Abstract
In the title compound, C11H9FN2O3, the benzene ring is almost coplanar with the heterocyclic ring, making a dihedral angle of 14.0 (1)°. The plane of the carboxyl group is rotated by 14.7 (3)° with respect to the 1,2,4-oxadiazole ring plane. The aliphatic chain exhibits a standard zigzag arrangement. Two intermolecular O—H⋯O hydrogen bonds between the carboxyl groups related by an inversion centre promote a dimeric structure formation. The dimers are stacked along the crystallographic a axis.
Related literature
For general background, see: Gallardo et al. (2008 ▶); Jakopin & Dolenc (2008 ▶). For related structures, see: Wang et al. (2006 ▶, 2007 ▶); Yan, Xing et al. (2006 ▶); Yan et al. (2006a
▶,b
▶). For the method of preparation, see: Sindkhedkar et al. (2008 ▶); Srivastava & Seabra (1997 ▶).
Experimental
Crystal data
C11H9FN2O3
M r = 236.20
Triclinic,

a = 5.055 (1) Å
b = 5.905 (1) Å
c = 17.967 (1) Å
α = 85.769 (5)°
β = 87.965 (7)°
γ = 81.252 (7)°
V = 528.47 (14) Å3
Z = 2
Mo Kα radiation
μ = 0.12 mm−1
T = 293 (2) K
0.50 × 0.33 × 0.07 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: none
2136 measured reflections
2066 independent reflections
1557 reflections with I > 2σ(I)
R int = 0.010
3 standard reflections every 200 reflections intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.112
S = 1.06
2066 reflections
158 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.21 e Å−3
Δρmin = −0.21 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 Software; data reduction: HELENA (Spek, 1996 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2003 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808042001/is2367sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808042001/is2367Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O10—H10⋯O9i | 0.97 (3) | 1.68 (3) | 2.650 (2) | 179 (3) |
Symmetry code: (i)  .
.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial assistance. SKMS thanks the Programa Institucional de Bolsas de Iniciação Científica (PIBIC/CNPq) for an Undergraduate fellowship.
supplementary crystallographic information
Comment
1,2,4-Oxadiazoles are well known compounds, which exhibit a large number of biological activities (Jakopin & Dolenc, 2008). Recently, the use of this heterocycle as core for luminescent liquid crystals has also been described (Gallardo et al., 2008).
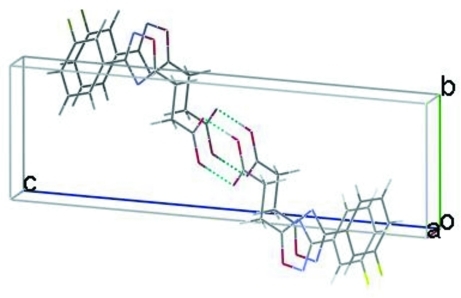
In the title compound (Fig. 1), the bond lengths and angles are in agreement with the values previously reported for 1,2,4-oxadiazole-containing molecules (Wang et al., 2006, 2007; Yan, Xing et al., 2006; Yan et al., 2006a,b). The torsion angle N2—C3—C11—C16 between the benzene ring attached to C-3 of the 1,2,4-oxadiazole system is -13.6 (2)°, thus, both rings are almost coplanar. The C-5 side-chain containing a carboxylic acid group shows a zigzag arrangement, having the torsion angle C5—C6—C7—C8 of -179.4 (1)°. In addition, the plane of the carboxylic group is also rotated by 14.7 (3)° with respect to the mean plane of the 1,2,4-oxadiazole five-membered ring, but in opposite direction of deviation of the fluoro-phenyl ring. This makes the molecular structure to be slightly twisted. Carboxylic groups are involved in centrosymmetric intermolecular hydrogen-bonding forming a dimeric structure (Fig. 2). The dimmers are perfectly stacked along the crystallographic a axis (Fig. 3).
Experimental
The title compound was synthesized following the procedure reported earlier for the analogous compounds (Srivastava & Seabra, 1997; Sindkhedkar et al., 2008). A mixture of 3-fluorbenzamidoxime (2.0 mmol) and succinic anhydride (2.2 mmol) was heated in a domestic microwave oven for 10 min. The crude material was purified by column chromatography. Crystallization of pure material from chloroform, from which a suitable crystal was chosen for the X-ray crystallographic experiment.
Refinement
H atoms attached to C atoms were added at their calculated positions and included in the structure factors calculations, with C—H = 0.93 (aromatic) and 0.97 Å (methylene), and with Uiso(H) = 1.2Ueq(C). The H atom of carboxylic acid was located in a difference Fourier map and treated as a free atom.
Figures
Fig. 1.
The molecular structure of (I) with labeling scheme. Displacement ellipsoids are shown at the 40% probability level.
Fig. 2.
Dimeric strucuture formed by hydrogen bonding.
Fig. 3.
Molecules of (I) stacked along the a axis.
Crystal data
| C11H9FN2O3 | Z = 2 |
| Mr = 236.20 | F(000) = 244 |
| Triclinic, P1 | Dx = 1.484 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71069 Å |
| a = 5.055 (1) Å | Cell parameters from 25 reflections |
| b = 5.905 (1) Å | θ = 5.0–18.8° |
| c = 17.967 (1) Å | µ = 0.12 mm−1 |
| α = 85.769 (5)° | T = 293 K |
| β = 87.965 (7)° | Irregular plate, colorless |
| γ = 81.252 (7)° | 0.50 × 0.33 × 0.07 mm |
| V = 528.47 (14) Å3 |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.010 |
| Radiation source: fine-focus sealed tube | θmax = 26.0°, θmin = 1.1° |
| graphite | h = −6→6 |
| ω–2θ scans | k = −7→7 |
| 2136 measured reflections | l = −22→0 |
| 2066 independent reflections | 3 standard reflections every 200 reflections |
| 1557 reflections with I > 2σ(I) | intensity decay: 1% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.112 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0583P)2 + 0.0868P] where P = (Fo2 + 2Fc2)/3 |
| 2066 reflections | (Δ/σ)max < 0.001 |
| 158 parameters | Δρmax = 0.21 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C3 | 0.1073 (3) | −0.0359 (3) | 0.24108 (9) | 0.0403 (4) | |
| C5 | −0.1973 (3) | 0.0022 (3) | 0.32245 (9) | 0.0412 (4) | |
| C6 | −0.4105 (3) | 0.0731 (3) | 0.37879 (10) | 0.0463 (4) | |
| H6A | −0.3429 | 0.0278 | 0.4284 | 0.056* | |
| H6B | −0.5616 | −0.0064 | 0.3722 | 0.056* | |
| C7 | −0.5043 (3) | 0.3291 (3) | 0.37270 (10) | 0.0476 (4) | |
| H7A | −0.3529 | 0.4086 | 0.3788 | 0.057* | |
| H7B | −0.5738 | 0.3741 | 0.3233 | 0.057* | |
| C8 | −0.7169 (3) | 0.4009 (3) | 0.42998 (9) | 0.0422 (4) | |
| C11 | 0.3022 (3) | 0.0236 (3) | 0.18257 (9) | 0.0415 (4) | |
| C12 | 0.2778 (4) | 0.2461 (3) | 0.15009 (10) | 0.0522 (4) | |
| H12 | 0.1426 | 0.3584 | 0.1660 | 0.063* | |
| C13 | 0.4575 (4) | 0.2996 (4) | 0.09349 (11) | 0.0618 (5) | |
| H13 | 0.4413 | 0.4485 | 0.0715 | 0.074* | |
| C14 | 0.6588 (4) | 0.1352 (4) | 0.06958 (11) | 0.0594 (5) | |
| H14 | 0.7785 | 0.1708 | 0.0316 | 0.071* | |
| C15 | 0.6783 (3) | −0.0823 (3) | 0.10322 (10) | 0.0534 (5) | |
| C16 | 0.5063 (3) | −0.1432 (3) | 0.15924 (10) | 0.0483 (4) | |
| H16 | 0.5256 | −0.2923 | 0.1811 | 0.058* | |
| N2 | 0.0849 (3) | −0.2473 (3) | 0.26222 (9) | 0.0552 (4) | |
| N4 | −0.0667 (3) | 0.1270 (2) | 0.27700 (8) | 0.0437 (3) | |
| O1 | −0.1230 (2) | −0.2237 (2) | 0.31750 (7) | 0.0555 (4) | |
| O9 | −0.8599 (2) | 0.2685 (2) | 0.45969 (7) | 0.0547 (3) | |
| O10 | −0.7395 (3) | 0.6168 (2) | 0.44456 (8) | 0.0551 (3) | |
| F17 | 0.8776 (2) | −0.2448 (2) | 0.07982 (7) | 0.0822 (4) | |
| H10 | −0.885 (6) | 0.660 (5) | 0.4800 (16) | 0.102 (8)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C3 | 0.0353 (8) | 0.0424 (8) | 0.0424 (8) | −0.0037 (6) | 0.0030 (7) | −0.0040 (7) |
| C5 | 0.0372 (8) | 0.0418 (8) | 0.0440 (9) | −0.0059 (6) | 0.0037 (7) | −0.0008 (7) |
| C6 | 0.0424 (9) | 0.0479 (9) | 0.0476 (9) | −0.0091 (7) | 0.0116 (7) | 0.0011 (7) |
| C7 | 0.0432 (9) | 0.0465 (9) | 0.0510 (10) | −0.0062 (7) | 0.0137 (7) | 0.0019 (7) |
| C8 | 0.0359 (8) | 0.0454 (9) | 0.0442 (9) | −0.0060 (7) | 0.0050 (7) | 0.0014 (7) |
| C11 | 0.0373 (8) | 0.0482 (9) | 0.0402 (8) | −0.0093 (7) | 0.0040 (6) | −0.0076 (7) |
| C12 | 0.0533 (10) | 0.0504 (10) | 0.0511 (10) | −0.0047 (8) | 0.0130 (8) | −0.0059 (8) |
| C13 | 0.0734 (13) | 0.0582 (12) | 0.0543 (11) | −0.0167 (10) | 0.0160 (9) | −0.0023 (9) |
| C14 | 0.0573 (11) | 0.0746 (14) | 0.0489 (10) | −0.0210 (10) | 0.0186 (9) | −0.0092 (9) |
| C15 | 0.0414 (9) | 0.0669 (12) | 0.0518 (10) | −0.0044 (8) | 0.0114 (8) | −0.0165 (9) |
| C16 | 0.0445 (9) | 0.0497 (10) | 0.0503 (10) | −0.0057 (7) | 0.0045 (8) | −0.0070 (7) |
| N2 | 0.0529 (9) | 0.0452 (8) | 0.0633 (10) | −0.0008 (6) | 0.0206 (7) | 0.0004 (7) |
| N4 | 0.0412 (7) | 0.0429 (7) | 0.0468 (8) | −0.0076 (6) | 0.0108 (6) | −0.0045 (6) |
| O1 | 0.0560 (7) | 0.0418 (7) | 0.0646 (8) | −0.0033 (5) | 0.0208 (6) | 0.0038 (5) |
| O9 | 0.0488 (7) | 0.0519 (7) | 0.0636 (8) | −0.0129 (5) | 0.0229 (6) | −0.0052 (6) |
| O10 | 0.0517 (7) | 0.0471 (7) | 0.0665 (8) | −0.0103 (5) | 0.0198 (6) | −0.0077 (6) |
| F17 | 0.0635 (7) | 0.0919 (10) | 0.0841 (9) | 0.0081 (6) | 0.0308 (6) | −0.0145 (7) |
Geometric parameters (Å, °)
| C3—N2 | 1.298 (2) | C11—C12 | 1.387 (2) |
| C3—N4 | 1.382 (2) | C11—C16 | 1.389 (2) |
| C3—C11 | 1.475 (2) | C12—C13 | 1.390 (3) |
| C5—N4 | 1.291 (2) | C12—H12 | 0.9300 |
| C5—O1 | 1.338 (2) | C13—C14 | 1.376 (3) |
| C5—C6 | 1.486 (2) | C13—H13 | 0.9300 |
| C6—C7 | 1.511 (2) | C14—C15 | 1.370 (3) |
| C6—H6A | 0.9700 | C14—H14 | 0.9300 |
| C6—H6B | 0.9700 | C15—F17 | 1.359 (2) |
| C7—C8 | 1.497 (2) | C15—C16 | 1.370 (2) |
| C7—H7A | 0.9700 | C16—H16 | 0.9300 |
| C7—H7B | 0.9700 | N2—O1 | 1.4183 (19) |
| C8—O9 | 1.2252 (19) | O10—H10 | 0.97 (3) |
| C8—O10 | 1.308 (2) | ||
| N2—C3—N4 | 114.80 (14) | C12—C11—C3 | 119.61 (15) |
| N2—C3—C11 | 122.14 (14) | C16—C11—C3 | 120.18 (15) |
| N4—C3—C11 | 123.06 (14) | C11—C12—C13 | 119.36 (17) |
| N4—C5—O1 | 113.59 (14) | C11—C12—H12 | 120.3 |
| N4—C5—C6 | 129.61 (15) | C13—C12—H12 | 120.3 |
| O1—C5—C6 | 116.79 (13) | C14—C13—C12 | 120.87 (18) |
| C5—C6—C7 | 112.30 (13) | C14—C13—H13 | 119.6 |
| C5—C6—H6A | 109.1 | C12—C13—H13 | 119.6 |
| C7—C6—H6A | 109.1 | C15—C14—C13 | 118.25 (17) |
| C5—C6—H6B | 109.1 | C15—C14—H14 | 120.9 |
| C7—C6—H6B | 109.1 | C13—C14—H14 | 120.9 |
| H6A—C6—H6B | 107.9 | F17—C15—C14 | 118.33 (16) |
| C8—C7—C6 | 112.31 (14) | F17—C15—C16 | 118.68 (18) |
| C8—C7—H7A | 109.1 | C14—C15—C16 | 122.99 (17) |
| C6—C7—H7A | 109.1 | C15—C16—C11 | 118.33 (17) |
| C8—C7—H7B | 109.1 | C15—C16—H16 | 120.8 |
| C6—C7—H7B | 109.1 | C11—C16—H16 | 120.8 |
| H7A—C7—H7B | 107.9 | C3—N2—O1 | 102.97 (13) |
| O9—C8—O10 | 123.07 (15) | C5—N4—C3 | 102.40 (13) |
| O9—C8—C7 | 122.54 (15) | C5—O1—N2 | 106.23 (12) |
| O10—C8—C7 | 114.38 (14) | C8—O10—H10 | 112.6 (16) |
| C12—C11—C16 | 120.20 (15) | ||
| N4—C5—C6—C7 | 8.6 (3) | C13—C14—C15—C16 | 0.2 (3) |
| O1—C5—C6—C7 | −172.42 (15) | F17—C15—C16—C11 | −179.68 (16) |
| C5—C6—C7—C8 | −179.36 (14) | C14—C15—C16—C11 | 0.3 (3) |
| C6—C7—C8—O9 | −23.4 (2) | C12—C11—C16—C15 | −0.8 (3) |
| C6—C7—C8—O10 | 157.66 (15) | C3—C11—C16—C15 | 178.08 (15) |
| N2—C3—C11—C12 | 165.28 (17) | N4—C3—N2—O1 | −0.10 (19) |
| N4—C3—C11—C12 | −14.0 (2) | C11—C3—N2—O1 | −179.48 (14) |
| N2—C3—C11—C16 | −13.6 (2) | O1—C5—N4—C3 | −0.79 (18) |
| N4—C3—C11—C16 | 167.08 (15) | C6—C5—N4—C3 | 178.25 (16) |
| C16—C11—C12—C13 | 0.7 (3) | N2—C3—N4—C5 | 0.54 (19) |
| C3—C11—C12—C13 | −178.15 (17) | C11—C3—N4—C5 | 179.91 (15) |
| C11—C12—C13—C14 | −0.2 (3) | N4—C5—O1—N2 | 0.77 (19) |
| C12—C13—C14—C15 | −0.3 (3) | C6—C5—O1—N2 | −178.40 (14) |
| C13—C14—C15—F17 | −179.80 (17) | C3—N2—O1—C5 | −0.37 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O10—H10···O9i | 0.97 (3) | 1.68 (3) | 2.650 (2) | 179 (3) |
Symmetry codes: (i) −x−2, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2367).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Gallardo, H., Cristiano, R., Vieira, A. A., Neves Filho, R. A. W. & Srivastava, R. M. (2008). Synthesis, pp. 605–609.
- Jakopin, Z. & Dolenc, M. S. (2008). Curr. Org. Chem.12, 850–898.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sindkhedkar, M. D., Desai, V. N., Loriya, R. M., Patel, M. V., Trivedi, B. K., Bora, R. O., Diwakar, S. D., Jadhav, G. R. & Pawar, S. S. (2008). PCT Int. Patent Appl. 123pp.
- Spek, A. L. (1996). HELENA University of Utrecht, The Netherlands.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Srivastava, R. M. & Seabra, G. M. (1997). J. Braz. Chem. Soc.8, 397–405.
- Wang, P., Li, H., Kang, S. & Wang, H. (2007). Acta Cryst. E63, o4411.
- Wang, H.-B., Liu, Z.-Q., Wang, H.-B. & Yan, X.-C. (2006). Acta Cryst. E62, o4715–o4716.
- Yan, X.-C., Wang, H.-B. & Liu, Z.-Q. (2006a). Acta Cryst. E62, o3007–o3008.
- Yan, X.-C., Wang, H.-B. & Liu, Z.-Q. (2006b). Acta Cryst. E62, o1302–o1303.
- Yan, X.-C., Xing, Z.-T., Liu, Z.-Q. & Wang, H.-B. (2006). Acta Cryst. E62, o4640–o4641.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808042001/is2367sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808042001/is2367Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report