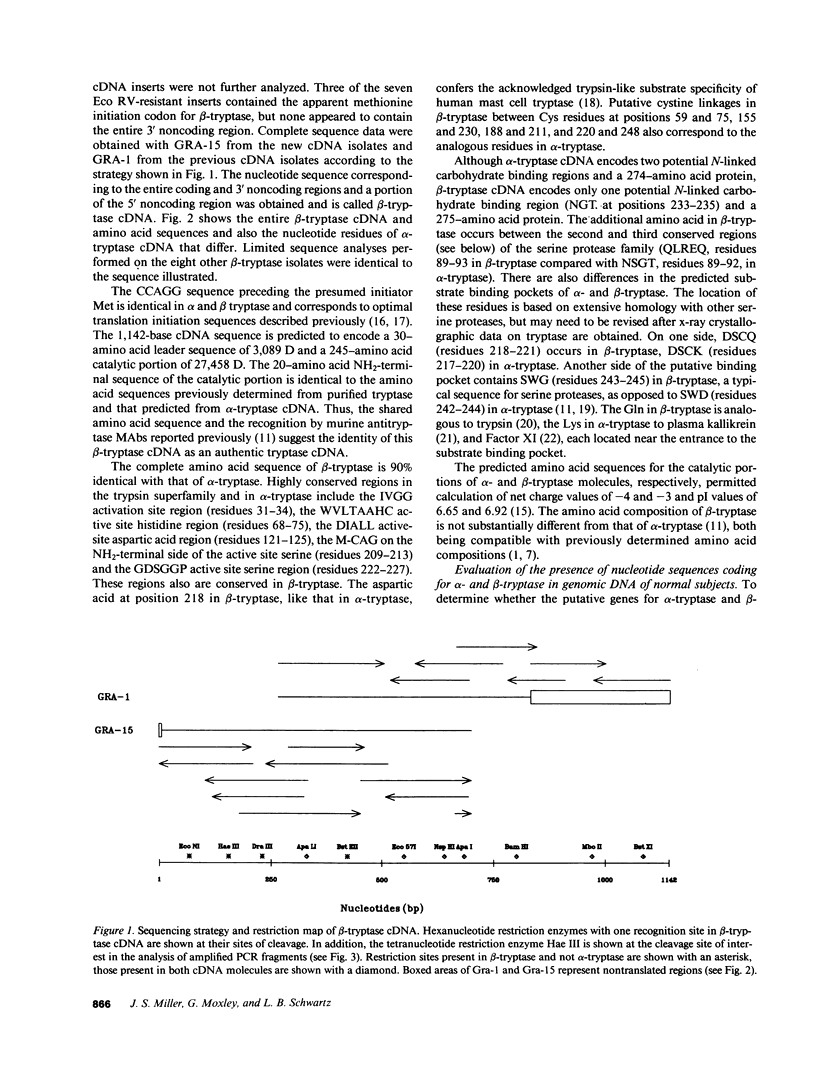
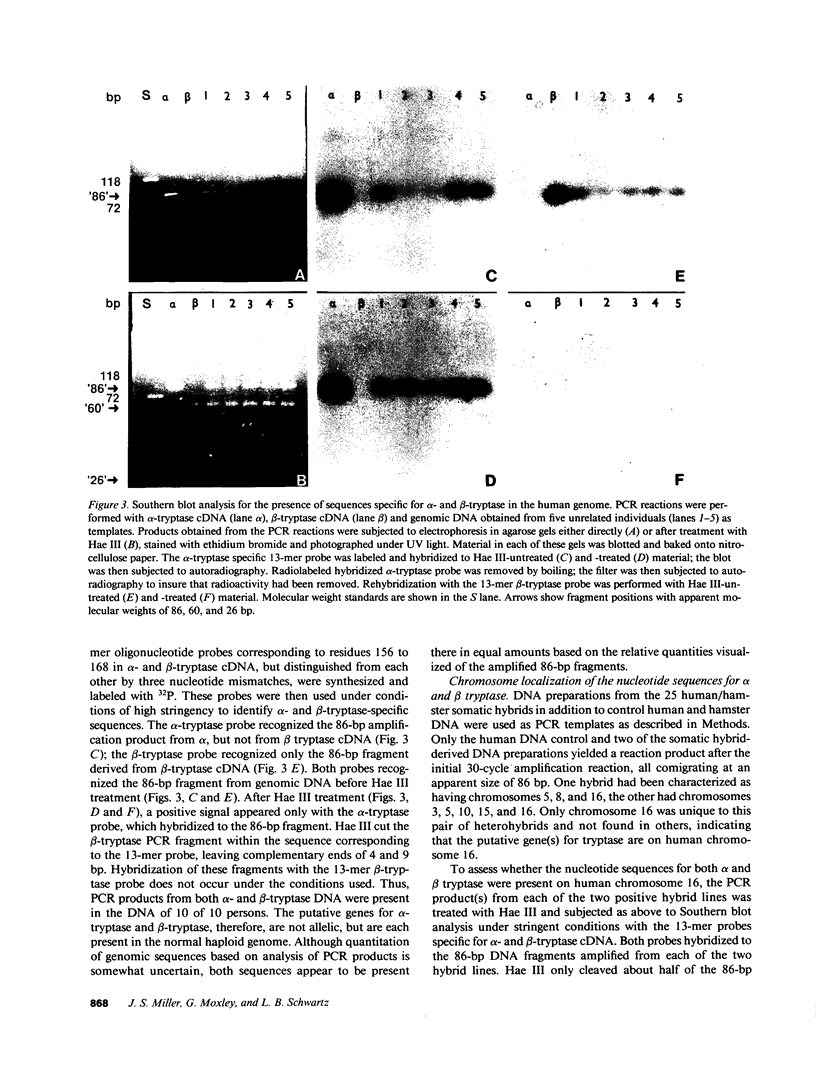
Abstract
A second cDNA for human tryptase, called beta-tryptase, was cloned from a mast cell cDNA library in lambda ZAP. Its nucleotide sequence and corresponding amino acid sequence were determined and compared with those of a previously cloned tryptase cDNA, now called alpha-tryptase. The 1,142-base sequence of beta-tryptase encodes a 30-amino acid leader sequence of 3,089 D and a 245-amino acid catalytic region of 27,458 D. The amino acid sequence of beta-tryptase is 90% identical with that of alpha-tryptase, the first 20 amino acids of the catalytic portions being 100% identical. This identity, together with recognition of each recombinant protein by monoclonal antibodies directed against purified tryptase validate the tryptase identity of both alpha-tryptase and beta-tryptase cDNA molecules. Modest differences between the nucleic acid sequences of alpha- and beta-tryptase occurred throughout the cDNA molecules except in the 3' noncoding regions, which were identical. Although most highly conserved regions of amino acid sequence in the trypsin superfamily are conserved in both tryptase molecules, beta-tryptase has one carbohydrate binding site compared to two in alpha-tryptase, and one additional amino acid in the catalytic sequence. Regions of the substrate binding pocket in beta-tryptase (DSCQ, residues 218-221; SWG, residues 243-245) differ slightly from those in alpha-tryptase (DSCK, residues 217-220; SWD, residues 242-244). The presence of both alpha- and beta-tryptase sequences in each haploid genome was indicated by finding alpha- and beta-tryptase specific fragments after amplification by PCR of genomic DNA in 10 unrelated individuals. Localization of both alpha- and beta-tryptase sequences to human chromosome 16 was then performed by analysis of DNA preparations from 25 human/hamster somatic hybrids by PCR. It is now possible to assess the expression of each tryptase cDNA by mast cells and the relationship of each gene product to the active enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham S., Stevens R. L., Nicodemus C. F., Gartner M. C., Austen K. F., Weis J. H. Molecular cloning of a cDNA that encodes the peptide core of a mouse mast cell secretory granule proteoglycan and comparison with the analogous rat and human cDNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3763–3767. doi: 10.1073/pnas.86.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey G. H., Viro N. F., Ramachandran J., Lazarus S. C., Borson D. B., Nadel J. A. Dog mastocytoma tryptase: affinity purification, characterization, and amino-terminal sequence. Arch Biochem Biophys. 1987 Nov 1;258(2):555–563. doi: 10.1016/0003-9861(87)90377-8. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Fujikawa K., McMullen B. A., Davie E. W. Human plasma prekallikrein, a zymogen to a serine protease that contains four tandem repeats. Biochemistry. 1986 May 6;25(9):2410–2417. doi: 10.1021/bi00357a017. [DOI] [PubMed] [Google Scholar]
- Craig S. S., Schechter N. M., Schwartz L. B. Ultrastructural analysis of human T and TC mast cells identified by immunoelectron microscopy. Lab Invest. 1988 Jun;58(6):682–691. [PubMed] [Google Scholar]
- Cromlish J. A., Seidah N. G., Marcinkiewicz M., Hamelin J., Johnson D. A., Chrétien M. Human pituitary tryptase: molecular forms, NH2-terminal sequence, immunocytochemical localization, and specificity with prohormone and fluorogenic substrates. J Biol Chem. 1987 Jan 25;262(3):1363–1373. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Chung D. W., Hendrickson L. E., Davie E. W. Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry. 1986 May 6;25(9):2417–2424. doi: 10.1021/bi00357a018. [DOI] [PubMed] [Google Scholar]
- Furie B., Bing D. H., Feldmann R. J., Robison D. J., Burnier J. P., Furie B. C. Computer-generated models of blood coagulation factor Xa, factor IXa, and thrombin based upon structural homology with other serine proteases. J Biol Chem. 1982 Apr 10;257(7):3875–3882. [PubMed] [Google Scholar]
- GLENNER G. G., COHEN L. A. Histochemical demonstration of a species-specific trypsin-like enzyme in mast cells. Nature. 1960 Mar 19;185:846–847. doi: 10.1038/185846a0. [DOI] [PubMed] [Google Scholar]
- Harvima I. T., Schechter N. M., Harvima R. J., Fräki J. E. Human skin tryptase: purification, partial characterization and comparison with human lung tryptase. Biochim Biophys Acta. 1988 Nov 2;957(1):71–80. doi: 10.1016/0167-4838(88)90158-6. [DOI] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido H., Fukusen N., Katunuma N. Chymotrypsin- and trypsin-type serine proteases in rat mast cells: properties and functions. Arch Biochem Biophys. 1985 Jun;239(2):436–443. doi: 10.1016/0003-9861(85)90709-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krieger M., Kay L. M., Stroud R. M. Structure and specific binding of trypsin: comparison of inhibited derivatives and a model for substrate binding. J Mol Biol. 1974 Feb 25;83(2):209–230. doi: 10.1016/0022-2836(74)90388-x. [DOI] [PubMed] [Google Scholar]
- LAGUNOFF D., BENDITT E. P. Proteolytic enzymes of mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:185–198. doi: 10.1111/j.1749-6632.1963.tb53698.x. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Westin E. H., Schwartz L. B. Cloning and characterization of complementary DNA for human tryptase. J Clin Invest. 1989 Oct;84(4):1188–1195. doi: 10.1172/JCI114284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley G. DNA polymorphism of immunoglobulin kappa confers risk of rheumatoid arthritis. Arthritis Rheum. 1989 May;32(5):634–637. doi: 10.1002/anr.1780320518. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Seldin D., Austen K. F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981 Apr;126(4):1290–1294. [PubMed] [Google Scholar]
- Schwartz L. B., Metcalfe D. D., Miller J. S., Earl H., Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987 Jun 25;316(26):1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B. Monoclonal antibodies against human mast cell tryptase demonstrate shared antigenic sites on subunits of tryptase and selective localization of the enzyme to mast cells. J Immunol. 1985 Jan;134(1):526–531. [PubMed] [Google Scholar]
- Schwartz L. B., Yunginger J. W., Miller J., Bokhari R., Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989 May;83(5):1551–1555. doi: 10.1172/JCI114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Hougland M. W., Johnson D. A. Human lung tryptase. Purification and characterization. J Biol Chem. 1984 Sep 10;259(17):11046–11051. [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Glynn S., Levi E., Mjolsness S., Hayday A. Use of a chemically modified T7 DNA polymerase for manual and automated sequencing of supercoiled DNA. Biotechniques. 1988 May;6(5):460–469. [PubMed] [Google Scholar]
- Vanderslice P., Craik C. S., Nadel J. A., Caughey G. H. Molecular cloning of dog mast cell tryptase and a related protease: structural evidence of a unique mode of serine protease activation. Biochemistry. 1989 May 16;28(10):4148–4155. doi: 10.1021/bi00436a004. [DOI] [PubMed] [Google Scholar]





