Abstract
The title zinc complex, [Zn(C8H8NO2)2(C6H6N2O)2], is composed of two monodentate 4-(methylamino)benzoate and two monodentate nicotinamide ligands. The coordination around the Zn atom is distorted tetrahedral. The dihedral angles between the two benzene rings and the two pyridine rings are 78.30 (6) and 68.86 (5)°. In the crystal structure, intermolecular N—H⋯O hydrogen bonds link the molecules into an infinite three-dimensional network.
Related literature
For general backgroud, see: Antolini et al. (1982 ▶); Krishnamachari (1974 ▶); Nadzhafov et al. (1981 ▶). For related structures, see: Necefoğlu et al. (2002 ▶); Hökelek et al. (2007 ▶).
Experimental
Crystal data
[Zn(C8H8NO2)2(C6H6N2O)2]
M r = 609.93
Monoclinic,
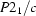
a = 8.085 (4) Å
b = 16.036 (7) Å
c = 21.333 (4) Å
β = 95.78 (3)°
V = 2751.8 (19) Å3
Z = 4
Mo Kα radiation
μ = 0.95 mm−1
T = 294 (2) K
0.55 × 0.20 × 0.10 mm
Data collection
Rigaku AFC-7S diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.624, T max = 0.911
17939 measured reflections
17079 independent reflections
7521 reflections with I > 2σ(I)
R int = 0.045
3 standard reflections every 150 reflections intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.148
S = 1.00
17079 reflections
380 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.67 e Å−3
Δρmin = −0.58 e Å−3
Data collection: MSC/AFC Diffractometer Control Software (Molecular Structure Corporation, 1994 ▶); cell refinement: MSC/AFC Diffractometer Control Software; data reduction: TEXSAN for Windows (Molecular Structure Corporation, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and Mercury (Macrae, 2006 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808042852/su2084sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808042852/su2084Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Zn1—O1 | 1.9584 (14) |
| Zn1—O3 | 1.9210 (14) |
| Zn1—N1 | 2.0722 (15) |
| Zn1—N3 | 2.0854 (15) |
| O1—Zn1—N1 | 96.85 (6) |
| O1—Zn1—N3 | 105.85 (6) |
| O3—Zn1—O1 | 143.41 (6) |
| O3—Zn1—N1 | 105.91 (5) |
| O3—Zn1—N3 | 95.81 (6) |
| N1—Zn1—N3 | 104.58 (5) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2B⋯O2i | 0.86 | 2.00 | 2.839 (3) | 166 |
| N4—H4B⋯O6ii | 0.86 | 2.12 | 2.950 (3) | 161 |
| N6—H61⋯O4ii | 0.88 (2) | 2.10 (2) | 2.955 (3) | 166 (2) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
supplementary crystallographic information
Comment
Transition metal complexes with biochemical molecules show interesting physical and/or chemical properties, through which they may find applications in biological systems (Antolini et al., 1982). The structure-function-coordination relationships of the arylcarboxylate ion in ZnII complexes of benzoic acid derivatives may be changed, depending on the nature and position of the substituted groups on the benzene ring, the nature of the additional ligand molecule or solvent, and the medium of the synthesis (Nadzhafov et al., 1981). Nicotinamide (NA) is one form of niacin, and a deficiency of this vitamin leads to loss of copper from the body, known as pellagra disease. Victims of pellagra show unusually high serum and urinary copper levels (Krishnamachari, 1974).
The structure determination of the title compound, a zinc complex with two methylaminobenzoate (MAB) and two nicotinamide (NA) ligands, was undertaken in order to determine the properties of the MAB and NA ligands and also to compare the results obtained with those reported previously.
The molecular structure of the title monomeric complex, containing two monodenate MAB and two monodentate NA ligands, is illustrated in Fig. 1. The coordination around the Zn atom is distorted tetrahedral (Table 1).
The near equalities of the C1—O1 [1.284 (2) Å], C1—O2 [1.248 (2) Å] and C9—O3 [1.278 (2) Å], C9—O4 [1.241 (2) Å] bonds in the carboxylate groups indicate delocalized bonding arrangements, rather than localized single and double bonds, as in the other zinc(II) complexes: bis(4-hydroxybenzoato-κO)bis(nicotinamide-κN)zinc(II) (Necefoğlu et al., 2002) and diaquabis(N,N'-diethylnicotinamide-κN)bis(4-\ fluorobenzoato-κO)- zinc(II) (Hökelek et al., 2007). This may be due to the intramolecular N—H···O hydrogen bonding of the carboxylate O atoms (Table 2).
The Zn atom is displaced out of the least-squares plane of the carboxylate groups (O1/C1/O2) and (O3/C9/O4) by -0.1070 (3) Å and -0.0967 (4) Å, respectively. The dihedral angles between the planar carboxylate groups (O1/C1/O2) and (O3/C9/O4) and the adjacent benzene rings, A (C2—C7) and B (C10—C15), are 7.92 (13)° and 6.60 (13)°, respectively. The dihedral angles between the two benzene rings, A and B, and the two pyridine rings, C (N1/C17—C21) and D (N3/C23—C27), are A/B = 78.30 (6)° and C/D = 68.86 (5)°. The benzene rings are oriented with respect to the pyridine rings at dihedral angles of A/C = 20.95 (5)°, A/D = 77.94 (6)°, B/C = 80.59 (5)° and B/D = 33.66 (6)°.
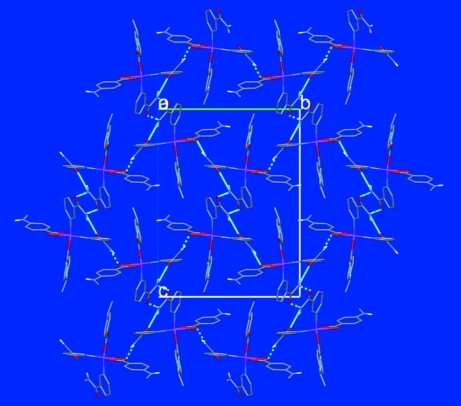
As can be seen from the crystal packing diagram (Fig. 2), the molecules are linked by intermolecular N—H···O hydrogen bonds to form an infinite three-dimensional network (Table 2).
Experimental
The title compound was prepared by the reaction of ZnNO4 (1.61 g, 10 mmol) in H2O (25 ml) and nicotinamide (2.44 g, 20 mmol) in H2O (100 ml), with sodium 4-N-methylaminobenzoate (3.00 g, 20 mmol) in H2O (100 ml), at room temperature. The mixture was filtered and set aside to crystallize at ambient temperature for one week, giving colorless single crystals.
Refinement
The NH H-atoms, H51 and H61, were located in difference Fourier maps and freely refined [N—H = 0.88 (2) and 0.90 (3) Å, resepectively]. The remaining H atoms were positioned geometrically and treated as riding atoms: N-H = 0.86 Å, C-H = 0.93 - 0.96 Å, with Uiso(H) = 1.2 or 1.5Ueq(parent N- or C-atom).
Figures
Fig. 1.
The molecular structure of the title complex, with the atom-numbering scheme and displacement ellipsoids drawn at the 40% probability level.
Fig. 2.
A view down the a axis of the crystal packing of the title compound, showing the N-H···O hydrogen bonds (blue dashed lines) linking the molecules to form an infinite three-dimensional network (H atoms not involved in hydrogen bonding have been omitted for clarity).
Crystal data
| [Zn(C8H8NO2)2(C6H6N2O)2] | F(000) = 1264 |
| Mr = 609.93 | Dx = 1.472 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 25 reflections |
| a = 8.085 (4) Å | θ = 20.1–27.1° |
| b = 16.036 (7) Å | µ = 0.95 mm−1 |
| c = 21.333 (4) Å | T = 294 K |
| β = 95.78 (3)° | Plate, colorless |
| V = 2751.8 (19) Å3 | 0.55 × 0.20 × 0.10 mm |
| Z = 4 |
Data collection
| Rigaku AFC-7S diffractometer | 7521 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.045 |
| graphite | θmax = 40.0°, θmin = 2.3° |
| ω/2θ scans | h = 0→14 |
| Absorption correction: ψ scan (North et al., 1968) | k = 0→29 |
| Tmin = 0.624, Tmax = 0.911 | l = −38→38 |
| 17939 measured reflections | 3 standard reflections every 150 reflections |
| 17079 independent reflections | intensity decay: 1% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.148 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.064P)2] where P = (Fo2 + 2Fc2)/3 |
| 17079 reflections | (Δ/σ)max = 0.003 |
| 380 parameters | Δρmax = 0.67 e Å−3 |
| 0 restraints | Δρmin = −0.58 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Zn1 | 0.70261 (2) | 0.883707 (11) | 0.824978 (8) | 0.02982 (5) | |
| O1 | 0.48643 (15) | 0.83449 (8) | 0.83709 (6) | 0.0433 (3) | |
| O2 | 0.65060 (16) | 0.72567 (9) | 0.83365 (7) | 0.0499 (3) | |
| O3 | 0.89177 (14) | 0.87348 (8) | 0.77778 (6) | 0.0399 (3) | |
| O4 | 0.72134 (14) | 0.85533 (10) | 0.69085 (7) | 0.0480 (3) | |
| O5 | 1.3622 (2) | 0.92075 (12) | 1.03306 (7) | 0.0696 (5) | |
| O6 | 0.12579 (14) | 1.06871 (10) | 0.83276 (6) | 0.0501 (3) | |
| N1 | 0.62073 (14) | 1.00559 (8) | 0.81250 (6) | 0.0294 (2) | |
| N2 | 0.14054 (17) | 1.16197 (10) | 0.75439 (8) | 0.0461 (4) | |
| H2A | 0.0346 | 1.1691 | 0.7494 | 0.055* | |
| H2B | 0.2033 | 1.1889 | 0.7312 | 0.055* | |
| N3 | 0.83802 (17) | 0.88321 (8) | 0.91350 (6) | 0.0332 (3) | |
| N4 | 1.31932 (19) | 0.98898 (11) | 0.94118 (7) | 0.0474 (4) | |
| H4A | 1.4181 | 1.0095 | 0.9451 | 0.057* | |
| H4B | 1.2521 | 1.0007 | 0.9085 | 0.057* | |
| N5 | −0.0098 (3) | 0.54968 (13) | 0.91042 (12) | 0.0686 (6) | |
| H51 | 0.006 (3) | 0.4943 (18) | 0.9059 (13) | 0.081 (9)* | |
| N6 | 1.44462 (19) | 0.85451 (14) | 0.58922 (8) | 0.0509 (4) | |
| H61 | 1.538 (3) | 0.8568 (15) | 0.6141 (11) | 0.056 (7)* | |
| C1 | 0.51107 (19) | 0.75548 (11) | 0.84074 (7) | 0.0343 (3) | |
| C2 | 0.37136 (19) | 0.70213 (10) | 0.85572 (7) | 0.0323 (3) | |
| C3 | 0.3855 (2) | 0.61500 (11) | 0.85629 (9) | 0.0419 (4) | |
| H3 | 0.4828 | 0.5904 | 0.8454 | 0.050* | |
| C4 | 0.2581 (2) | 0.56531 (11) | 0.87261 (10) | 0.0487 (4) | |
| H4 | 0.2692 | 0.5076 | 0.8713 | 0.058* | |
| C5 | 0.1117 (2) | 0.60028 (11) | 0.89119 (9) | 0.0430 (4) | |
| C6 | 0.0946 (2) | 0.68736 (11) | 0.88884 (9) | 0.0418 (4) | |
| H6 | −0.0029 | 0.7123 | 0.8992 | 0.050* | |
| C7 | 0.2234 (2) | 0.73610 (10) | 0.87094 (9) | 0.0373 (3) | |
| H7 | 0.2099 | 0.7937 | 0.8691 | 0.045* | |
| C8 | −0.1605 (3) | 0.57827 (18) | 0.93347 (14) | 0.0713 (7) | |
| H8A | −0.1340 | 0.6133 | 0.9696 | 0.107* | |
| H8B | −0.2241 | 0.5312 | 0.9452 | 0.107* | |
| H8C | −0.2243 | 0.6095 | 0.9011 | 0.107* | |
| C9 | 0.86333 (18) | 0.86285 (10) | 0.71831 (8) | 0.0315 (3) | |
| C10 | 1.01355 (17) | 0.85983 (10) | 0.68309 (7) | 0.0298 (3) | |
| C11 | 1.00398 (19) | 0.84079 (11) | 0.61932 (8) | 0.0373 (3) | |
| H11 | 0.9007 | 0.8295 | 0.5977 | 0.045* | |
| C12 | 1.1442 (2) | 0.83816 (12) | 0.58699 (7) | 0.0391 (4) | |
| H12 | 1.1342 | 0.8246 | 0.5444 | 0.047* | |
| C13 | 1.30180 (19) | 0.85588 (11) | 0.61844 (7) | 0.0342 (3) | |
| C14 | 1.31028 (18) | 0.87565 (12) | 0.68256 (8) | 0.0384 (4) | |
| H14 | 1.4128 | 0.8882 | 0.7043 | 0.046* | |
| C15 | 1.17060 (18) | 0.87688 (11) | 0.71403 (7) | 0.0359 (3) | |
| H15 | 1.1805 | 0.8893 | 0.7568 | 0.043* | |
| C16 | 1.4524 (3) | 0.8232 (2) | 0.52666 (11) | 0.0807 (9) | |
| H16A | 1.4162 | 0.7662 | 0.5247 | 0.121* | |
| H16B | 1.5648 | 0.8265 | 0.5160 | 0.121* | |
| H16C | 1.3815 | 0.8560 | 0.4974 | 0.121* | |
| C17 | 0.45776 (16) | 1.02117 (9) | 0.80810 (7) | 0.0292 (3) | |
| H17 | 0.3849 | 0.9764 | 0.8089 | 0.035* | |
| C18 | 0.39254 (17) | 1.10066 (9) | 0.80236 (7) | 0.0286 (3) | |
| C19 | 0.50145 (18) | 1.16809 (10) | 0.80272 (8) | 0.0333 (3) | |
| H19 | 0.4616 | 1.2225 | 0.7995 | 0.040* | |
| C20 | 0.66992 (19) | 1.15209 (11) | 0.80794 (9) | 0.0377 (3) | |
| H20 | 0.7458 | 1.1958 | 0.8086 | 0.045* | |
| C21 | 0.72488 (17) | 1.07040 (11) | 0.81222 (8) | 0.0339 (3) | |
| H21 | 0.8386 | 1.0601 | 0.8150 | 0.041* | |
| C22 | 0.20678 (17) | 1.10968 (10) | 0.79763 (8) | 0.0324 (3) | |
| C23 | 0.99457 (19) | 0.91195 (10) | 0.92055 (7) | 0.0328 (3) | |
| H23 | 1.0367 | 0.9371 | 0.8862 | 0.039* | |
| C24 | 1.0964 (2) | 0.90585 (10) | 0.97668 (7) | 0.0347 (3) | |
| C25 | 1.0320 (3) | 0.86721 (12) | 1.02707 (8) | 0.0469 (4) | |
| H25 | 1.0975 | 0.8605 | 1.0651 | 0.056* | |
| C26 | 0.8706 (3) | 0.83885 (14) | 1.02045 (9) | 0.0505 (5) | |
| H26 | 0.8254 | 0.8139 | 1.0542 | 0.061* | |
| C27 | 0.7764 (2) | 0.84774 (12) | 0.96324 (8) | 0.0418 (4) | |
| H27 | 0.6673 | 0.8286 | 0.9590 | 0.050* | |
| C28 | 1.2709 (2) | 0.93956 (12) | 0.98526 (8) | 0.0414 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn1 | 0.02093 (7) | 0.03526 (10) | 0.03313 (9) | 0.00195 (6) | 0.00203 (6) | 0.00011 (7) |
| N1 | 0.0195 (5) | 0.0333 (6) | 0.0351 (6) | 0.0005 (4) | 0.0016 (4) | 0.0020 (5) |
| N2 | 0.0223 (6) | 0.0578 (10) | 0.0581 (9) | 0.0050 (6) | 0.0030 (6) | 0.0255 (8) |
| N3 | 0.0313 (6) | 0.0355 (7) | 0.0326 (6) | 0.0026 (5) | 0.0018 (5) | −0.0002 (5) |
| N4 | 0.0339 (7) | 0.0644 (10) | 0.0413 (8) | −0.0028 (7) | −0.0083 (6) | 0.0072 (7) |
| N5 | 0.0552 (11) | 0.0446 (10) | 0.1095 (18) | −0.0141 (9) | 0.0262 (11) | 0.0041 (11) |
| N6 | 0.0287 (7) | 0.0872 (13) | 0.0372 (7) | −0.0010 (8) | 0.0052 (6) | −0.0064 (8) |
| O1 | 0.0325 (6) | 0.0377 (6) | 0.0597 (8) | −0.0058 (5) | 0.0043 (5) | 0.0075 (6) |
| O2 | 0.0329 (6) | 0.0584 (8) | 0.0607 (8) | −0.0003 (6) | 0.0155 (6) | −0.0087 (7) |
| O3 | 0.0262 (5) | 0.0568 (8) | 0.0372 (6) | 0.0011 (5) | 0.0063 (4) | −0.0029 (5) |
| O4 | 0.0200 (5) | 0.0701 (9) | 0.0529 (7) | −0.0037 (5) | −0.0012 (5) | 0.0024 (7) |
| O5 | 0.0570 (9) | 0.0906 (12) | 0.0546 (9) | −0.0181 (9) | −0.0264 (7) | 0.0248 (9) |
| O6 | 0.0218 (5) | 0.0739 (9) | 0.0547 (7) | −0.0007 (6) | 0.0043 (5) | 0.0282 (7) |
| C1 | 0.0303 (7) | 0.0408 (8) | 0.0317 (7) | −0.0034 (6) | 0.0027 (6) | −0.0036 (6) |
| C2 | 0.0310 (7) | 0.0336 (7) | 0.0325 (7) | −0.0014 (6) | 0.0033 (5) | −0.0029 (6) |
| C3 | 0.0384 (8) | 0.0342 (8) | 0.0539 (10) | 0.0043 (7) | 0.0092 (7) | −0.0062 (7) |
| C4 | 0.0495 (10) | 0.0287 (8) | 0.0689 (13) | −0.0028 (7) | 0.0109 (9) | −0.0041 (8) |
| C5 | 0.0397 (9) | 0.0375 (9) | 0.0517 (10) | −0.0086 (7) | 0.0045 (8) | 0.0005 (7) |
| C6 | 0.0297 (7) | 0.0391 (9) | 0.0575 (10) | 0.0003 (6) | 0.0088 (7) | 0.0012 (8) |
| C7 | 0.0332 (7) | 0.0291 (7) | 0.0501 (9) | 0.0011 (6) | 0.0063 (7) | −0.0005 (6) |
| C8 | 0.0428 (11) | 0.0760 (17) | 0.097 (2) | −0.0142 (11) | 0.0142 (12) | 0.0192 (15) |
| C9 | 0.0221 (6) | 0.0322 (7) | 0.0401 (8) | −0.0007 (5) | 0.0031 (5) | 0.0007 (6) |
| C10 | 0.0203 (5) | 0.0355 (7) | 0.0332 (7) | −0.0007 (5) | 0.0009 (5) | −0.0025 (6) |
| C11 | 0.0242 (6) | 0.0515 (10) | 0.0350 (7) | −0.0034 (6) | −0.0030 (5) | −0.0069 (7) |
| C12 | 0.0316 (7) | 0.0558 (10) | 0.0292 (7) | −0.0009 (7) | −0.0011 (6) | −0.0079 (7) |
| C13 | 0.0252 (6) | 0.0447 (8) | 0.0329 (7) | 0.0001 (6) | 0.0038 (5) | −0.0012 (6) |
| C14 | 0.0224 (6) | 0.0578 (11) | 0.0346 (7) | −0.0053 (6) | 0.0011 (5) | −0.0065 (7) |
| C15 | 0.0224 (6) | 0.0536 (10) | 0.0314 (7) | −0.0048 (6) | 0.0012 (5) | −0.0073 (7) |
| C16 | 0.0495 (12) | 0.149 (3) | 0.0462 (12) | 0.0039 (16) | 0.0179 (10) | −0.0182 (15) |
| C17 | 0.0199 (5) | 0.0319 (7) | 0.0355 (7) | −0.0026 (5) | 0.0019 (5) | 0.0010 (6) |
| C18 | 0.0195 (5) | 0.0337 (7) | 0.0322 (7) | −0.0002 (5) | 0.0014 (5) | 0.0029 (5) |
| C19 | 0.0253 (6) | 0.0315 (7) | 0.0423 (8) | −0.0018 (5) | −0.0003 (6) | 0.0026 (6) |
| C20 | 0.0239 (6) | 0.0362 (8) | 0.0520 (9) | −0.0088 (6) | −0.0003 (6) | 0.0030 (7) |
| C21 | 0.0188 (5) | 0.0403 (8) | 0.0421 (8) | −0.0030 (5) | 0.0002 (5) | 0.0042 (6) |
| C22 | 0.0192 (5) | 0.0391 (8) | 0.0384 (7) | 0.0013 (5) | 0.0010 (5) | 0.0053 (6) |
| C23 | 0.0325 (7) | 0.0360 (7) | 0.0290 (6) | 0.0027 (6) | −0.0009 (5) | 0.0006 (6) |
| C24 | 0.0382 (8) | 0.0352 (7) | 0.0293 (7) | 0.0044 (6) | −0.0043 (6) | −0.0014 (6) |
| C25 | 0.0577 (12) | 0.0508 (10) | 0.0301 (7) | −0.0012 (9) | −0.0062 (7) | 0.0040 (7) |
| C26 | 0.0612 (12) | 0.0563 (12) | 0.0341 (8) | −0.0096 (10) | 0.0042 (8) | 0.0078 (8) |
| C27 | 0.0425 (9) | 0.0460 (10) | 0.0372 (8) | −0.0031 (8) | 0.0055 (7) | 0.0016 (7) |
| C28 | 0.0390 (8) | 0.0473 (10) | 0.0352 (8) | 0.0023 (7) | −0.0091 (6) | −0.0010 (7) |
Geometric parameters (Å, °)
| Zn1—O1 | 1.9584 (14) | C8—H8C | 0.9600 |
| Zn1—O3 | 1.9210 (14) | C10—C11 | 1.389 (2) |
| Zn1—N1 | 2.0722 (15) | C10—C9 | 1.492 (2) |
| Zn1—N3 | 2.0854 (15) | C11—C12 | 1.386 (2) |
| O1—C1 | 1.284 (2) | C11—H11 | 0.9300 |
| O2—C1 | 1.248 (2) | C12—H12 | 0.9300 |
| O3—C9 | 1.278 (2) | C13—N6 | 1.366 (2) |
| O4—C9 | 1.2410 (19) | C13—C12 | 1.408 (2) |
| O5—C28 | 1.235 (2) | C14—C13 | 1.399 (2) |
| N1—C17 | 1.3350 (18) | C14—H14 | 0.9300 |
| N1—C21 | 1.338 (2) | C15—C14 | 1.371 (2) |
| N2—H2A | 0.8600 | C15—C10 | 1.398 (2) |
| N2—H2B | 0.8600 | C15—H15 | 0.9300 |
| N3—C23 | 1.341 (2) | C16—H16A | 0.9600 |
| N3—C27 | 1.343 (2) | C16—H16B | 0.9600 |
| N4—C28 | 1.319 (2) | C16—H16C | 0.9600 |
| N4—H4A | 0.8600 | C17—H17 | 0.9300 |
| N4—H4B | 0.8600 | C18—C17 | 1.380 (2) |
| N5—C8 | 1.434 (3) | C18—C22 | 1.502 (2) |
| N5—H51 | 0.90 (3) | C19—C20 | 1.379 (2) |
| N6—C16 | 1.433 (3) | C19—C18 | 1.394 (2) |
| N6—H61 | 0.88 (2) | C19—H19 | 0.9300 |
| C1—C2 | 1.477 (2) | C20—H20 | 0.9300 |
| C2—C7 | 1.382 (2) | C21—C20 | 1.383 (3) |
| C2—C3 | 1.402 (2) | C21—H21 | 0.9300 |
| C3—C4 | 1.374 (3) | C22—O6 | 1.2333 (19) |
| C3—H3 | 0.9300 | C22—N2 | 1.320 (2) |
| C4—H4 | 0.9300 | C23—C24 | 1.387 (2) |
| C5—N5 | 1.369 (3) | C23—H23 | 0.9300 |
| C5—C4 | 1.403 (3) | C24—C25 | 1.387 (3) |
| C6—C5 | 1.404 (3) | C24—C28 | 1.505 (3) |
| C6—H6 | 0.9300 | C25—C26 | 1.376 (3) |
| C7—C6 | 1.386 (2) | C25—H25 | 0.9300 |
| C7—H7 | 0.9300 | C26—H26 | 0.9300 |
| C8—H8A | 0.9600 | C27—C26 | 1.379 (3) |
| C8—H8B | 0.9600 | C27—H27 | 0.9300 |
| O1—Zn1—N1 | 96.85 (6) | C15—C10—C9 | 120.17 (14) |
| O1—Zn1—N3 | 105.85 (6) | C12—C11—C10 | 121.78 (14) |
| O3—Zn1—O1 | 143.41 (6) | C12—C11—H11 | 119.1 |
| O3—Zn1—N1 | 105.91 (5) | C10—C11—H11 | 119.1 |
| O3—Zn1—N3 | 95.81 (6) | C11—C12—C13 | 120.21 (15) |
| N1—Zn1—N3 | 104.58 (5) | C11—C12—H12 | 119.9 |
| C1—O1—Zn1 | 105.71 (11) | C13—C12—H12 | 119.9 |
| C9—O3—Zn1 | 117.30 (10) | N6—C13—C14 | 119.28 (15) |
| C17—N1—Zn1 | 119.06 (10) | N6—C13—C12 | 123.04 (15) |
| C17—N1—C21 | 118.13 (14) | C14—C13—C12 | 117.68 (14) |
| C21—N1—Zn1 | 122.69 (10) | C15—C14—C13 | 121.36 (14) |
| C22—N2—H2A | 120.0 | C15—C14—H14 | 119.3 |
| C22—N2—H2B | 120.0 | C13—C14—H14 | 119.3 |
| H2A—N2—H2B | 120.0 | C14—C15—C10 | 121.32 (15) |
| C23—N3—C27 | 118.54 (15) | C14—C15—H15 | 119.3 |
| C23—N3—Zn1 | 120.24 (11) | C10—C15—H15 | 119.3 |
| C27—N3—Zn1 | 120.97 (12) | N6—C16—H16A | 109.5 |
| C28—N4—H4A | 120.0 | N6—C16—H16B | 109.5 |
| C28—N4—H4B | 120.0 | H16A—C16—H16B | 109.5 |
| H4A—N4—H4B | 120.0 | N6—C16—H16C | 109.5 |
| C5—N5—C8 | 125.0 (2) | H16A—C16—H16C | 109.5 |
| C5—N5—H51 | 115.8 (18) | H16B—C16—H16C | 109.5 |
| C8—N5—H51 | 119.1 (18) | N1—C17—C18 | 122.99 (13) |
| C13—N6—C16 | 123.28 (17) | N1—C17—H17 | 118.5 |
| C13—N6—H61 | 116.0 (16) | C18—C17—H17 | 118.5 |
| C16—N6—H61 | 117.5 (16) | C17—C18—C19 | 118.71 (13) |
| O2—C1—O1 | 120.47 (15) | C17—C18—C22 | 117.73 (13) |
| O2—C1—C2 | 121.63 (16) | C19—C18—C22 | 123.54 (14) |
| O1—C1—C2 | 117.86 (14) | C20—C19—C18 | 118.31 (15) |
| C7—C2—C3 | 117.54 (15) | C20—C19—H19 | 120.8 |
| C7—C2—C1 | 121.41 (15) | C18—C19—H19 | 120.8 |
| C3—C2—C1 | 121.04 (15) | C19—C20—C21 | 119.31 (14) |
| C4—C3—C2 | 121.17 (16) | C19—C20—H20 | 120.3 |
| C4—C3—H3 | 119.4 | C21—C20—H20 | 120.3 |
| C2—C3—H3 | 119.4 | N1—C21—C20 | 122.53 (14) |
| C3—C4—C5 | 121.00 (17) | N1—C21—H21 | 118.7 |
| C3—C4—H4 | 119.5 | C20—C21—H21 | 118.7 |
| C5—C4—H4 | 119.5 | O6—C22—N2 | 124.08 (14) |
| N5—C5—C4 | 119.96 (18) | O6—C22—C18 | 119.76 (14) |
| N5—C5—C6 | 121.96 (18) | N2—C22—C18 | 116.16 (14) |
| C4—C5—C6 | 118.08 (16) | N3—C23—C24 | 122.98 (16) |
| C7—C6—C5 | 119.76 (16) | N3—C23—H23 | 118.5 |
| C7—C6—H6 | 120.1 | C24—C23—H23 | 118.5 |
| C5—C6—H6 | 120.1 | C23—C24—C25 | 117.63 (17) |
| C2—C7—C6 | 122.31 (16) | C23—C24—C28 | 123.21 (16) |
| C2—C7—H7 | 118.8 | C25—C24—C28 | 119.15 (15) |
| C6—C7—H7 | 118.8 | C26—C25—C24 | 119.65 (17) |
| N5—C8—H8A | 109.5 | C26—C25—H25 | 120.2 |
| N5—C8—H8B | 109.5 | C24—C25—H25 | 120.2 |
| H8A—C8—H8B | 109.5 | C25—C26—C27 | 119.34 (18) |
| N5—C8—H8C | 109.5 | C25—C26—H26 | 120.3 |
| H8A—C8—H8C | 109.5 | C27—C26—H26 | 120.3 |
| H8B—C8—H8C | 109.5 | N3—C27—C26 | 121.83 (18) |
| O4—C9—O3 | 123.17 (15) | N3—C27—H27 | 119.1 |
| O4—C9—C10 | 121.35 (15) | C26—C27—H27 | 119.1 |
| O3—C9—C10 | 115.48 (13) | O5—C28—N4 | 122.70 (18) |
| C11—C10—C15 | 117.64 (14) | O5—C28—C24 | 119.20 (18) |
| C11—C10—C9 | 122.18 (13) | N4—C28—C24 | 118.09 (15) |
| O3—Zn1—O1—C1 | 46.26 (16) | C4—C5—N5—C8 | 176.6 (2) |
| N1—Zn1—O1—C1 | 174.95 (11) | C6—C5—N5—C8 | −3.9 (4) |
| N3—Zn1—O1—C1 | −77.74 (11) | C7—C6—C5—N5 | 178.0 (2) |
| O1—Zn1—O3—C9 | 46.46 (17) | C7—C6—C5—C4 | −2.6 (3) |
| N1—Zn1—O3—C9 | −79.85 (13) | C2—C7—C6—C5 | −0.8 (3) |
| N3—Zn1—O3—C9 | 173.16 (12) | C11—C10—C9—O4 | −6.2 (3) |
| O3—Zn1—N1—C17 | 143.78 (11) | C15—C10—C9—O4 | 173.48 (16) |
| O1—Zn1—N1—C17 | −7.29 (12) | C11—C10—C9—O3 | 173.75 (16) |
| N3—Zn1—N1—C17 | −115.68 (12) | C15—C10—C9—O3 | −6.6 (2) |
| O3—Zn1—N1—C21 | −40.14 (14) | C15—C10—C11—C12 | 0.3 (3) |
| O1—Zn1—N1—C21 | 168.79 (13) | C9—C10—C11—C12 | 179.99 (17) |
| N3—Zn1—N1—C21 | 60.41 (14) | C10—C11—C12—C13 | −0.7 (3) |
| O3—Zn1—N3—C23 | 28.53 (13) | C14—C13—N6—C16 | 170.4 (2) |
| O1—Zn1—N3—C23 | 178.74 (12) | C12—C13—N6—C16 | −9.6 (3) |
| N1—Zn1—N3—C23 | −79.60 (13) | N6—C13—C12—C11 | −179.86 (19) |
| O3—Zn1—N3—C27 | −145.61 (14) | C14—C13—C12—C11 | 0.2 (3) |
| O1—Zn1—N3—C27 | 4.61 (15) | C15—C14—C13—N6 | −179.25 (19) |
| N1—Zn1—N3—C27 | 106.26 (14) | C15—C14—C13—C12 | 0.7 (3) |
| Zn1—O1—C1—O2 | −3.24 (19) | C14—C15—C10—C11 | 0.6 (3) |
| Zn1—O1—C1—C2 | 174.63 (11) | C14—C15—C10—C9 | −179.10 (16) |
| Zn1—O3—C9—O4 | −3.2 (2) | C10—C15—C14—C13 | −1.1 (3) |
| Zn1—O3—C9—C10 | 176.82 (10) | C19—C18—C17—N1 | −1.6 (2) |
| C21—N1—C17—C18 | 1.0 (2) | C22—C18—C17—N1 | 179.83 (14) |
| Zn1—N1—C17—C18 | 177.25 (12) | C17—C18—C22—O6 | 43.7 (2) |
| C17—N1—C21—C20 | 0.4 (2) | C19—C18—C22—O6 | −134.76 (18) |
| Zn1—N1—C21—C20 | −175.73 (13) | C17—C18—C22—N2 | −135.66 (17) |
| C27—N3—C23—C24 | 0.8 (2) | C19—C18—C22—N2 | 45.9 (2) |
| Zn1—N3—C23—C24 | −173.51 (12) | C20—C19—C18—C17 | 0.9 (2) |
| C23—N3—C27—C26 | −1.3 (3) | C20—C19—C18—C22 | 179.33 (15) |
| Zn1—N3—C27—C26 | 172.97 (15) | C18—C19—C20—C21 | 0.4 (3) |
| O2—C1—C2—C7 | 172.14 (16) | N1—C21—C20—C19 | −1.1 (3) |
| O1—C1—C2—C7 | −5.7 (2) | N3—C23—C24—C25 | 0.8 (3) |
| O2—C1—C2—C3 | −6.5 (2) | N3—C23—C24—C28 | −178.69 (16) |
| O1—C1—C2—C3 | 175.65 (16) | C23—C24—C25—C26 | −1.8 (3) |
| C7—C2—C3—C4 | −1.3 (3) | C28—C24—C25—C26 | 177.67 (18) |
| C1—C2—C3—C4 | 177.42 (17) | C23—C24—C28—O5 | −169.02 (19) |
| C3—C2—C7—C6 | 2.7 (3) | C25—C24—C28—O5 | 11.5 (3) |
| C1—C2—C7—C6 | −176.01 (16) | C23—C24—C28—N4 | 11.6 (3) |
| C2—C3—C4—C5 | −2.0 (3) | C25—C24—C28—N4 | −167.84 (18) |
| N5—C5—C4—C3 | −176.6 (2) | C24—C25—C26—C27 | 1.4 (3) |
| C6—C5—C4—C3 | 3.9 (3) | N3—C27—C26—C25 | 0.2 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2B···O2i | 0.86 | 2.00 | 2.839 (3) | 166 |
| N4—H4B···O6ii | 0.86 | 2.12 | 2.950 (3) | 161 |
| N6—H61···O4ii | 0.88 (2) | 2.10 (2) | 2.955 (3) | 166 (2) |
Symmetry codes: (i) −x+1, y+1/2, −z+3/2; (ii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2084).
References
- Antolini, L., Battaglia, L. P., Corradi, A. B., Marcotrigiano, G., Menabue, L., Pellacani, G. C. & Saladini, M. (1982). Inorg. Chem.21, 1391–1395.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Hökelek, T., Çaylak, N. & Necefoğlu, H. (2007). Acta Cryst. E63, m2561–m2562.
- Krishnamachari, K. A. V. R. (1974). Am. J. Clin. Nutr.27, 108–111. [DOI] [PubMed]
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Molecular Structure Corporation (1994). MSC/AFC Diffractometer Control Software MSC, The Woodlands, Texas, USA.
- Molecular Structure Corporation (1997). TEXSAN for Windows MSC, The Woodlands, Texas, USA.
- Nadzhafov, G. N., Shnulin, A. N. & Mamedov, Kh. S. (1981). Zh. Strukt. Khim.22, 124–128.
- Necefoğlu, H., Hökelek, T., Ersanlı, C. C. & Erdönmez, A. (2002). Acta Cryst. E58, m758–m761.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808042852/su2084sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808042852/su2084Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report