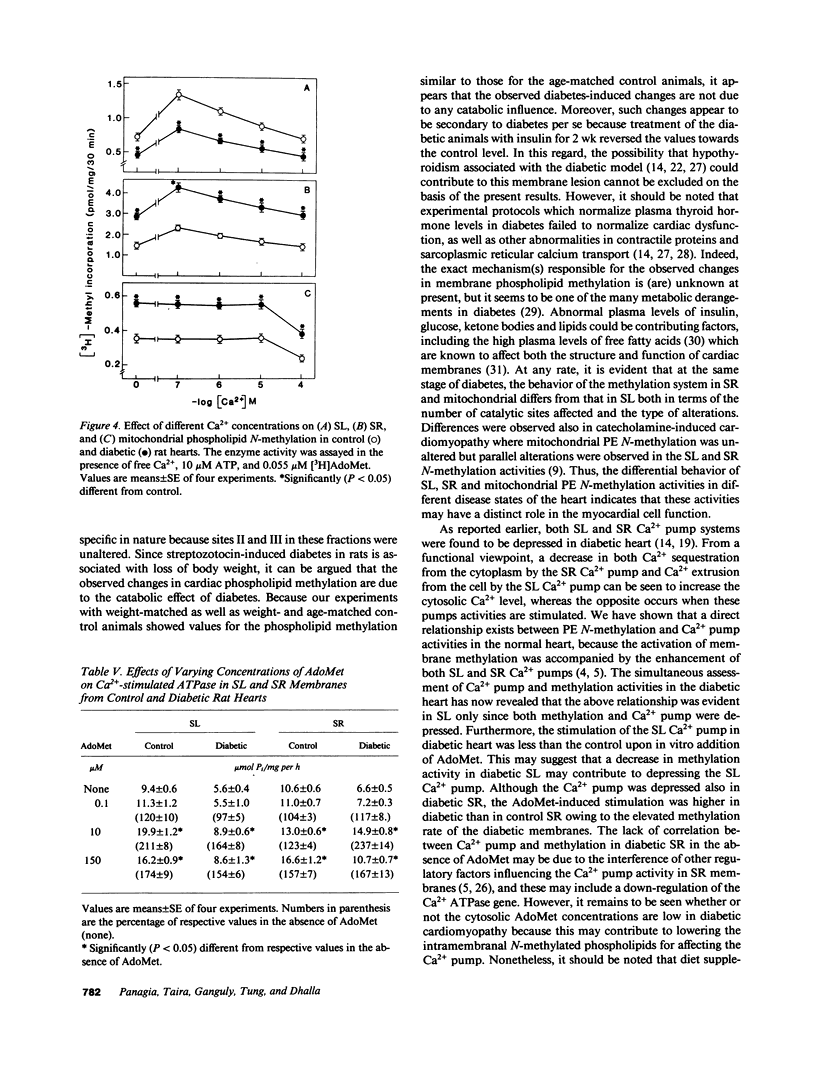
Phosphatidylethanolamine N-methylation was examined in cardiac subcellular membranes after inducing chronic experimental diabetes in rats (65 mg streptozotocin/kg, i.v.). The incorporation of radiolabeled methyl groups from S-adenosyl-L-methionine in diabetic sarcolemma was significantly depressed at all three catalytic sites (I, II, and III) of the methyltransferase system. An increase in methyl group incorporation was evident at site I without any changes at sites II and III in diabetic sarcoplasmic reticulum and mitochondria. Similar changes were also seen for the individual N-methylated lipids (monomethyl-, dimethylphosphatidylethanolamine, and phosphatidylcholine) specifically formed at each catalytic site in all cardiac membranes from diabetic animals. These alterations in N-methylation were reversible by a 14-d insulin therapy to the diabetic animals. In the presence of 10 microM ATP and 0.1 microM Ca2+, N-methylation was maximally activated at site I in both control and diabetic sarcolemma and sarcoplasmic reticulum, but not in mitochondria. Incubation of cardiac membranes with of S-adenosyl-L-methionine showed that Ca2(+)-stimulated ATPase activities in both sarcolemma and sarcoplasmic reticulum were augmented; however, the activation of diabetic sarcolemma was lesser and that of diabetic sarcoplasmic reticulum was greater in comparison with the control preparations. These results identify alterations in phosphatidylethanolamine N-methylation in subcellular membranes from diabetic heart, and it is suggested that these defects may be crucial in the development of cardiac dysfunction in chronic diabetes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany S., Varela I., Harper J. F., Mato J. M. Calmodulin regulation of phospholipid and fatty acid methylation by rat liver microsomes. J Biol Chem. 1982 Aug 25;257(16):9249–9251. [PubMed] [Google Scholar]
- Barbee R. W., Shepherd R. E., Burns A. H. T3 treatment does not prevent myocardial dysfunction in chronically diabetic rats. Am J Physiol. 1988 Feb;254(2 Pt 2):H265–H273. doi: 10.1152/ajpheart.1988.254.2.H265. [DOI] [PubMed] [Google Scholar]
- Chen V., Ianuzzo C. D., Fong B. C., Spitzer J. J. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984 Nov;33(11):1078–1084. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Pierce G. N., Panagia V., Singal P. K., Beamish R. E. Calcium movements in relation to heart function. Basic Res Cardiol. 1982 Mar-Apr;77(2):117–139. doi: 10.1007/BF01908167. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Dhalla K. S., Innes I. R., Beamish R. E., Dhalla N. S. Altered norepinephrine turnover and metabolism in diabetic cardiomyopathy. Circ Res. 1986 Dec;59(6):684–693. doi: 10.1161/01.res.59.6.684. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Panagia V., Okumura K., Dhalla N. S. Activation of Ca2+-stimulated ATPase by phospholipid N-methylation in cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1985 Jul 16;130(1):472–478. doi: 10.1016/0006-291x(85)90441-3. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Pierce G. N., Dhalla K. S., Dhalla N. S. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983 Jun;244(6):E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Rice K. M., Panagia V., Dhalla N. S. Sarcolemmal phosphatidylethanolamine N-methylation in diabetic cardiomyopathy. Circ Res. 1984 Oct;55(4):504–512. doi: 10.1161/01.res.55.4.504. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Heyliger C. E., Rodrigues B., McNeill J. H. Effect of choline and methionine treatment on cardiac dysfunction of diabetic rats. Diabetes. 1986 Oct;35(10):1152–1157. doi: 10.2337/diab.35.10.1152. [DOI] [PubMed] [Google Scholar]
- Lamers J. M., Hartog J. M., Verdouw P. D., Hülsmann W. C. Dietary fatty acids and myocardial function. Basic Res Cardiol. 1987;82 (Suppl 1):209–221. doi: 10.1007/978-3-662-08390-1_25. [DOI] [PubMed] [Google Scholar]
- Makino N., Dhalla K. S., Elimban V., Dhalla N. S. Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol. 1987 Aug;253(2 Pt 1):E202–E207. doi: 10.1152/ajpendo.1987.253.2.E202. [DOI] [PubMed] [Google Scholar]
- Malhotra A., Penpargkul S., Fein F. S., Sonnenblick E. H., Scheuer J. The effect of streptozotocin-induced diabetes in rats on cardiac contractile proteins. Circ Res. 1981 Dec;49(6):1243–1250. doi: 10.1161/01.res.49.6.1243. [DOI] [PubMed] [Google Scholar]
- Okumura K., Panagia V., Beamish R. E., Dhalla N. S. Biphasic changes in the sarcolemmal phosphatidylethanolamine N-methylation activity in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol. 1987 Apr;19(4):357–366. doi: 10.1016/s0022-2828(87)80581-3. [DOI] [PubMed] [Google Scholar]
- Panagia V., Ganguly P. K., Dhalla N. S. Characterization of heart sarcolemmal phospholipid methylation. Biochim Biophys Acta. 1984 Mar 7;792(3):245–253. doi: 10.1016/0005-2760(84)90192-9. [DOI] [PubMed] [Google Scholar]
- Panagia V., Ganguly P. K., Okumura K., Dhalla N. S. Subcellular localization of phosphatidylethanolamine N-methylation activity in rat heart. J Mol Cell Cardiol. 1985 Dec;17(12):1151–1159. doi: 10.1016/s0022-2828(85)80111-5. [DOI] [PubMed] [Google Scholar]
- Panagia V., Lamers J. M., Singal P. K., Dhalla N. S. Ca2+- and Mg2+-dependent ATPase activities in the deoxycholate-treated rat heart sarcolemma. Int J Biochem. 1982;14(5):387–397. doi: 10.1016/0020-711x(82)90024-6. [DOI] [PubMed] [Google Scholar]
- Panagia V., Makino N., Ganguly P. K., Dhalla N. S. Inhibition of Na+-Ca2+ exchange in heart sarcolemmal vesicles by phosphatidylethanolamine N-methylation. Eur J Biochem. 1987 Aug 3;166(3):597–603. doi: 10.1111/j.1432-1033.1987.tb13555.x. [DOI] [PubMed] [Google Scholar]
- Panagia V., Okumura K., Makino N., Dhalla N. S. Stimulation of Ca2+-pump in rat heart sarcolemma by phosphatidylethanolamine N-methylation. Biochim Biophys Acta. 1986 Apr 14;856(2):383–387. doi: 10.1016/0005-2736(86)90049-0. [DOI] [PubMed] [Google Scholar]
- Panagia V., Okumura K., Shah K. R., Dhalla N. S. Modification of sarcolemmal phosphatidylethanolamine N-methylation during heart hypertrophy. Am J Physiol. 1987 Jul;253(1 Pt 2):H8–15. doi: 10.1152/ajpheart.1987.253.1.H8. [DOI] [PubMed] [Google Scholar]
- Penpargkul S., Schaible T., Yipintsoi T., Scheuer J. The effect of diabetes on performance and metabolism of rat hearts. Circ Res. 1980 Dec;47(6):911–921. doi: 10.1161/01.res.47.6.911. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Kutryk M. J., Dhalla N. S. Alterations in Ca2+ binding by and composition of the cardiac sarcolemmal membrane in chronic diabetes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5412–5416. doi: 10.1073/pnas.80.17.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts B. J. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem. 1979 Jul 25;254(14):6232–6235. [PubMed] [Google Scholar]
- Prasad C., Edwards R. M. Increased phospholipid methylation in the myocardium of alcoholic rats. Biochem Biophys Res Commun. 1983 Mar 16;111(2):710–716. doi: 10.1016/0006-291x(83)90363-7. [DOI] [PubMed] [Google Scholar]
- Seager M. J., Singal P. K., Orchard R., Pierce G. N., Dhalla N. S. Cardiac cell damage: a primary myocardial disease in streptozotocin-induced chronic diabetes. Br J Exp Pathol. 1984 Oct;65(5):613–623. [PMC free article] [PubMed] [Google Scholar]
- Taira Y., Ganguly P. K., Panagia V., Dhalla N. S. Increased SR phospholipid N-methylation in skeletal muscle of diabetic rats. Am J Physiol. 1988 Sep;255(3 Pt 1):E347–E352. doi: 10.1152/ajpendo.1988.255.3.E347. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Ridgway N. D. The methylation of phosphatidylethanolamine. Prog Lipid Res. 1988;27(1):61–79. doi: 10.1016/0163-7827(88)90005-7. [DOI] [PubMed] [Google Scholar]



