Abstract
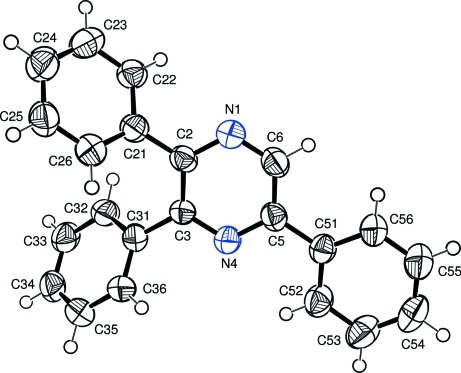
In the title molecule, C22H16N2, the pyrazine ring deviates very slightly from planarity [maximum deviation 0.044 (3) Å], tending towards a twist-boat conformation. The phenyl ring at position 3 makes dihedral angles of 64.0 (2) and 45.8 (2)°, respectively, with the phenyl rings at positions 2 and 5. The dihedral angle between the phenyl rings at positions 2 and 5 is 49.7 (2)°. A C—H⋯π interaction is found in the crystal structure, but no classical hydrogen bonds form.
Related literature
For the biological properties of pyrazines, see: Foks et al. (2004 ▶); Premkumar & Govindarajan (2005 ▶); Sondhi et al. (2005 ▶).
Experimental
Crystal data
C22H16N2
M r = 308.37
Orthorhombic,
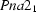
a = 15.563 (2) Å
b = 6.2005 (9) Å
c = 16.845 (3) Å
V = 1625.5 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.07 mm−1
T = 296 (2) K
0.44 × 0.35 × 0.21 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2004 ▶) T min = 0.968, T max = 0.985
17890 measured reflections
1730 independent reflections
1109 reflections with I > 2σ(I)
R int = 0.091
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.129
S = 1.02
1730 reflections
218 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.13 e Å−3
Δρmin = −0.13 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT-NT (Bruker, 2004 ▶); data reduction: SAINT-NT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808041627/wn2297sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808041627/wn2297Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C53—H53⋯Cgi | 0.93 | 2.98 | 3.909 (5) | 174 |
Symmetry code: (i) 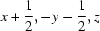 . Cg is the centroid of the C21–C26 phenyl ring.
. Cg is the centroid of the C21–C26 phenyl ring.
Acknowledgments
AT thanks the UGC, India, for the award of a Minor Research Project [File No. MRP-2355/06(UGC-SERO), Link No. 2355, 10/01/2007]. RJB acknowledges the NSF–MRI program for funding to purchase the X-ray CCD diffractometer.
supplementary crystallographic information
Comment
Pyrazines are heterocycles which exhibit nutty, roasted or earth flavouring agents. 2-Cyanopyrazine derivatives show anticancer, anti-inflammatory and analgesic activities (Sondhi et al., 2005). Pyrazine derivatives exhibit tuberculostatic activity (Foks et al., 2004) and antimicrobial activity (Premkumar & Govindarajan, 2005).
In the title molecule, C22H16N2, (Fig. 1), the pyrazine ring deviates very slightly from planarity, tending towards a twist-boat conformation. The phenyl ring at position 3 makes dihedral angles of 64.0 (2)° and 45.8 (2)° with the phenyl rings at positions 2 and 5, respectively. The dihedral angle between the phenyl rings at positions 2 and 5 is 49.7 (2)°. A C53—H53···π interaction involving the phenyl (C21–C26) ring at position 2 is found in the crystal structure, but no classical hydrogen bonds.
Experimental
To a homogeneous solution of benzil (1.05 g, 0.005 mol) and 1-phenylethanediamine dihydrochloride (1.04 g, 0.005 mol) in ethanol, sodium acetate trihydrate (2.04 g, 0.015 mol) was added. The precipitated sodium chloride was filtered off and the filtrate was refluxed for 2 h. On completion of the reaction, as indicated by TLC, the reaction mixture was poured into crushed ice and the resulting solid was filtered off and purified by column chromatography on silica gel. Elution with benzene: petroleum ether (4:1 v/v) at 333–353 K gave the product in pure form. Yield 1.6 g (80%).
Refinement
H atoms were positioned geometrically and allowed to ride on their parent atoms, with C—H = 0.93 Å and Uiso(H) = 1.2 times Ueq(C). In the absence of significant anomalous scattering effects Friedel pairs have been merged.
Figures
Fig. 1.
The molecular structure of the title compound, showing the atom-numbering scheme and displacement ellipsoids drawn at the 50% probability level. H atoms are shown as small spheres of arbitrary radius.
Crystal data
| C22H16N2 | Dx = 1.260 Mg m−3 |
| Mr = 308.37 | Melting point: 421 K |
| Orthorhombic, Pna21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2c -2n | Cell parameters from 2955 reflections |
| a = 15.563 (2) Å | θ = 2.4–21.2° |
| b = 6.2005 (9) Å | µ = 0.07 mm−1 |
| c = 16.845 (3) Å | T = 296 K |
| V = 1625.5 (4) Å3 | Chunk, colourless |
| Z = 4 | 0.44 × 0.35 × 0.21 mm |
| F(000) = 648 |
Data collection
| Bruker APEXII CCD diffractometer | 1730 independent reflections |
| Radiation source: fine-focus sealed tube | 1109 reflections with I > 2σ(I) |
| graphite | Rint = 0.091 |
| φ and ω scans | θmax = 26.5°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | h = −19→19 |
| Tmin = 0.968, Tmax = 0.985 | k = −7→7 |
| 17890 measured reflections | l = −20→19 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.050 | H-atom parameters constrained |
| wR(F2) = 0.129 | w = 1/[σ2(Fo2) + (0.0653P)2 + 0.1081P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max = 0.001 |
| 1730 reflections | Δρmax = 0.13 e Å−3 |
| 218 parameters | Δρmin = −0.13 e Å−3 |
| 1 restraint | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.015 (3) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.2355 (2) | 0.1296 (5) | 0.19242 (19) | 0.0547 (12) | |
| N4 | 0.3436 (2) | −0.1252 (5) | 0.10173 (19) | 0.0487 (11) | |
| C2 | 0.2412 (3) | 0.1612 (5) | 0.1141 (2) | 0.0463 (12) | |
| C3 | 0.3009 (3) | 0.0395 (6) | 0.0686 (2) | 0.0447 (12) | |
| C5 | 0.3329 (3) | −0.1640 (6) | 0.1791 (2) | 0.0477 (12) | |
| C6 | 0.2822 (3) | −0.0252 (7) | 0.2244 (3) | 0.0557 (14) | |
| C21 | 0.1810 (3) | 0.3188 (6) | 0.0793 (2) | 0.0480 (12) | |
| C22 | 0.1658 (3) | 0.5110 (6) | 0.1192 (3) | 0.0547 (16) | |
| C23 | 0.1074 (3) | 0.6577 (7) | 0.0897 (3) | 0.0670 (17) | |
| C24 | 0.0622 (3) | 0.6125 (8) | 0.0212 (3) | 0.0717 (17) | |
| C25 | 0.0760 (3) | 0.4218 (8) | −0.0185 (3) | 0.0683 (19) | |
| C26 | 0.1359 (3) | 0.2775 (8) | 0.0110 (3) | 0.0610 (17) | |
| C31 | 0.3243 (3) | 0.0908 (7) | −0.0142 (2) | 0.0500 (14) | |
| C32 | 0.3486 (3) | 0.2977 (7) | −0.0358 (3) | 0.0583 (16) | |
| C33 | 0.3772 (3) | 0.3414 (7) | −0.1116 (3) | 0.0653 (17) | |
| C34 | 0.3783 (3) | 0.1810 (8) | −0.1676 (3) | 0.0660 (17) | |
| C35 | 0.3525 (3) | −0.0229 (8) | −0.1474 (3) | 0.0687 (17) | |
| C36 | 0.3261 (3) | −0.0692 (6) | −0.0714 (2) | 0.0577 (16) | |
| C51 | 0.3770 (3) | −0.3528 (6) | 0.2136 (2) | 0.0483 (12) | |
| C52 | 0.4302 (3) | −0.4792 (7) | 0.1662 (3) | 0.0610 (17) | |
| C53 | 0.4682 (3) | −0.6620 (7) | 0.1961 (3) | 0.0730 (19) | |
| C54 | 0.4543 (3) | −0.7247 (8) | 0.2734 (3) | 0.0747 (19) | |
| C55 | 0.4017 (4) | −0.6012 (8) | 0.3210 (3) | 0.0770 (19) | |
| C56 | 0.3635 (3) | −0.4195 (7) | 0.2911 (3) | 0.0667 (17) | |
| H6 | 0.28114 | −0.04259 | 0.27921 | 0.0669* | |
| H22 | 0.19510 | 0.54089 | 0.16601 | 0.0653* | |
| H23 | 0.09840 | 0.78757 | 0.11605 | 0.0803* | |
| H24 | 0.02234 | 0.71109 | 0.00186 | 0.0859* | |
| H25 | 0.04549 | 0.39011 | −0.06447 | 0.0819* | |
| H26 | 0.14584 | 0.14928 | −0.01612 | 0.0727* | |
| H32 | 0.34552 | 0.40842 | 0.00137 | 0.0699* | |
| H33 | 0.39584 | 0.47943 | −0.12464 | 0.0783* | |
| H34 | 0.39640 | 0.21058 | −0.21902 | 0.0788* | |
| H35 | 0.35281 | −0.13135 | −0.18555 | 0.0820* | |
| H36 | 0.30936 | −0.20876 | −0.05833 | 0.0693* | |
| H52 | 0.44015 | −0.43949 | 0.11371 | 0.0731* | |
| H53 | 0.50365 | −0.74446 | 0.16366 | 0.0872* | |
| H54 | 0.48007 | −0.84883 | 0.29325 | 0.0894* | |
| H55 | 0.39220 | −0.64112 | 0.37352 | 0.0927* | |
| H56 | 0.32749 | −0.33888 | 0.32367 | 0.0800* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.062 (2) | 0.052 (2) | 0.050 (2) | 0.0055 (17) | 0.0027 (16) | −0.0004 (16) |
| N4 | 0.0533 (19) | 0.0458 (18) | 0.047 (2) | 0.0009 (15) | 0.0024 (15) | 0.0030 (15) |
| C2 | 0.057 (2) | 0.042 (2) | 0.040 (2) | −0.0052 (18) | 0.0015 (17) | −0.0036 (18) |
| C3 | 0.055 (2) | 0.039 (2) | 0.040 (2) | 0.0020 (18) | 0.0004 (16) | −0.0025 (16) |
| C5 | 0.055 (2) | 0.047 (2) | 0.041 (2) | −0.0069 (18) | −0.0003 (17) | 0.0022 (18) |
| C6 | 0.069 (3) | 0.057 (2) | 0.041 (2) | −0.002 (2) | −0.0020 (19) | −0.001 (2) |
| C21 | 0.051 (2) | 0.047 (2) | 0.046 (2) | −0.0008 (17) | 0.0017 (18) | −0.0008 (18) |
| C22 | 0.060 (3) | 0.050 (2) | 0.054 (3) | 0.000 (2) | 0.0030 (19) | −0.0013 (19) |
| C23 | 0.067 (3) | 0.055 (3) | 0.079 (3) | 0.011 (2) | 0.007 (3) | 0.001 (2) |
| C24 | 0.066 (3) | 0.077 (3) | 0.072 (3) | 0.013 (3) | 0.002 (3) | 0.008 (3) |
| C25 | 0.067 (3) | 0.085 (4) | 0.053 (3) | 0.012 (3) | −0.006 (2) | 0.004 (2) |
| C26 | 0.067 (3) | 0.062 (3) | 0.054 (3) | 0.002 (2) | −0.004 (2) | −0.002 (2) |
| C31 | 0.054 (2) | 0.054 (3) | 0.042 (2) | 0.0078 (19) | −0.0010 (18) | 0.0036 (18) |
| C32 | 0.077 (3) | 0.047 (2) | 0.051 (3) | 0.003 (2) | 0.009 (2) | 0.002 (2) |
| C33 | 0.077 (3) | 0.056 (3) | 0.063 (3) | 0.006 (2) | 0.012 (2) | 0.015 (2) |
| C34 | 0.076 (3) | 0.076 (3) | 0.046 (3) | 0.015 (3) | 0.009 (2) | 0.014 (3) |
| C35 | 0.089 (3) | 0.070 (3) | 0.047 (3) | 0.017 (3) | 0.001 (2) | −0.006 (2) |
| C36 | 0.079 (3) | 0.046 (2) | 0.048 (3) | 0.009 (2) | 0.001 (2) | −0.004 (2) |
| C51 | 0.049 (2) | 0.045 (2) | 0.051 (2) | −0.0034 (18) | −0.0025 (18) | 0.0050 (19) |
| C52 | 0.064 (3) | 0.056 (3) | 0.063 (3) | −0.001 (2) | 0.005 (2) | 0.013 (2) |
| C53 | 0.066 (3) | 0.061 (3) | 0.092 (4) | 0.012 (2) | 0.008 (3) | 0.011 (3) |
| C54 | 0.075 (3) | 0.056 (3) | 0.093 (4) | 0.007 (3) | −0.019 (3) | 0.019 (3) |
| C55 | 0.098 (4) | 0.072 (3) | 0.061 (3) | 0.010 (3) | −0.013 (3) | 0.012 (3) |
| C56 | 0.085 (3) | 0.064 (3) | 0.051 (3) | 0.013 (2) | −0.010 (2) | 0.006 (2) |
Geometric parameters (Å, °)
| N1—C2 | 1.337 (5) | C51—C56 | 1.386 (6) |
| N1—C6 | 1.319 (6) | C52—C53 | 1.374 (6) |
| N4—C3 | 1.340 (5) | C53—C54 | 1.376 (7) |
| N4—C5 | 1.336 (5) | C54—C55 | 1.378 (7) |
| C2—C3 | 1.421 (6) | C55—C56 | 1.370 (7) |
| C2—C21 | 1.475 (6) | C6—H6 | 0.9300 |
| C3—C31 | 1.476 (5) | C22—H22 | 0.9300 |
| C5—C6 | 1.395 (6) | C23—H23 | 0.9300 |
| C5—C51 | 1.476 (6) | C24—H24 | 0.9300 |
| C21—C22 | 1.389 (6) | C25—H25 | 0.9300 |
| C21—C26 | 1.372 (6) | C26—H26 | 0.9300 |
| C22—C23 | 1.379 (6) | C32—H32 | 0.9300 |
| C23—C24 | 1.380 (7) | C33—H33 | 0.9300 |
| C24—C25 | 1.375 (7) | C34—H34 | 0.9300 |
| C25—C26 | 1.384 (7) | C35—H35 | 0.9300 |
| C31—C32 | 1.386 (6) | C36—H36 | 0.9300 |
| C31—C36 | 1.383 (5) | C52—H52 | 0.9300 |
| C32—C33 | 1.379 (7) | C53—H53 | 0.9300 |
| C33—C34 | 1.371 (7) | C54—H54 | 0.9300 |
| C34—C35 | 1.370 (7) | C55—H55 | 0.9300 |
| C35—C36 | 1.375 (6) | C56—H56 | 0.9300 |
| C51—C52 | 1.392 (6) | ||
| N1···N4 | 2.768 (4) | C34···H54viii | 3.0900 |
| N4···N1 | 2.768 (4) | C35···H54viii | 2.9000 |
| N1···H22 | 2.6600 | C35···H56v | 3.0600 |
| N1···H35i | 2.8800 | C51···H22ii | 3.0200 |
| N4···H36 | 2.8000 | C52···H23vii | 3.0000 |
| N4···H52 | 2.4700 | C56···H6 | 2.6700 |
| C5···C22ii | 3.441 (6) | H6···C56 | 2.6700 |
| C6···C34iii | 3.587 (7) | H6···H56 | 2.1100 |
| C6···C54iv | 3.366 (7) | H22···N1 | 2.6600 |
| C21···C32 | 3.253 (6) | H22···C5iv | 2.8300 |
| C22···C5iv | 3.441 (6) | H22···C51iv | 3.0200 |
| C26···C32 | 3.405 (7) | H23···C52ix | 3.0000 |
| C26···C31 | 3.181 (7) | H25···C33ix | 3.0900 |
| C31···C26 | 3.181 (7) | H26···C3 | 2.8900 |
| C32···C26 | 3.405 (7) | H26···C31 | 2.8000 |
| C32···C21 | 3.253 (6) | H32···C2 | 2.9300 |
| C34···C6v | 3.587 (7) | H32···C21 | 2.9300 |
| C35···C56v | 3.576 (7) | H32···H52iv | 2.5800 |
| C54···C6ii | 3.366 (7) | H35···N1x | 2.8800 |
| C56···C35iii | 3.576 (7) | H36···N4 | 2.8000 |
| C2···H32 | 2.9300 | H52···N4 | 2.4700 |
| C3···H26 | 2.8900 | H52···H32ii | 2.5800 |
| C5···H22ii | 2.8300 | H54···C34xi | 3.0900 |
| C6···H56 | 2.6600 | H54···C35xi | 2.9000 |
| C21···H32 | 2.9300 | H55···C24xii | 3.0000 |
| C24···H55vi | 3.0000 | H56···C6 | 2.6600 |
| C31···H26 | 2.8000 | H56···H6 | 2.1100 |
| C33···H25vii | 3.0900 | H56···C35iii | 3.0600 |
| C2—N1—C6 | 118.2 (4) | C54—C55—C56 | 120.1 (5) |
| C3—N4—C5 | 118.8 (3) | C51—C56—C55 | 121.8 (4) |
| N1—C2—C3 | 119.9 (3) | N1—C6—H6 | 119.00 |
| N1—C2—C21 | 116.6 (3) | C5—C6—H6 | 119.00 |
| C3—C2—C21 | 123.5 (3) | C21—C22—H22 | 120.00 |
| N4—C3—C2 | 120.3 (3) | C23—C22—H22 | 120.00 |
| N4—C3—C31 | 115.8 (4) | C22—C23—H23 | 120.00 |
| C2—C3—C31 | 123.8 (3) | C24—C23—H23 | 120.00 |
| N4—C5—C6 | 119.6 (4) | C23—C24—H24 | 120.00 |
| N4—C5—C51 | 118.0 (3) | C25—C24—H24 | 120.00 |
| C6—C5—C51 | 122.5 (3) | C24—C25—H25 | 120.00 |
| N1—C6—C5 | 122.5 (4) | C26—C25—H25 | 120.00 |
| C2—C21—C22 | 119.0 (3) | C21—C26—H26 | 119.00 |
| C2—C21—C26 | 122.3 (4) | C25—C26—H26 | 119.00 |
| C22—C21—C26 | 118.6 (4) | C31—C32—H32 | 119.00 |
| C21—C22—C23 | 120.3 (4) | C33—C32—H32 | 120.00 |
| C22—C23—C24 | 120.2 (4) | C32—C33—H33 | 120.00 |
| C23—C24—C25 | 120.1 (4) | C34—C33—H33 | 120.00 |
| C24—C25—C26 | 119.1 (5) | C33—C34—H34 | 120.00 |
| C21—C26—C25 | 121.7 (4) | C35—C34—H34 | 120.00 |
| C3—C31—C32 | 121.0 (4) | C34—C35—H35 | 120.00 |
| C3—C31—C36 | 120.6 (4) | C36—C35—H35 | 120.00 |
| C32—C31—C36 | 118.4 (4) | C31—C36—H36 | 120.00 |
| C31—C32—C33 | 120.9 (4) | C35—C36—H36 | 120.00 |
| C32—C33—C34 | 119.9 (4) | C51—C52—H52 | 120.00 |
| C33—C34—C35 | 119.7 (5) | C53—C52—H52 | 120.00 |
| C34—C35—C36 | 120.8 (4) | C52—C53—H53 | 120.00 |
| C31—C36—C35 | 120.3 (4) | C54—C53—H53 | 120.00 |
| C5—C51—C52 | 119.8 (3) | C53—C54—H54 | 120.00 |
| C5—C51—C56 | 122.5 (4) | C55—C54—H54 | 120.00 |
| C52—C51—C56 | 117.6 (4) | C54—C55—H55 | 120.00 |
| C51—C52—C53 | 120.7 (4) | C56—C55—H55 | 120.00 |
| C52—C53—C54 | 120.8 (4) | C51—C56—H56 | 119.00 |
| C53—C54—C55 | 119.1 (5) | C55—C56—H56 | 119.00 |
| C6—N1—C2—C3 | −4.3 (6) | C2—C21—C22—C23 | −177.5 (4) |
| C6—N1—C2—C21 | 173.4 (4) | C26—C21—C22—C23 | −0.9 (7) |
| C2—N1—C6—C5 | −3.9 (6) | C2—C21—C26—C25 | 176.3 (4) |
| C5—N4—C3—C2 | −4.0 (6) | C22—C21—C26—C25 | −0.2 (7) |
| C5—N4—C3—C31 | 172.3 (4) | C21—C22—C23—C24 | 1.4 (7) |
| C3—N4—C5—C6 | −4.0 (6) | C22—C23—C24—C25 | −0.8 (7) |
| C3—N4—C5—C51 | 176.9 (4) | C23—C24—C25—C26 | −0.3 (7) |
| N1—C2—C3—N4 | 8.5 (6) | C24—C25—C26—C21 | 0.8 (7) |
| N1—C2—C3—C31 | −167.5 (4) | C3—C31—C32—C33 | 174.6 (4) |
| C21—C2—C3—N4 | −169.0 (4) | C36—C31—C32—C33 | −2.6 (7) |
| C21—C2—C3—C31 | 15.0 (6) | C3—C31—C36—C35 | −176.4 (4) |
| N1—C2—C21—C22 | 42.2 (6) | C32—C31—C36—C35 | 0.8 (7) |
| N1—C2—C21—C26 | −134.2 (4) | C31—C32—C33—C34 | 2.8 (7) |
| C3—C2—C21—C22 | −140.2 (4) | C32—C33—C34—C35 | −1.3 (7) |
| C3—C2—C21—C26 | 43.4 (6) | C33—C34—C35—C36 | −0.5 (7) |
| N4—C3—C31—C32 | −126.1 (4) | C34—C35—C36—C31 | 0.7 (7) |
| N4—C3—C31—C36 | 51.0 (6) | C5—C51—C52—C53 | −176.5 (4) |
| C2—C3—C31—C32 | 50.0 (7) | C56—C51—C52—C53 | −0.6 (7) |
| C2—C3—C31—C36 | −132.9 (5) | C5—C51—C56—C55 | 176.7 (5) |
| N4—C5—C6—N1 | 8.4 (7) | C52—C51—C56—C55 | 0.9 (7) |
| C51—C5—C6—N1 | −172.6 (4) | C51—C52—C53—C54 | 0.3 (7) |
| N4—C5—C51—C52 | 2.3 (6) | C52—C53—C54—C55 | −0.2 (7) |
| N4—C5—C51—C56 | −173.5 (4) | C53—C54—C55—C56 | 0.5 (8) |
| C6—C5—C51—C52 | −176.8 (4) | C54—C55—C56—C51 | −0.9 (8) |
| C6—C5—C51—C56 | 7.5 (7) |
Symmetry codes: (i) −x+1/2, y+1/2, z+1/2; (ii) x, y−1, z; (iii) −x+1/2, y−1/2, z+1/2; (iv) x, y+1, z; (v) −x+1/2, y+1/2, z−1/2; (vi) −x+1/2, y+3/2, z−1/2; (vii) x+1/2, −y+1/2, z; (viii) −x+1, −y−1, z−1/2; (ix) x−1/2, −y+1/2, z; (x) −x+1/2, y−1/2, z−1/2; (xi) −x+1, −y−1, z+1/2; (xii) −x+1/2, y−3/2, z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C53—H53···Cgxiii | 0.93 | 2.98 | 3.909 (5) | 174 |
Symmetry codes: (xiii) x+1/2, −y−1/2, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2297).
References
- Bruker (2004). APEX2, SAINT-NT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Foks, H., Trapkaoska, I., Janowiec, M., Zwolska, Z. & Augustynowicz-Kopec, E. (2004). Chem. Heterocycl. Cmpd, 40, 1185–1193.
- Premkumar, T. & Govindarajan, S. (2005). World J. Microbiol. Biotechnol.21, 479–480.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sondhi, S. M., Singh, N., Rajvanshi, S. & Johar, M. (2005). Indian J. Chem.44B, 387–399.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808041627/wn2297sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808041627/wn2297Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report