Abstract
In the title compound, C11H11F6N·C4H6O5, a key intermediate in the synthesis of the NK1 receptor antagonist of casopitant, the F atoms of the trifluoromethyl groups are disordered over two sites with equal occupancies. In the crystal, the components are linked by bifurcated N—H⋯(O,O) hydrogen bonds.
Related literature
The title compound is a key intermediate for the synthesis of casopitant, which is an NK1 receptor antagonist (Humphrey, 2003 ▶) for the treatment of chemotheraphy-induced nausea and vomiting (CINV) (Lohr, 2008 ▶).
Experimental
Crystal data
C11H11F6N·C4H6O5
M r = 405.30
Monoclinic,

a = 6.6770 (13) Å
b = 8.4510 (17) Å
c = 16.366 (3) Å
β = 100.05 (3)°
V = 909.3 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.15 mm−1
T = 298 (2) K
0.30 × 0.10 × 0.10 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.957, T max = 0.985
1915 measured reflections
1757 independent reflections
1067 reflections with I > 2σ(I)
R int = 0.058
3 standard reflections every 200 reflections intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.063
wR(F 2) = 0.154
S = 1.00
1757 reflections
220 parameters
2 restraints
H-atom parameters constrained
Δρmax = 0.18 e Å−3
Δρmin = −0.27 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius,1989 ▶); cell refinement: CAD-4 Software; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808043201/at2692sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808043201/at2692Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N—H0A⋯O4i | 0.86 | 2.37 | 2.914 (7) | 122 |
| N—H0A⋯O3ii | 0.86 | 2.20 | 2.887 (7) | 137 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors thank the China Postdoctoral Research Fund (20070411010) and the Young Teachers’ Starting Fund of Southeast University for support.
supplementary crystallographic information
Comment
The title compound, C11H11F6N.C4H6O5, is a key intermediate for the synthesis of casopitant, which is an NK1 receptor antagonist (Humphrey, 2003) for the treatment of chemotheraphy-induced nausea and vomiting (CINV) (Lohr, 2008).
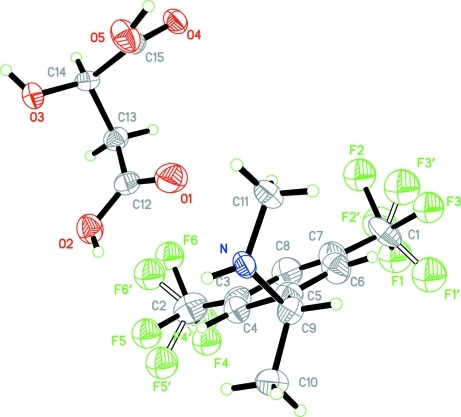
The molecular structure of the title compound is shown in Fig.1. The F atoms of the trifluoromethyl group are disordered over two sites in a 0.50:0.50 ratio. N—H···O hydrogen bonding interactions occur between (S)-1-(3, 5-bis(trifluoromethyl)-phenyl)ethylamine N-monomethyl and (R)-2-hydroxybutanedioic acid (Table 1).
Experimental
To a solution of 3, 5-bis(trifluoromethyl)-phenyl)ethylamine N-monomethyl (2.71 g, 10 mmol) in EtOAc (25 ml), (R)-2-hydroxybutanedioic acid (1.34,10 mmol) was added portionwise. The suspension was stirred for 2h at 298 K, then for 3 h at 273 K. The suspension was filtered and the cake was washed with EtOAc (20 ml). The solid was dried under vacucum obtaining the crude title compound (1.48 g). Single crystal of the title compound suitable for X-ray diffraction was obtained by slow evaporation of the EtOAc solution of the title compound.
Refinement
All H atoms were positoned geometrically and allowed to ride on their parent atoms, with C—H = 0.93Å for aromatic H atoms, 0.96Å for methyl H atoms, 0.97Å for methylene H atoms, 0.98Å for methine H atoms and O—H = 0.82 Å, N—H = 0.86 Å, respectively. [Uiso (H) = 1.2Ueq(C) for aromatic, methylene and methine; Uiso(H) = 1.5 Ueq(C) for methyl, Uiso(H) = 1.2Ueq(N); Uiso(H) = 1.5 Ueq(O).]
Figures
Fig. 1.
The molecular structure of the title compound, drawn with 30% probability ellipsoids.
Crystal data
| C11H11F6N·C4H6O5 | F(000) = 416 |
| Mr = 405.30 | Dx = 1.480 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 25 reflections |
| a = 6.6770 (13) Å | θ = 9–12° |
| b = 8.4510 (17) Å | µ = 0.15 mm−1 |
| c = 16.366 (3) Å | T = 298 K |
| β = 100.05 (3)° | Needle, colourless |
| V = 909.3 (3) Å3 | 0.30 × 0.10 × 0.10 mm |
| Z = 2 |
Data collection
| Enraf–Nonius CAD-4 diffractometer | 1067 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.058 |
| graphite | θmax = 25.3°, θmin = 1.3° |
| ω/2θ scans | h = 0→7 |
| Absorption correction: ψ scan (North et al., 1968) | k = 0→10 |
| Tmin = 0.957, Tmax = 0.985 | l = −19→19 |
| 1915 measured reflections | 3 standard reflections every 200 reflections |
| 1757 independent reflections | intensity decay: 1% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.063 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.154 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.060P)2 + 0.3P] where P = (Fo2 + 2Fc2)/3 |
| 1757 reflections | (Δ/σ)max < 0.001 |
| 220 parameters | Δρmax = 0.18 e Å−3 |
| 2 restraints | Δρmin = −0.27 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| F1 | 0.9058 (17) | 0.4852 (16) | 0.9973 (8) | 0.112 | 0.50 |
| F3 | 0.9270 (13) | 0.6270 (13) | 0.8772 (6) | 0.083 | 0.50 |
| F2 | 1.0892 (15) | 0.4013 (14) | 0.9084 (6) | 0.101 | 0.50 |
| F1' | 0.7985 (16) | 0.6070 (16) | 0.9651 (7) | 0.111 | 0.50 |
| F2' | 1.000 (2) | 0.4376 (16) | 0.9758 (7) | 0.111 | 0.50 |
| F3' | 0.9991 (17) | 0.5461 (16) | 0.8769 (7) | 0.115 | 0.50 |
| F4 | 0.5055 (13) | −0.0158 (14) | 0.9651 (7) | 0.085 | 0.50 |
| F5 | 0.3781 (15) | −0.0926 (12) | 0.8429 (6) | 0.080 | 0.50 |
| F6 | 0.6975 (13) | −0.0918 (12) | 0.8664 (6) | 0.078 | 0.50 |
| F4' | 0.5831 (15) | −0.0443 (15) | 0.9665 (8) | 0.097 | 0.50 |
| F5' | 0.3083 (15) | −0.0556 (14) | 0.8707 (6) | 0.095 | 0.50 |
| F6' | 0.6174 (16) | −0.1374 (14) | 0.8609 (7) | 0.093 | 0.50 |
| N | 0.3387 (7) | 0.3503 (6) | 0.6045 (3) | 0.0464 (12) | |
| H0A | 0.2534 | 0.2888 | 0.5744 | 0.056* | |
| C1 | 0.8859 (12) | 0.4933 (11) | 0.9164 (5) | 0.081 | |
| C2 | 0.5244 (14) | −0.0104 (14) | 0.8816 (5) | 0.094 (3) | |
| C3 | 0.5326 (11) | 0.1454 (10) | 0.8548 (4) | 0.063 (2) | |
| C4 | 0.4157 (10) | 0.2037 (8) | 0.7869 (4) | 0.0543 (17) | |
| H4A | 0.3136 | 0.1408 | 0.7574 | 0.065* | |
| C5 | 0.4445 (9) | 0.3611 (9) | 0.7587 (4) | 0.0527 (16) | |
| C6 | 0.5931 (11) | 0.4465 (10) | 0.8000 (4) | 0.072 (2) | |
| H6A | 0.6137 | 0.5488 | 0.7821 | 0.087* | |
| C7 | 0.7249 (13) | 0.3856 (12) | 0.8723 (5) | 0.080 (2) | |
| C8 | 0.6962 (12) | 0.2426 (12) | 0.8978 (5) | 0.079 (3) | |
| H8A | 0.7817 | 0.2026 | 0.9441 | 0.095* | |
| C9 | 0.3017 (8) | 0.4239 (8) | 0.6855 (3) | 0.0482 (15) | |
| H9A | 0.3274 | 0.5377 | 0.6823 | 0.058* | |
| C10 | 0.0783 (9) | 0.4033 (9) | 0.6903 (4) | 0.0646 (19) | |
| H10A | 0.0507 | 0.4508 | 0.7405 | 0.097* | |
| H10B | 0.0461 | 0.2926 | 0.6902 | 0.097* | |
| H10C | −0.0033 | 0.4535 | 0.6433 | 0.097* | |
| C11 | 0.5373 (9) | 0.3961 (9) | 0.5832 (4) | 0.0606 (18) | |
| H11A | 0.5542 | 0.3455 | 0.5323 | 0.091* | |
| H11B | 0.6447 | 0.3636 | 0.6269 | 0.091* | |
| H11C | 0.5419 | 0.5088 | 0.5766 | 0.091* | |
| O1 | 0.6777 (8) | 0.0742 (7) | 0.6474 (4) | 0.0869 (18) | |
| O2 | 0.5220 (7) | −0.1471 (5) | 0.6689 (3) | 0.0652 (13) | |
| H2A | 0.4226 | −0.0912 | 0.6526 | 0.098* | |
| O3 | 0.8263 (6) | −0.2740 (5) | 0.5576 (3) | 0.0579 (12) | |
| H3A | 0.8825 | −0.3437 | 0.5351 | 0.087* | |
| O4 | 1.1965 (6) | 0.0264 (5) | 0.6157 (3) | 0.0540 (11) | |
| O5 | 0.9516 (7) | 0.0076 (6) | 0.5086 (3) | 0.0678 (15) | |
| H5A | 1.0184 | 0.0743 | 0.4888 | 0.102* | |
| C12 | 0.6858 (11) | −0.0671 (9) | 0.6676 (4) | 0.0551 (17) | |
| C13 | 0.8797 (9) | −0.1508 (8) | 0.6891 (4) | 0.0522 (15) | |
| H13A | 0.8587 | −0.2481 | 0.7179 | 0.063* | |
| H13B | 0.9744 | −0.0857 | 0.7265 | 0.063* | |
| C14 | 0.9715 (8) | −0.1898 (7) | 0.6122 (3) | 0.0428 (14) | |
| H14A | 1.0894 | −0.2588 | 0.6292 | 0.051* | |
| C15 | 1.0461 (9) | −0.0360 (7) | 0.5766 (4) | 0.0464 (15) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| F1 | 0.112 | 0.112 | 0.112 | 0.000 | 0.020 | 0.000 |
| F3 | 0.083 | 0.083 | 0.083 | 0.000 | 0.015 | 0.000 |
| F2 | 0.101 | 0.101 | 0.101 | 0.000 | 0.018 | 0.000 |
| F1' | 0.111 | 0.111 | 0.111 | 0.000 | 0.019 | 0.000 |
| F2' | 0.111 | 0.111 | 0.111 | 0.000 | 0.019 | 0.000 |
| F3' | 0.115 | 0.115 | 0.115 | 0.000 | 0.020 | 0.000 |
| F4 | 0.085 | 0.085 | 0.085 | 0.000 | 0.015 | 0.000 |
| F5 | 0.080 | 0.080 | 0.080 | 0.000 | 0.014 | 0.000 |
| F6 | 0.078 | 0.078 | 0.078 | 0.000 | 0.014 | 0.000 |
| F4' | 0.097 | 0.097 | 0.097 | 0.000 | 0.017 | 0.000 |
| F5' | 0.095 | 0.095 | 0.095 | 0.000 | 0.017 | 0.000 |
| F6' | 0.093 | 0.093 | 0.093 | 0.000 | 0.016 | 0.000 |
| N | 0.050 (3) | 0.044 (3) | 0.047 (3) | −0.015 (3) | 0.010 (2) | −0.004 (3) |
| C1 | 0.072 | 0.100 | 0.067 | −0.033 | −0.003 | 0.033 |
| C2 | 0.097 (7) | 0.114 (8) | 0.069 (6) | 0.014 (7) | 0.012 (5) | 0.000 (6) |
| C3 | 0.063 (4) | 0.065 (5) | 0.059 (4) | 0.016 (4) | 0.005 (4) | 0.000 (4) |
| C4 | 0.055 (4) | 0.058 (4) | 0.048 (3) | −0.002 (4) | 0.002 (3) | 0.002 (3) |
| C5 | 0.053 (4) | 0.065 (5) | 0.042 (3) | −0.004 (4) | 0.013 (3) | −0.002 (3) |
| C6 | 0.075 (5) | 0.084 (6) | 0.055 (4) | −0.014 (5) | 0.003 (4) | −0.012 (4) |
| C7 | 0.068 (5) | 0.076 (6) | 0.089 (6) | −0.003 (5) | −0.006 (4) | 0.001 (5) |
| C8 | 0.068 (5) | 0.102 (7) | 0.061 (5) | 0.000 (5) | −0.007 (4) | −0.014 (5) |
| C9 | 0.053 (3) | 0.045 (3) | 0.045 (3) | 0.009 (3) | 0.003 (3) | 0.007 (3) |
| C10 | 0.054 (4) | 0.069 (5) | 0.073 (4) | 0.005 (4) | 0.017 (3) | 0.014 (4) |
| C11 | 0.047 (3) | 0.062 (4) | 0.072 (4) | −0.015 (3) | 0.010 (3) | 0.003 (4) |
| O1 | 0.081 (4) | 0.067 (4) | 0.120 (5) | 0.011 (3) | 0.037 (3) | 0.010 (4) |
| O2 | 0.068 (3) | 0.053 (3) | 0.074 (3) | −0.002 (3) | 0.009 (2) | 0.002 (3) |
| O3 | 0.059 (3) | 0.043 (2) | 0.074 (3) | −0.012 (2) | 0.018 (2) | −0.011 (2) |
| O4 | 0.046 (2) | 0.053 (3) | 0.062 (3) | −0.011 (2) | 0.009 (2) | −0.002 (2) |
| O5 | 0.082 (3) | 0.066 (3) | 0.052 (2) | −0.022 (3) | 0.003 (2) | 0.015 (3) |
| C12 | 0.062 (4) | 0.061 (4) | 0.046 (4) | −0.003 (4) | 0.022 (3) | −0.005 (3) |
| C13 | 0.060 (4) | 0.051 (4) | 0.048 (3) | 0.005 (4) | 0.016 (3) | 0.003 (3) |
| C14 | 0.041 (3) | 0.048 (4) | 0.042 (3) | 0.007 (3) | 0.014 (3) | 0.006 (3) |
| C15 | 0.046 (3) | 0.036 (3) | 0.061 (4) | 0.005 (3) | 0.020 (3) | −0.004 (3) |
Geometric parameters (Å, °)
| F1—C1 | 1.309 (14) | C7—C8 | 1.304 (13) |
| F3—C1 | 1.352 (13) | C8—H8A | 0.9300 |
| F2—C1 | 1.589 (14) | C9—C10 | 1.517 (8) |
| F1'—C1 | 1.436 (13) | C9—H9A | 0.9800 |
| F2'—C1 | 1.219 (13) | C10—H10A | 0.9600 |
| F3'—C1 | 1.167 (12) | C10—H10B | 0.9600 |
| F4—C2 | 1.395 (13) | C10—H10C | 0.9600 |
| F5—C2 | 1.274 (12) | C11—H11A | 0.9600 |
| F6—C2 | 1.404 (11) | C11—H11B | 0.9600 |
| F4'—C2 | 1.405 (14) | C11—H11C | 0.9600 |
| F5'—C2 | 1.474 (13) | O1—C12 | 1.237 (9) |
| F6'—C2 | 1.313 (15) | O2—C12 | 1.290 (8) |
| N—C11 | 1.480 (7) | O2—H2A | 0.8200 |
| N—C9 | 1.523 (7) | O3—C14 | 1.394 (7) |
| N—H0A | 0.8600 | O3—H3A | 0.8200 |
| C1—C7 | 1.495 (11) | O4—C15 | 1.213 (7) |
| C2—C3 | 1.392 (13) | O5—C15 | 1.236 (7) |
| C3—C4 | 1.336 (9) | O5—H5A | 0.8200 |
| C3—C8 | 1.447 (11) | C12—C13 | 1.463 (9) |
| C4—C5 | 1.432 (10) | C13—C14 | 1.530 (8) |
| C4—H4A | 0.9300 | C13—H13A | 0.9700 |
| C5—C6 | 1.315 (9) | C13—H13B | 0.9700 |
| C5—C9 | 1.492 (8) | C14—C15 | 1.543 (8) |
| C6—C7 | 1.441 (10) | C14—H14A | 0.9800 |
| C6—H6A | 0.9300 | ||
| C11—N—C9 | 112.7 (5) | C5—C6—C7 | 121.6 (8) |
| C11—N—H0A | 123.6 | C5—C6—H6A | 119.2 |
| C9—N—H0A | 123.6 | C7—C6—H6A | 119.2 |
| F3'—C1—F2' | 102.5 (11) | C8—C7—C6 | 119.4 (8) |
| F3'—C1—F1 | 128.3 (11) | C8—C7—C1 | 122.9 (8) |
| F2'—C1—F3 | 123.5 (10) | C6—C7—C1 | 117.6 (8) |
| F1—C1—F3 | 122.1 (10) | C7—C8—C3 | 120.8 (8) |
| F3'—C1—F1' | 114.7 (11) | C7—C8—H8A | 119.6 |
| F2'—C1—F1' | 94.2 (9) | C3—C8—H8A | 119.6 |
| F1—C1—F1' | 56.8 (8) | C5—C9—C10 | 114.4 (5) |
| F3—C1—F1' | 80.8 (8) | C5—C9—N | 112.1 (5) |
| F3'—C1—C7 | 116.5 (10) | C10—C9—N | 108.0 (5) |
| F2'—C1—C7 | 116.5 (9) | C5—C9—H9A | 107.3 |
| F1—C1—C7 | 113.2 (9) | C10—C9—H9A | 107.3 |
| F3—C1—C7 | 117.9 (7) | N—C9—H9A | 107.3 |
| F1'—C1—C7 | 110.3 (8) | C9—C10—H10A | 109.5 |
| F3'—C1—F2 | 59.7 (8) | C9—C10—H10B | 109.5 |
| F2'—C1—F2 | 56.9 (8) | H10A—C10—H10B | 109.5 |
| F1—C1—F2 | 96.9 (8) | C9—C10—H10C | 109.5 |
| F3—C1—F2 | 97.0 (8) | H10A—C10—H10C | 109.5 |
| F1'—C1—F2 | 144.0 (8) | H10B—C10—H10C | 109.5 |
| C7—C1—F2 | 102.4 (8) | N—C11—H11A | 109.5 |
| F5—C2—F6' | 77.4 (9) | N—C11—H11B | 109.5 |
| F5—C2—C3 | 115.3 (9) | H11A—C11—H11B | 109.5 |
| F6'—C2—C3 | 130.1 (9) | N—C11—H11C | 109.5 |
| F5—C2—F4 | 106.1 (10) | H11A—C11—H11C | 109.5 |
| F6'—C2—F4 | 110.7 (10) | H11B—C11—H11C | 109.5 |
| C3—C2—F4 | 110.8 (10) | C12—O2—H2A | 109.5 |
| F5—C2—F6 | 103.2 (10) | C14—O3—H3A | 109.5 |
| C3—C2—F6 | 109.2 (9) | C15—O5—H5A | 109.5 |
| F4—C2—F6 | 112.1 (8) | O1—C12—O2 | 120.8 (7) |
| F5—C2—F4' | 116.0 (10) | O1—C12—C13 | 121.8 (7) |
| F6'—C2—F4' | 91.5 (9) | O2—C12—C13 | 117.4 (7) |
| C3—C2—F4' | 119.0 (10) | C12—C13—C14 | 111.8 (5) |
| F6—C2—F4' | 88.8 (8) | C12—C13—H13A | 109.3 |
| F6'—C2—F5' | 104.7 (10) | C14—C13—H13A | 109.3 |
| C3—C2—F5' | 107.4 (9) | C12—C13—H13B | 109.3 |
| F4—C2—F5' | 81.7 (8) | C14—C13—H13B | 109.3 |
| F6—C2—F5' | 132.1 (11) | H13A—C13—H13B | 107.9 |
| F4'—C2—F5' | 99.5 (9) | O3—C14—C13 | 107.7 (4) |
| C4—C3—C2 | 124.2 (8) | O3—C14—C15 | 115.0 (5) |
| C4—C3—C8 | 118.5 (8) | C13—C14—C15 | 109.4 (5) |
| C2—C3—C8 | 116.8 (8) | O3—C14—H14A | 108.2 |
| C3—C4—C5 | 121.2 (7) | C13—C14—H14A | 108.2 |
| C3—C4—H4A | 119.4 | C15—C14—H14A | 108.2 |
| C5—C4—H4A | 119.4 | O4—C15—O5 | 126.3 (6) |
| C6—C5—C4 | 118.4 (7) | O4—C15—C14 | 117.3 (5) |
| C6—C5—C9 | 122.5 (7) | O5—C15—C14 | 116.3 (5) |
| C4—C5—C9 | 119.1 (6) | ||
| F5—C2—C3—C4 | −8.6 (14) | F2—C1—C7—C8 | 63.3 (12) |
| F6'—C2—C3—C4 | 86.2 (14) | F3'—C1—C7—C6 | −56.5 (14) |
| F4—C2—C3—C4 | −129.1 (9) | F2'—C1—C7—C6 | −177.7 (11) |
| F6—C2—C3—C4 | 107.0 (10) | F1—C1—C7—C6 | 138.1 (10) |
| F4'—C2—C3—C4 | −153.3 (8) | F3—C1—C7—C6 | −13.7 (14) |
| F5'—C2—C3—C4 | −41.5 (11) | F1'—C1—C7—C6 | 76.5 (10) |
| F5—C2—C3—C8 | 179.6 (8) | F2—C1—C7—C6 | −118.7 (8) |
| F6'—C2—C3—C8 | −85.6 (13) | C6—C7—C8—C3 | −0.6 (14) |
| F4—C2—C3—C8 | 59.1 (11) | C1—C7—C8—C3 | 177.4 (8) |
| F6—C2—C3—C8 | −64.8 (10) | C4—C3—C8—C7 | 2.0 (13) |
| F4'—C2—C3—C8 | 34.8 (13) | C2—C3—C8—C7 | 174.3 (9) |
| F5'—C2—C3—C8 | 146.6 (8) | C6—C5—C9—C10 | −128.2 (7) |
| C2—C3—C4—C5 | −174.3 (8) | C4—C5—C9—C10 | 49.9 (8) |
| C8—C3—C4—C5 | −2.6 (11) | C6—C5—C9—N | 108.5 (7) |
| C3—C4—C5—C6 | 1.8 (11) | C4—C5—C9—N | −73.5 (7) |
| C3—C4—C5—C9 | −176.3 (6) | C11—N—C9—C5 | −66.8 (7) |
| C4—C5—C6—C7 | −0.3 (11) | C11—N—C9—C10 | 166.3 (5) |
| C9—C5—C6—C7 | 177.8 (7) | O1—C12—C13—C14 | −75.2 (9) |
| C5—C6—C7—C8 | −0.3 (13) | O2—C12—C13—C14 | 103.5 (7) |
| C5—C6—C7—C1 | −178.4 (8) | C12—C13—C14—O3 | −55.0 (7) |
| F3'—C1—C7—C8 | 125.5 (13) | C12—C13—C14—C15 | 70.6 (7) |
| F2'—C1—C7—C8 | 4.3 (17) | O3—C14—C15—O4 | −167.3 (5) |
| F1—C1—C7—C8 | −39.9 (15) | C13—C14—C15—O4 | 71.4 (6) |
| F3—C1—C7—C8 | 168.3 (10) | O3—C14—C15—O5 | 9.4 (7) |
| F1'—C1—C7—C8 | −101.5 (12) | C13—C14—C15—O5 | −111.9 (6) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N—H0A···O4i | 0.86 | 2.37 | 2.914 (7) | 122 |
| N—H0A···O3ii | 0.86 | 2.20 | 2.887 (7) | 137 |
Symmetry codes: (i) x−1, y, z; (ii) −x+1, y+1/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AT2692).
References
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- Humphrey, J. M. (2003). Curr. Topics Med. Chem.3, 1423–1435. [DOI] [PubMed]
- Lohr, L. (2008). Cancer J.14, 85–93. [DOI] [PubMed]
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808043201/at2692sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808043201/at2692Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report