Abstract
In the title salt, C26H29N2 +·Br−, which may serve as a precursor for N-heterocyclic carbenes, the imidazolidine ring adopts a twist conformation with a pseudo-twofold axis passing through the N—C—N carbon and the opposite C—C bond. The N—C—N bond angle [113.0 (4)°] and C—N bond lengths [1.313 (6) and 1.305 (6) Å] confirm the existence of strong resonance in this part of the molecule. In the crystal, a C—H⋯Br interaction is present. The dihedral angle between the biphenyl rings is 64.3 (2)° and the phenyl rings make angles of 76.6 (3) and 18.5 (3)° with the plane of the imidazolidine ring.
Related literature
For the synthesis, see: Özdemir et al. (2005b
▶,d
▶). For general background, see: Herrmann (2002 ▶); Scott & Nolan (2005 ▶). For puckering and asymmetry parameters, see: Cremer & Pople (1975 ▶); Nardelli (1983 ▶). For related compounds, see: Arduengo et al. (1995a
▶,b
▶); Hagos et al. (2008 ▶). For bond-length data, see: Allen et al. (1987 ▶). For related literature, see: Arslan et al. (2004a
▶,b
▶, 2005a
▶,b
▶, 2007a
▶,b
▶,c
▶); Cavell & Guinness (2004 ▶); Hahn (2006 ▶); Kirmse (2004 ▶); Nair et al. (2004 ▶); Rangits & Kollar (2006 ▶); Richmond (2000 ▶); Özdemir et al. (2004 ▶, 2005a
▶,c
▶).
Experimental
Crystal data
C26H29N2 +·Br−
M r = 449.42
Monoclinic,
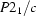
a = 18.626 (4) Å
b = 13.793 (3) Å
c = 8.8181 (18) Å
β = 95.08 (3)°
V = 2256.6 (8) Å3
Z = 4
Mo Kα radiation
μ = 1.84 mm−1
T = 183 (2) K
0.36 × 0.19 × 0.12 mm
Data collection
Rigaku AFC-8S Mercury CCD diffractometer
Absorption correction: multi-scan (REQAB; Jacobson, 1998 ▶) T min = 0.615, T max = 0.798
18802 measured reflections
4010 independent reflections
3051 reflections with I > 2σ(I)
R int = 0.060
Refinement
R[F 2 > 2σ(F 2)] = 0.078
wR(F 2) = 0.241
S = 1.09
4010 reflections
266 parameters
H-atom parameters constrained
Δρmax = 0.75 e Å−3
Δρmin = −1.13 e Å−3
Data collection: CrystalClear (Rigaku/MSC, 2006 ▶); cell refinement: CrystalClear; data reduction: CrystalClear (Rigaku/MSC, 2006 ▶); program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808042086/hg2448sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808042086/hg2448Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯Br1i | 0.96 | 2.52 | 3.472 (5) | 170 |
Symmetry code: (i) 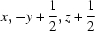 .
.
Acknowledgments
This work was supported financially by the Technological and Scientific Research Council of Turkey TÜBİTAK [TÜBITAK(106 T106)] and İnönü University Research Fund (BAP:2006/Güdümlü-7).
supplementary crystallographic information
Comment
Recently, N-heterocyclic carbene compounds have become universal ligands in inorganic and organometallic chemistry (Hahn, 2006; Kirmse, 2004). The role of N-heterocyclic carbenes as alternative compounds to tertiary phosphines in homogeneous catalysis is now well established (Scott & Nolan, 2005; Herrmann, 2002). At the same time, it has become increasingly apparent that carbenes (and/or their imidazolium precursors) are quite susceptible to metal induced bond activation reactions, which may be crucial to both catalytic activity and possible deactivation pathways. The chemistry of N-heterocyclic carbenes has become a major area of research as these stable carbenes (Nair et al., 2004) have proven to be outstanding ligands for transition metals (Cavell & Guinness, 2004), as well as potent nucleophilic organic catalysts (Rangits & Kollar, 2006). In this field, the conjugate acids play a very important role. Indeed, they are by far the most frequently used precursors of N-heterocyclic carbenes, and oxidative addition of the CH bond to transition metal centers can even directly generate the carbine complexes (Herrmann, 2002). Moreover, the conjugate acids of N-heterocyclic carbenes (H+) have found numerous applications as ionic liquids (Richmond, 2000), which are important components of green chemistry.
We have previously reported the use of in situ formed imidazolidin-2-ylidene, tetrahydropyrimidin-2-ylidene and tetrahydrodiazepin-2-ylidene palladium (II) systems that exhibit high activity for various coupling reactions of aryl bromides and aryl chlorides (Özdemir et al., 2005a, 2005b). In order to obtain more stable, efficient and active systems, we have also investigated benzo-annelated derivatives (Özdemir et al., 2004). Recently our group reported that novel complexes of rhodium(I) 1,3-dialkyimidazolidin-2-ylidenes gave secondary alcohols in good yields by the addition of phenylboronic acid to aldehydes (Özdemir et al., 2005c). In recent years, we also investigated the crystal structures of new N-heterocyclic carbene derivatives (Arslan et al., 2004a, 2004b, 2005a, 2005b, 2007a, 2007b, 2007c).
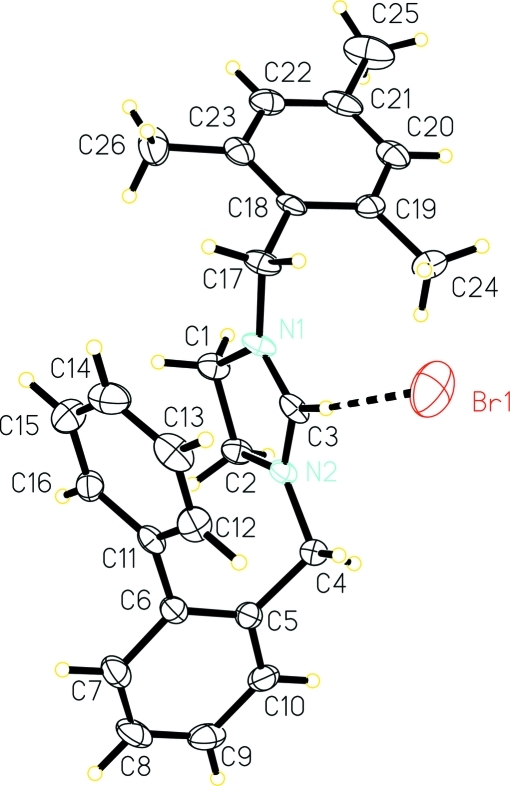
Due to our interest in preparing stable ligands for selected catalytic processes, we prepared 1-(2,4,6-trimethylbenzyl)-3-(2-phenylbenzyl)-imidazolidinium bromide, (I) as a carbene precursor. The title compound was purified by re-crystallization from an ethanol:diethylether mixture (2:1) and characterized by elemental analysis, 1H and 13C-NMR and IR spectroscopy (Özdemir et al. 2005d). The analytical and spectroscopic data are consistent with the proposed structure given in Scheme1. The structure consists of a 1-(2,4,6-trimethylbenzyl)-3-(2-phenylbenzyl)-imidazolidinium cation and a Br-anion. The structure of (I) is shown in Fig. 1.
The crystallographic data for (I) will provide valuable information for assessing its electronic conjugation properties and a possible intra- and inter-molecular hydrogen bond, which may be correlated with the reactivity of the carben carbonatom. N—Ccarben—N bond angles and Ccarben—N bond lengths are related with the proximity of the proton in the carben compounds (Arduengo et al., 1995b). Though N—Ccarben—N bond angles are observed as 101.4 (2) ° and 108.7 (4) ° for 1,3-dimesityl-2,3-dihydro-1H-imidazole-2-ylidene and 1,3-dimesityl-1H-imidazol-3-ium, respectively, (Arduengo et al., 1995b), N—Ccarben—N bond angle is 113.0 (4) ° in the title compound. According to this result, the larger N—Ccarben—N bond angle is more relaxed than the other carben derivatives. In addition, C3—H3···Br1 intermolecular hydrogen bond and Ccarben—N bond lengths have confirmed this result (Arduengo et al., 1995a, 1995b). The same geometric behaviors are observed for 1,3-dimesitylimidazolidinium tetrachloridogold(III) and 1,3-dimesityl-4,5-dihydro-1H-imidazol-3-ium chloride where the N—Ccarben—N bond angles are 113.9 (6) ° and 113.1 (4) °, respectively (Hagos et al., 2008; Arduengo et al., 1995b).
The Ccarben—N bond distances in the imidazolidine ring are not the expected single and double bond lengths as reported in other imidazolidine derivatives (Arduengo et al., 1995a, 1995b). Although the Ccarben—N bond distances (N1—C3 = 1.313 (6) Å and N2—C3 = 1.305 (6) Å) in the title compound are shorter than the average Ccarben—N bond length for free carbene derivatives which have imidazolidine rings is 1.360 Å. The C—N bond lengths have intermediate values between the single and double C—N bond lengths (Allen et al., 1987) and agree with Ccarben—N bond lengths values of 1,3-dimesitylimidazolidinium tetrachloridogold(III) and 1,3-dimesityl-4,5-dihydro-1H-imidazol-3-ium chloride (1.294 (7) and 1.320 (8) Å) (Hagos et al., 2008), (1.327 (5) and 1.310 (5) Å) (Arduengo et al., 1995b), respectively. Also, the sum of the bond angles around N1 (360.0 °) and N2 (360.0 °) indicate sp2 hybridization. These results can be explained by existence of resonance in this part of the molecule. All the other bond lengths are in normal ranges (Allen et al., 1987).
The deviations from planarity of imidazolidine ring are C1 0.056 (5), C2 0.051 (5), N2 0.027 (4), C3 0.008 (5) and N1 0.039 (4) Å. The puckering parameters (Cremer & Pople, 1975) and the smallest displacement asymmetry parameters (Nardelli, 1983) for the imidazolidine ringare q2 = 0.090 (5) Å, φ2= 46 (3)° and ΔC2(C3) = 13.0 (5), ΔCs(C3) = 5.1 (5). According to these results, the imidazolidine ring adopts a twisted conformation with a pseudo-twofold axis passing through C3 and the C1—C2 bond.
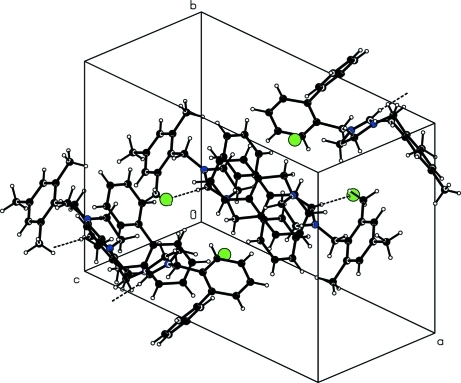
The crystal packing is shown in Fig. 2. The crystal packing is dominated by intermolecular C3—H3···Br1 (x,1/2 - y,1/2 + z) hydrogen bonds, with H···Br = 2.52 Å and C—H···Br 170 o (Table 1).
Experimental
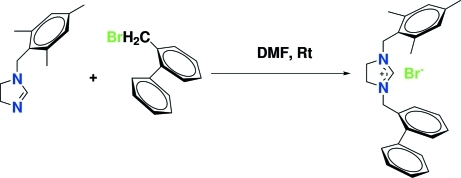
To a solution of 1-(2,4,6-trimethylbenzyl) imidazoline (2.02 g, 10 mmol) in DMF (5 ml) was added slowly 2-phenylbenzyl bromide (2.49 g, 10.07 mmol) at 25 °C and the resulting mixture was stirred at room temperature for 8 h (Fig. 3). Diethylether (15 ml) was added to obtain a white crystalline solid which was filtered off. The solid was washed with diethylether (3 x 10 ml), dried under vacuum and the crude product was recrystallized from ethanol:diethylether mixture (2:1) (Özdemir et al., 2005 d). M.p.: 241–241.5 °C. Yield: 4.22 g, 94%. υ(CN) = 1444 cm-1. 1H NMR (δ, CDCl3): 2.26 and 2.27 (s, 9H, CH2C6H2(CH3)3-2,4,6), 6.32 (s, 2H, CH2C6H2(CH3)3-2,4,6), 4.66 (s, 2H, CH2C6H2(CH3)3-2,4,6), 3.51 and 3.61 (m, 4H, NCH2CH2N), 4.88 (s, 2H, CH2C6H4(o-C6H5), 7.21–7.54 (m, 9H,CH2C6H4(o-C6H5), 8.78 (s, 1H, NCHN). 13C{H}NMR (δ, CDCl3): 20.5 and 21.2 (CH2C6H2(CH3)3-2,4,6), 125.5, 128.9, 138.0, and 139.3 (CH2C6H2(CH3)3-2,4,6), 46.5 (CH2C6H2(CH3)3-2,4,6), 47.9 and 48.3 (NCH2CH2N), 50.5 (CH2C6H4(o-C6H5), 127.9, 128.7, 129.0, 129.4, 129.9, 130.0, 130.4, 130.8, 139.9, 142.5 (CH2C6H4(o-C6H5), 157.5 (NCHN). Found: C 69.45, H 6.51, N 6.26%. Calcd. for C26H29N2Br: C 69.48, H 6.50, N 6.23%.
Figures
Fig. 1.
The molecular structure of the title compound, showing the atom-numbering scheme and displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
A view of the packing diagram of (I). Hydrogen bonds are shown as dashed lines.
Fig. 3.
Reaction scheme.
Crystal data
| C26H29N2+·Br− | F(000) = 936 |
| Mr = 449.42 | Dx = 1.323 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 8750 reflections |
| a = 18.626 (4) Å | θ = 2.2–26.0° |
| b = 13.793 (3) Å | µ = 1.84 mm−1 |
| c = 8.8181 (18) Å | T = 183 K |
| β = 95.08 (3)° | Rod, colorless |
| V = 2256.6 (8) Å3 | 0.36 × 0.19 × 0.12 mm |
| Z = 4 |
Data collection
| Rigaku AFC-8S Mercury CCD diffractometer | 4010 independent reflections |
| Radiation source: Sealed Tube | 3051 reflections with I > 2σ(I) |
| Graphite Monochromator | Rint = 0.060 |
| Detector resolution: 14.6199 pixels mm-1 | θmax = 25.1°, θmin = 2.2° |
| ω scans | h = −22→22 |
| Absorption correction: multi-scan (REQAB; Jacobson, 1998) | k = −16→16 |
| Tmin = 0.615, Tmax = 0.798 | l = −10→10 |
| 18802 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.078 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.241 | H-atom parameters constrained |
| S = 1.09 | w = 1/[σ2(Fo2) + (0.1604P)2 + 1.5464P] where P = (Fo2 + 2Fc2)/3 |
| 4010 reflections | (Δ/σ)max = 0.001 |
| 266 parameters | Δρmax = 0.75 e Å−3 |
| 0 restraints | Δρmin = −1.13 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.26262 (5) | 0.44009 (6) | 0.82641 (7) | 0.0611 (3) | |
| N1 | 0.18896 (19) | 0.0693 (3) | 0.8527 (4) | 0.0237 (8) | |
| N2 | 0.28839 (18) | 0.1536 (3) | 0.8601 (4) | 0.0199 (8) | |
| C1 | 0.1919 (2) | 0.0981 (4) | 0.6940 (5) | 0.0246 (10) | |
| H1A | 0.1486 | 0.1310 | 0.6561 | 0.030* | |
| H1B | 0.1990 | 0.0429 | 0.6306 | 0.030* | |
| C2 | 0.2573 (2) | 0.1665 (3) | 0.7014 (5) | 0.0233 (9) | |
| H2A | 0.2908 | 0.1477 | 0.6302 | 0.028* | |
| H2B | 0.2427 | 0.2325 | 0.6820 | 0.028* | |
| C3 | 0.2464 (2) | 0.0999 (4) | 0.9361 (5) | 0.0228 (9) | |
| H3 | 0.2565 | 0.0844 | 1.0420 | 0.027* | |
| C4 | 0.3567 (2) | 0.1972 (3) | 0.9213 (5) | 0.0238 (9) | |
| H4A | 0.3690 | 0.1732 | 1.0225 | 0.029* | |
| H4B | 0.3508 | 0.2662 | 0.9277 | 0.029* | |
| C5 | 0.4179 (2) | 0.1750 (3) | 0.8235 (5) | 0.0227 (9) | |
| C6 | 0.4328 (2) | 0.0805 (3) | 0.7758 (5) | 0.0219 (9) | |
| C7 | 0.4901 (3) | 0.0680 (4) | 0.6813 (6) | 0.0299 (11) | |
| H7 | 0.5011 | 0.0042 | 0.6464 | 0.036* | |
| C8 | 0.5299 (3) | 0.1449 (5) | 0.6396 (6) | 0.0355 (12) | |
| H8 | 0.5685 | 0.1346 | 0.5761 | 0.043* | |
| C9 | 0.5154 (3) | 0.2370 (4) | 0.6871 (6) | 0.0343 (12) | |
| H9 | 0.5437 | 0.2907 | 0.6574 | 0.041* | |
| C10 | 0.4585 (2) | 0.2522 (4) | 0.7797 (6) | 0.0277 (10) | |
| H10 | 0.4480 | 0.3166 | 0.8125 | 0.033* | |
| C11 | 0.3903 (2) | −0.0055 (3) | 0.8174 (5) | 0.0214 (9) | |
| C12 | 0.3899 (3) | −0.0353 (4) | 0.9702 (6) | 0.0289 (11) | |
| H12 | 0.4185 | −0.0014 | 1.0490 | 0.035* | |
| C13 | 0.3481 (3) | −0.1133 (4) | 1.0064 (6) | 0.0340 (11) | |
| H13 | 0.3475 | −0.1326 | 1.1108 | 0.041* | |
| C14 | 0.3074 (3) | −0.1638 (4) | 0.8945 (7) | 0.0368 (12) | |
| H14 | 0.2792 | −0.2185 | 0.9211 | 0.044* | |
| C15 | 0.3072 (3) | −0.1356 (4) | 0.7439 (6) | 0.0345 (11) | |
| H15 | 0.2786 | −0.1704 | 0.6660 | 0.041* | |
| C16 | 0.3487 (2) | −0.0567 (3) | 0.7052 (5) | 0.0247 (10) | |
| H16 | 0.3485 | −0.0375 | 0.6005 | 0.030* | |
| C17 | 0.1316 (2) | 0.0098 (4) | 0.9081 (6) | 0.0260 (10) | |
| H17A | 0.1407 | 0.0013 | 1.0162 | 0.031* | |
| H17B | 0.1319 | −0.0530 | 0.8614 | 0.031* | |
| C18 | 0.0574 (2) | 0.0560 (3) | 0.8730 (5) | 0.0220 (9) | |
| C19 | 0.0392 (2) | 0.1386 (3) | 0.9524 (6) | 0.0254 (10) | |
| C20 | −0.0292 (3) | 0.1791 (4) | 0.9211 (6) | 0.0308 (11) | |
| H20 | −0.0419 | 0.2359 | 0.9756 | 0.037* | |
| C21 | −0.0789 (2) | 0.1391 (4) | 0.8132 (6) | 0.0326 (11) | |
| C22 | −0.0600 (3) | 0.0574 (4) | 0.7337 (6) | 0.0303 (11) | |
| H22 | −0.0941 | 0.0300 | 0.6574 | 0.036* | |
| C23 | 0.0079 (2) | 0.0142 (4) | 0.7626 (5) | 0.0256 (10) | |
| C24 | 0.0914 (3) | 0.1861 (4) | 1.0706 (7) | 0.0422 (13) | |
| H24A | 0.1256 | 0.2240 | 1.0207 | 0.063* | |
| H24B | 0.1164 | 0.1371 | 1.1320 | 0.063* | |
| H24C | 0.0655 | 0.2275 | 1.1341 | 0.063* | |
| C25 | −0.1526 (3) | 0.1846 (5) | 0.7800 (9) | 0.0527 (17) | |
| H25A | −0.1505 | 0.2329 | 0.7022 | 0.079* | |
| H25B | −0.1671 | 0.2143 | 0.8709 | 0.079* | |
| H25C | −0.1868 | 0.1355 | 0.7460 | 0.079* | |
| C26 | 0.0244 (3) | −0.0751 (4) | 0.6756 (7) | 0.0387 (12) | |
| H26A | −0.0163 | −0.0912 | 0.6056 | 0.058* | |
| H26B | 0.0344 | −0.1279 | 0.7452 | 0.058* | |
| H26C | 0.0656 | −0.0635 | 0.6201 | 0.058* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0930 (6) | 0.0614 (5) | 0.0280 (4) | 0.0157 (4) | 0.0006 (3) | −0.0021 (3) |
| N1 | 0.0173 (17) | 0.032 (2) | 0.023 (2) | −0.0009 (15) | 0.0085 (15) | 0.0097 (16) |
| N2 | 0.0164 (16) | 0.028 (2) | 0.0162 (17) | −0.0001 (14) | 0.0043 (14) | 0.0024 (15) |
| C1 | 0.023 (2) | 0.030 (3) | 0.020 (2) | −0.0016 (18) | 0.0031 (17) | 0.0082 (19) |
| C2 | 0.021 (2) | 0.028 (2) | 0.020 (2) | −0.0010 (18) | 0.0039 (17) | 0.0074 (18) |
| C3 | 0.019 (2) | 0.030 (2) | 0.020 (2) | 0.0085 (17) | 0.0062 (17) | 0.0045 (18) |
| C4 | 0.020 (2) | 0.027 (2) | 0.025 (2) | −0.0013 (18) | 0.0031 (17) | −0.0032 (19) |
| C5 | 0.020 (2) | 0.032 (2) | 0.016 (2) | −0.0009 (17) | 0.0003 (16) | −0.0022 (18) |
| C6 | 0.0178 (19) | 0.031 (2) | 0.016 (2) | 0.0032 (18) | −0.0004 (16) | −0.0007 (18) |
| C7 | 0.025 (2) | 0.043 (3) | 0.022 (2) | 0.006 (2) | 0.0042 (19) | −0.004 (2) |
| C8 | 0.023 (2) | 0.061 (4) | 0.025 (2) | 0.000 (2) | 0.0106 (19) | 0.000 (2) |
| C9 | 0.027 (2) | 0.048 (3) | 0.028 (3) | −0.015 (2) | 0.004 (2) | 0.004 (2) |
| C10 | 0.024 (2) | 0.030 (3) | 0.029 (2) | −0.0073 (19) | −0.0001 (18) | −0.002 (2) |
| C11 | 0.0178 (19) | 0.023 (2) | 0.024 (2) | 0.0070 (16) | 0.0044 (16) | 0.0013 (18) |
| C12 | 0.028 (2) | 0.034 (3) | 0.024 (2) | 0.0054 (19) | −0.0028 (19) | 0.002 (2) |
| C13 | 0.033 (2) | 0.038 (3) | 0.033 (3) | 0.009 (2) | 0.009 (2) | 0.010 (2) |
| C14 | 0.028 (2) | 0.034 (3) | 0.050 (3) | −0.002 (2) | 0.008 (2) | 0.007 (2) |
| C15 | 0.032 (2) | 0.035 (3) | 0.035 (3) | −0.004 (2) | −0.001 (2) | −0.006 (2) |
| C16 | 0.026 (2) | 0.025 (2) | 0.023 (2) | 0.0010 (17) | 0.0027 (18) | −0.0029 (18) |
| C17 | 0.019 (2) | 0.027 (2) | 0.033 (3) | 0.0019 (18) | 0.0091 (19) | 0.013 (2) |
| C18 | 0.017 (2) | 0.022 (2) | 0.029 (2) | −0.0015 (16) | 0.0125 (18) | 0.0053 (17) |
| C19 | 0.024 (2) | 0.020 (2) | 0.033 (2) | −0.0066 (17) | 0.0092 (19) | 0.0006 (19) |
| C20 | 0.032 (2) | 0.021 (2) | 0.042 (3) | 0.0008 (18) | 0.018 (2) | 0.008 (2) |
| C21 | 0.024 (2) | 0.032 (3) | 0.044 (3) | 0.002 (2) | 0.015 (2) | 0.018 (2) |
| C22 | 0.026 (2) | 0.041 (3) | 0.024 (2) | −0.006 (2) | 0.0041 (19) | 0.008 (2) |
| C23 | 0.030 (2) | 0.026 (2) | 0.022 (2) | −0.0017 (18) | 0.0114 (18) | 0.0072 (19) |
| C24 | 0.034 (3) | 0.040 (3) | 0.053 (4) | −0.016 (2) | 0.010 (2) | −0.014 (3) |
| C25 | 0.026 (3) | 0.052 (4) | 0.081 (5) | 0.015 (3) | 0.007 (3) | 0.026 (3) |
| C26 | 0.047 (3) | 0.040 (3) | 0.030 (3) | 0.002 (2) | 0.010 (2) | −0.005 (2) |
Geometric parameters (Å, °)
| N1—C3 | 1.313 (6) | C13—C14 | 1.379 (8) |
| N1—C1 | 1.460 (6) | C13—H13 | 0.9600 |
| N1—C17 | 1.465 (6) | C14—C15 | 1.384 (8) |
| N2—C3 | 1.305 (6) | C14—H14 | 0.9600 |
| N2—C4 | 1.467 (6) | C15—C16 | 1.394 (7) |
| N2—C2 | 1.477 (6) | C15—H15 | 0.9600 |
| C1—C2 | 1.538 (6) | C16—H16 | 0.9600 |
| C1—H1A | 0.9600 | C17—C18 | 1.528 (6) |
| C1—H1B | 0.9600 | C17—H17A | 0.9600 |
| C2—H2A | 0.9600 | C17—H17B | 0.9600 |
| C2—H2B | 0.9600 | C18—C19 | 1.395 (7) |
| C3—H3 | 0.9600 | C18—C23 | 1.404 (7) |
| C4—C5 | 1.520 (6) | C19—C20 | 1.395 (7) |
| C4—H4A | 0.9600 | C19—C24 | 1.511 (7) |
| C4—H4B | 0.9600 | C20—C21 | 1.383 (8) |
| C5—C10 | 1.382 (7) | C20—H20 | 0.9600 |
| C5—C6 | 1.404 (7) | C21—C22 | 1.390 (8) |
| C6—C7 | 1.422 (7) | C21—C25 | 1.513 (7) |
| C6—C11 | 1.490 (6) | C22—C23 | 1.400 (7) |
| C7—C8 | 1.363 (8) | C22—H22 | 0.9600 |
| C7—H7 | 0.9600 | C23—C26 | 1.497 (7) |
| C8—C9 | 1.371 (8) | C24—H24A | 0.9599 |
| C8—H8 | 0.9600 | C24—H24B | 0.9599 |
| C9—C10 | 1.408 (7) | C24—H24C | 0.9599 |
| C9—H9 | 0.9600 | C25—H25A | 0.9599 |
| C10—H10 | 0.9600 | C25—H25B | 0.9599 |
| C11—C16 | 1.395 (6) | C25—H25C | 0.9599 |
| C11—C12 | 1.409 (7) | C26—H26A | 0.9599 |
| C12—C13 | 1.381 (8) | C26—H26B | 0.9599 |
| C12—H12 | 0.9600 | C26—H26C | 0.9599 |
| C3—N1—C1 | 110.6 (4) | C12—C13—H13 | 119.6 |
| C3—N1—C17 | 125.2 (4) | C13—C14—C15 | 119.9 (5) |
| C1—N1—C17 | 124.2 (4) | C13—C14—H14 | 120.0 |
| C3—N2—C4 | 125.7 (4) | C15—C14—H14 | 120.0 |
| C3—N2—C2 | 110.6 (4) | C14—C15—C16 | 120.0 (5) |
| C4—N2—C2 | 123.7 (3) | C14—C15—H15 | 120.0 |
| N1—C1—C2 | 102.9 (4) | C16—C15—H15 | 120.0 |
| N1—C1—H1A | 111.2 | C15—C16—C11 | 120.5 (4) |
| C2—C1—H1A | 111.2 | C15—C16—H16 | 119.8 |
| N1—C1—H1B | 111.2 | C11—C16—H16 | 119.8 |
| C2—C1—H1B | 111.2 | N1—C17—C18 | 111.9 (4) |
| H1A—C1—H1B | 109.1 | N1—C17—H17A | 109.2 |
| N2—C2—C1 | 102.1 (3) | C18—C17—H17A | 109.2 |
| N2—C2—H2A | 111.4 | N1—C17—H17B | 109.2 |
| C1—C2—H2A | 111.4 | C18—C17—H17B | 109.2 |
| N2—C2—H2B | 111.4 | H17A—C17—H17B | 107.9 |
| C1—C2—H2B | 111.4 | C19—C18—C23 | 120.6 (4) |
| H2A—C2—H2B | 109.2 | C19—C18—C17 | 119.6 (4) |
| N2—C3—N1 | 113.0 (4) | C23—C18—C17 | 119.8 (4) |
| N2—C3—H3 | 123.5 | C18—C19—C20 | 119.1 (4) |
| N1—C3—H3 | 123.5 | C18—C19—C24 | 122.0 (4) |
| N2—C4—C5 | 112.2 (4) | C20—C19—C24 | 118.9 (5) |
| N2—C4—H4A | 109.2 | C21—C20—C19 | 121.4 (5) |
| C5—C4—H4A | 109.2 | C21—C20—H20 | 119.3 |
| N2—C4—H4B | 109.2 | C19—C20—H20 | 119.3 |
| C5—C4—H4B | 109.2 | C20—C21—C22 | 119.0 (4) |
| H4A—C4—H4B | 107.9 | C20—C21—C25 | 120.6 (5) |
| C10—C5—C6 | 120.2 (4) | C22—C21—C25 | 120.4 (5) |
| C10—C5—C4 | 117.4 (4) | C21—C22—C23 | 121.2 (5) |
| C6—C5—C4 | 122.4 (4) | C21—C22—H22 | 119.4 |
| C5—C6—C7 | 117.9 (4) | C23—C22—H22 | 119.4 |
| C5—C6—C11 | 122.8 (4) | C22—C23—C18 | 118.6 (5) |
| C7—C6—C11 | 119.3 (4) | C22—C23—C26 | 118.6 (5) |
| C8—C7—C6 | 121.1 (5) | C18—C23—C26 | 122.7 (4) |
| C8—C7—H7 | 119.4 | C19—C24—H24A | 109.5 |
| C6—C7—H7 | 119.4 | C19—C24—H24B | 109.5 |
| C7—C8—C9 | 120.8 (4) | H24A—C24—H24B | 109.5 |
| C7—C8—H8 | 119.6 | C19—C24—H24C | 109.5 |
| C9—C8—H8 | 119.6 | H24A—C24—H24C | 109.5 |
| C8—C9—C10 | 119.6 (5) | H24B—C24—H24C | 109.5 |
| C8—C9—H9 | 120.2 | C21—C25—H25A | 109.5 |
| C10—C9—H9 | 120.2 | C21—C25—H25B | 109.5 |
| C5—C10—C9 | 120.4 (5) | H25A—C25—H25B | 109.5 |
| C5—C10—H10 | 119.8 | C21—C25—H25C | 109.5 |
| C9—C10—H10 | 119.8 | H25A—C25—H25C | 109.5 |
| C16—C11—C12 | 118.7 (4) | H25B—C25—H25C | 109.5 |
| C16—C11—C6 | 120.2 (4) | C23—C26—H26A | 109.5 |
| C12—C11—C6 | 121.1 (4) | C23—C26—H26B | 109.5 |
| C13—C12—C11 | 120.0 (5) | H26A—C26—H26B | 109.5 |
| C13—C12—H12 | 120.0 | C23—C26—H26C | 109.5 |
| C11—C12—H12 | 120.0 | H26A—C26—H26C | 109.5 |
| C14—C13—C12 | 120.9 (5) | H26B—C26—H26C | 109.5 |
| C14—C13—H13 | 119.6 | ||
| C3—N1—C1—C2 | −8.6 (5) | C16—C11—C12—C13 | −0.6 (7) |
| C17—N1—C1—C2 | 174.6 (4) | C6—C11—C12—C13 | 177.8 (4) |
| C3—N2—C2—C1 | −6.8 (5) | C11—C12—C13—C14 | 1.0 (7) |
| C4—N2—C2—C1 | 174.1 (4) | C12—C13—C14—C15 | −0.9 (8) |
| N1—C1—C2—N2 | 8.7 (4) | C13—C14—C15—C16 | 0.6 (8) |
| C4—N2—C3—N1 | −179.2 (4) | C14—C15—C16—C11 | −0.2 (8) |
| C2—N2—C3—N1 | 1.7 (5) | C12—C11—C16—C15 | 0.2 (7) |
| C1—N1—C3—N2 | 4.7 (5) | C6—C11—C16—C15 | −178.2 (4) |
| C17—N1—C3—N2 | −178.5 (4) | C3—N1—C17—C18 | 125.6 (5) |
| C3—N2—C4—C5 | 128.7 (5) | C1—N1—C17—C18 | −58.0 (6) |
| C2—N2—C4—C5 | −52.3 (6) | N1—C17—C18—C19 | −71.4 (6) |
| N2—C4—C5—C10 | 129.1 (4) | N1—C17—C18—C23 | 109.4 (5) |
| N2—C4—C5—C6 | −49.8 (6) | C23—C18—C19—C20 | 0.3 (7) |
| C10—C5—C6—C7 | −0.4 (6) | C17—C18—C19—C20 | −179.0 (4) |
| C4—C5—C6—C7 | 178.5 (4) | C23—C18—C19—C24 | −179.2 (5) |
| C10—C5—C6—C11 | −179.1 (4) | C17—C18—C19—C24 | 1.6 (7) |
| C4—C5—C6—C11 | −0.3 (6) | C18—C19—C20—C21 | −0.1 (7) |
| C5—C6—C7—C8 | 0.6 (7) | C24—C19—C20—C21 | 179.4 (5) |
| C11—C6—C7—C8 | 179.4 (4) | C19—C20—C21—C22 | −0.6 (7) |
| C6—C7—C8—C9 | −0.3 (8) | C19—C20—C21—C25 | −179.5 (5) |
| C7—C8—C9—C10 | −0.2 (8) | C20—C21—C22—C23 | 1.0 (7) |
| C6—C5—C10—C9 | −0.1 (7) | C25—C21—C22—C23 | 180.0 (5) |
| C4—C5—C10—C9 | −179.0 (4) | C21—C22—C23—C18 | −0.8 (7) |
| C8—C9—C10—C5 | 0.5 (7) | C21—C22—C23—C26 | 178.6 (5) |
| C5—C6—C11—C16 | 114.2 (5) | C19—C18—C23—C22 | 0.2 (6) |
| C7—C6—C11—C16 | −64.5 (6) | C17—C18—C23—C22 | 179.4 (4) |
| C5—C6—C11—C12 | −64.2 (6) | C19—C18—C23—C26 | −179.2 (4) |
| C7—C6—C11—C12 | 117.1 (5) | C17—C18—C23—C26 | 0.0 (7) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···Br1i | 0.96 | 2.52 | 3.472 (5) | 170 |
Symmetry codes: (i) x, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2448).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Arduengo, A. J. III, Gamper, S. F., Tamm, M., Calabrese, J. C., Davidson, F. & Craig, H. A. (1995a). J. Am. Chem. Soc.117, 572–573.
- Arduengo, A. J. III, Goerlich, J. R. & Marshall, W. J. (1995b). J. Am. Chem. Soc.117, 11027–11028.
- Arslan, H., VanDerveer, D., Özdemir, I., Çetinkaya, B. & Demir, S. (2004b). Z. Kristallogr. New Cryst. Struct.219, 377–378.
- Arslan, H., VanDerveer, D., Özdemir, I., Çetinkaya, B. & Demir, S. (2005a). J. Chem. Crystallogr.35, 491–495.
- Arslan, H., Vanderveer, D., Özdemir, I., Çetinkaya, B. & Yaşar, S. (2004a). Z. Kristallogr. New Cryst. Struct.219, 44–46.
- Arslan, H., VanDerveer, D., Özdemir, İ., Demir, S. & Çetinkaya, B. (2007a). Acta Cryst. E63, m770–m771.
- Arslan, H., VanDerveer, D., Özdemir, I., Yaşar, S. & Çetinkaya, B. (2005b). Acta Cryst. E61, m1873–m1875.
- Arslan, H., VanDerveer, D., Yaşar, S., Özdemir, I. & Çetinkaya, B. (2007b). Acta Cryst. E63, m942–m944.
- Arslan, H., VanDerveer, D., Yaşar, S., Özdemir, İ. & Çetinkaya, B. (2007c). Acta Cryst. E63, m1001–m1003.
- Cavell, K. J. & Guinness, D. S. (2004). Coord. Chem. Rev.248, 671–681.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Hagos, T. K., Nogai, S. D., Dobrzańska, L. & Cronje, S. (2008). Acta Cryst. E64, m1357. [DOI] [PMC free article] [PubMed]
- Hahn, F. E. (2006). Angew. Chem. Int. Ed.45, 1348–1352.
- Herrmann, W. A. (2002). Angew. Chem. Int. Ed.41, 1290–1311.
- Jacobson, R. (1998). REQAB Molecular Structure Corporation, The Woodlands, Texas, USA.
- Kirmse, W. (2004). Angew. Chem. Int. Ed.43, 1767–1769. [DOI] [PubMed]
- Nair, V., Bindu, S. & Sreekumar, V. (2004). Angew. Chem. Int. Ed.43, 5130–5135. [DOI] [PubMed]
- Nardelli, M. (1983). Acta Cryst. C39, 1141–1142.
- Özdemir, I., Demir, S. & Çetinkaya, B. (2005a). Tetrahedron, 61, 9791–9798.
- Özdemir, I., Gök, Y., Gürbüz, N., Çetinkaya, E. & Çetinkaya, B. (2004). Heteroatom. Chem.15, 419–423.
- Özdemir, I., Gürbüz, N., Gök, Y., Çetinkaya, E. & Çetinkaya, B. (2005b). Synlett, 15, 2394–2396.
- Özdemir, I., Gürbüz, N., Seçkin, T. & Çetinkaya, B. (2005c). Appl. Organomet. Chem.19, 633–638.
- Özdemir, I., Yaşar, S., Demir, S. & Çetinkaya, B. (2005d). Heteroatom. Chem.16, 557–561.
- Rangits, G. & Kollar, L. (2006). J. Mol. Catal. A.246, 59–64.
- Richmond, T. G. (2000). Angew. Chem.112, 3378–3380.
- Rigaku/MSC (2006). CrystalClear. Rigaku/MSC, The Woodlands, Texas, USA.
- Scott, N. M. & Nolan, S. P. (2005). Eur. J. Inorg. Chem.10, 1815–1828.
- Sheldrick, G. M. (2008). Acta Cryst A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808042086/hg2448sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808042086/hg2448Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report