Abstract
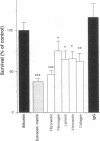
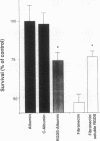
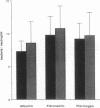
The activation patterns of surface adherent neutrophils are modulated via interaction of extracellular matrix proteins with neutrophil integrins. To evaluate neutrophil bactericidal activity, Staphylococcus aureus adherent to biological surfaces were incubated with neutrophils and serum, and the survival of surface bacteria was determined. When compared to albumin-coated surfaces, the bactericidal activity of neutrophils adherent to purified human extracellular matrix was markedly enhanced (mean survival: 34.2% +/- 9.0% of albumin, P less than 0.0001) despite similar efficient ingestion of extracellular bacteria. Enhancement of killing was observed when surfaces were coated with purified constituents of extracellular matrix, i.e., fibronectin, fibrinogen, laminin, vitronectin, or type IV collagen. In addition to matrix proteins, the tetrapeptide RGDS (the sequence recognized by integrins) crosslinked to surface bound albumin was also active (survival: 74.5% +/- 5.5% of albumin, P less than 0.02), and fibronectin-increased killing was inhibited by soluble RGDS. Chemiluminescence measurements and experiments with CGD neutrophils revealed that both oxygen-dependent and -independent bactericidal mechanisms are involved. In conclusion, matrix proteins enhance intracellular bactericidal activity of adherent neutrophils, presumably by integrin recognition of RGDS-containing ligands. These results indicate a role for extracellular matrix proteins in the enhancement of the host defense against pyogenic infections.
Full text
PDF


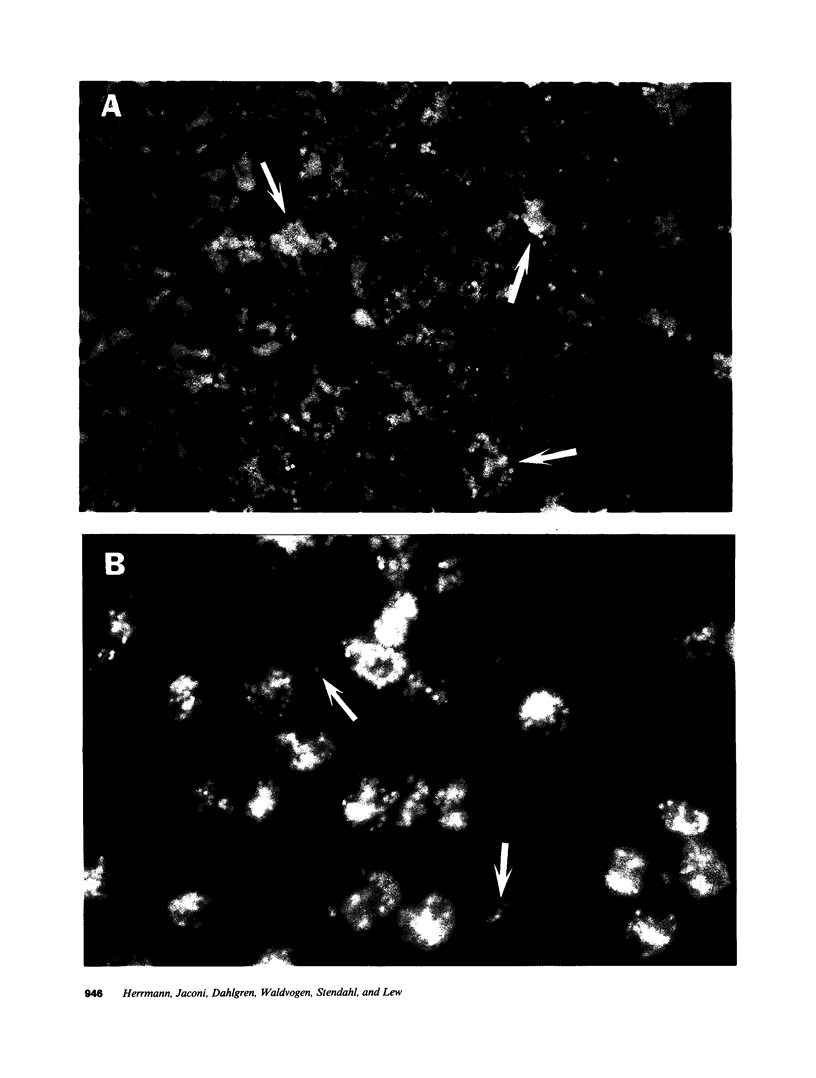
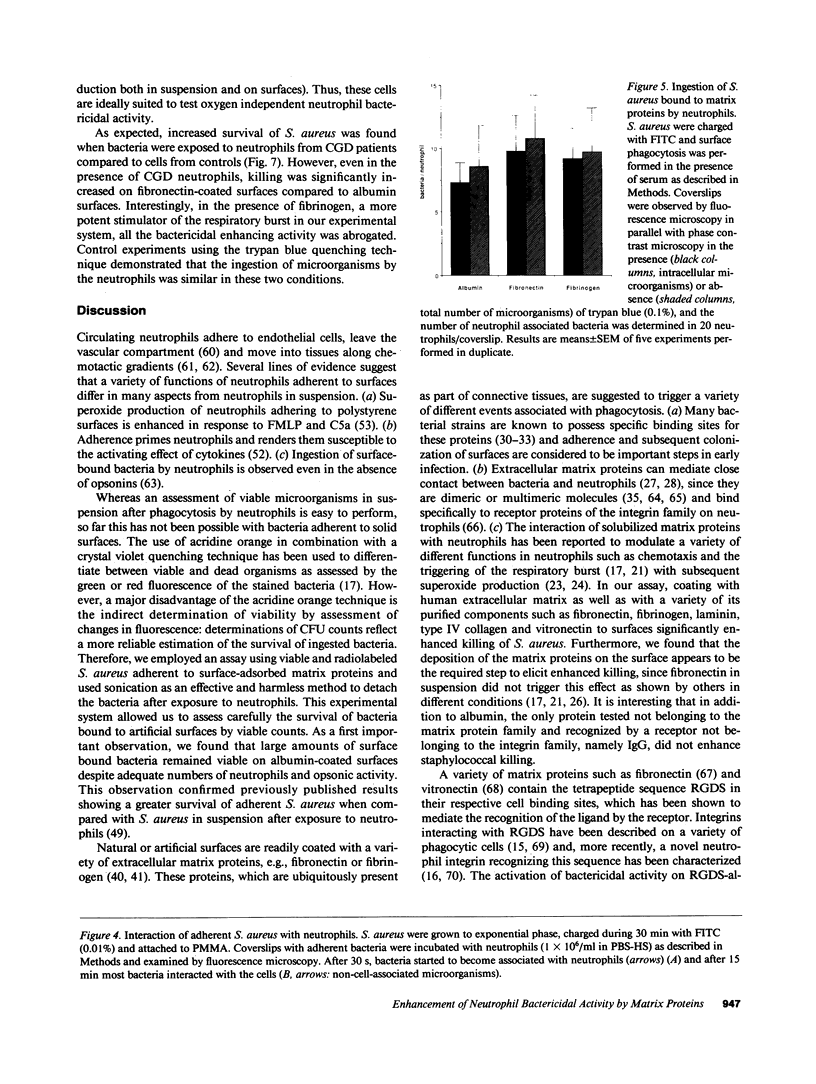
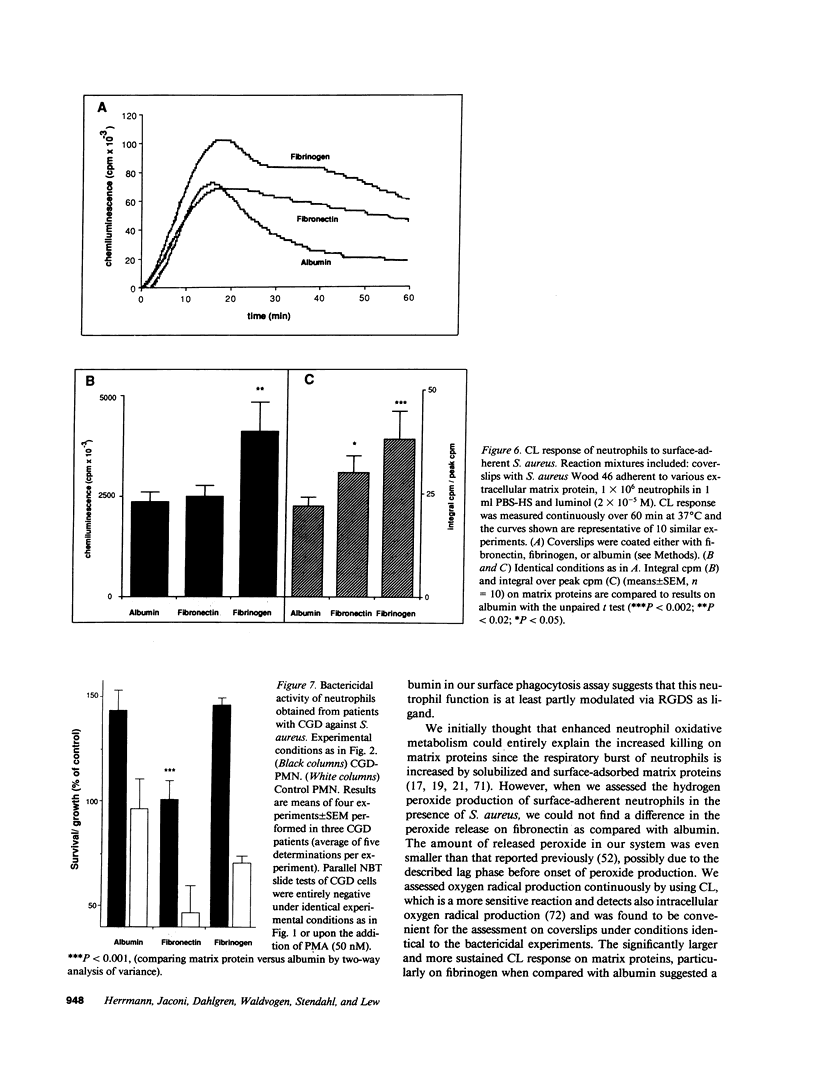



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldwin L., Nitecki D. E. A water-soluble, monitorable peptide and protein crosslinking agent. Anal Biochem. 1987 Aug 1;164(2):494–501. doi: 10.1016/0003-2697(87)90524-0. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Ruch W., Cooper P. H. Measurement of hydrogen peroxide production by phagocytes using homovanillic acid and horseradish peroxidase. Methods Enzymol. 1986;132:395–400. doi: 10.1016/s0076-6879(86)32024-x. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Differential control of azurophilic and specific granule exocytosis in Sendai-virus-permeabilized rabbit neutrophils. J Physiol. 1987 Feb;383:115–124. doi: 10.1113/jphysiol.1987.sp016399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briheim G., Coble B., Stendahl O., Dahlgren C. Exudate polymorphonuclear leukocytes isolated from skin chambers are primed for enhanced response to subsequent stimulation with chemoattractant f-Met-Leu-Phe and C3-opsonized yeast particles. Inflammation. 1988 Apr;12(2):141–152. doi: 10.1007/BF00916397. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Goodwin J. L. Fibronectin receptors of phagocytes. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J Exp Med. 1988 Mar 1;167(3):777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Preissner K. T., Müller-Berghaus G., Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987 Aug;55(8):1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Fuller R. A., Rosen J. J. Materials for medicine. Sci Am. 1986 Oct;255(4):118–125. doi: 10.1038/scientificamerican1086-118. [DOI] [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Gresham H. D., Goodwin J. L., Allen P. M., Anderson D. C., Brown E. J. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989 May;108(5):1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J., Hammond D. K., Timmons S., Budzynski A. Z. Interaction of human fibrinogen with staphylococci: presence of a binding region on normal and abnormal fibrinogen variants and fibrinogen derivatives. Blood. 1978 May;51(5):799–812. [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Horbett T. A., Cheng C. M., Ratner B. D., Hoffman A. S., Hanson S. R. The kinetics of baboon fibrinogen adsorption to polymers: in vitro and in vivo studies. J Biomed Mater Res. 1986 Jul-Aug;20(6):739–772. doi: 10.1002/jbm.820200608. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jaconi M. E., Rivest R. W., Schlegel W., Wollheim C. B., Pittet D., Lew P. D. Spontaneous and chemoattractant-induced oscillations of cytosolic free calcium in single adherent human neutrophils. J Biol Chem. 1988 Aug 5;263(22):10557–10560. [PubMed] [Google Scholar]
- Kishimoto T. K., Hollander N., Roberts T. M., Anderson D. C., Springer T. A. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987 Jul 17;50(2):193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Binding sites for streptococci and staphylococci in fibronectin. Infect Immun. 1984 Aug;45(2):433–436. doi: 10.1128/iai.45.2.433-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad B., Johansson A. 125I-fibrinogen uptake on peripheral venous cannulas: a comparison between different cannula materials and coatings. J Biomed Mater Res. 1987 Jan;21(1):99–105. doi: 10.1002/jbm.820210113. [DOI] [PubMed] [Google Scholar]
- Lindon J. N., McManama G., Kushner L., Merrill E. W., Salzman E. W. Does the conformation of adsorbed fibrinogen dictate platelet interactions with artificial surfaces? Blood. 1986 Aug;68(2):355–362. [PubMed] [Google Scholar]
- Lock R., Dahlgren C. Characteristics of the granulocyte chemiluminescence reaction following an interaction between human neutrophils and Salmonella typhimurium bacteria. APMIS. 1988 Apr;96(4):299–305. [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Monboisse J. C., Bellon G., Dufer J., Randoux A., Borel J. P. Collagen activates superoxide anion production by human polymorphonuclear neutrophils. Biochem J. 1987 Sep 15;246(3):599–603. doi: 10.1042/bj2460599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murabayashi S., Nosé Y. Biocompatibility: bioengineering aspects. Artif Organs. 1986 Apr;10(2):114–121. doi: 10.1111/j.1525-1594.1986.tb02529.x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Frame R. N., Elstad M. R. Vitronectin (S protein) augments the functional activity of monocyte receptors for IgG and complement C3b. Blood. 1988 Jan;71(1):86–93. [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D., Krause K. H., Wollheim C. B., Bruzzone R., Lew D. P. Nonselective inhibition of neutrophil functions by sphinganine. J Biol Chem. 1987 Jul 25;262(21):10072–10076. [PubMed] [Google Scholar]
- Preissner K. T., Wassmuth R., Müller-Berghaus G. Physicochemical characterization of human S-protein and its function in the blood coagulation system. Biochem J. 1985 Oct 15;231(2):349–355. doi: 10.1042/bj2310349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A. Fibronectin: an enhancer of phagocyte function. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S412–S419. doi: 10.1093/clinids/9.supplement_4.s412. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Prendergast E., Mosher D. F. Fibronectin mediates attachment of Staphylococcus aureus to human neutrophils. Blood. 1982 Apr;59(4):681–687. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Raynor R. H., Doran J. E., Reese A. C. A polymorphonuclear leukocyte monolayer system for studies of phagocytosis. J Reticuloendothel Soc. 1981 Jun;29(6):441–449. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Römer J., Morgenthaler J. J., Scherz R., Skvaril F. Characterization of various immunoglobulin preparations for intravenous application. I. Protein composition and antibody content. Vox Sang. 1982 Feb;42(2):62–73. doi: 10.1159/000460850. [DOI] [PubMed] [Google Scholar]
- Segal A. W. The molecular and cellular pathology of chronic granulomatous disease. Eur J Clin Invest. 1988 Oct;18(5):433–443. doi: 10.1111/j.1365-2362.1988.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Skogen W. F., Griffin G. L., Wilner G. D. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986 Mar;77(3):1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Coble B. I., Dahlgren C., Hed J., Molin L. Myeloperoxidase modulates the phagocytic activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J Clin Invest. 1984 Feb;73(2):366–373. doi: 10.1172/JCI111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Arai H., Languino L. R., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the vitronectin receptor alpha subunit and comparative expression of adhesion receptor mRNAs. J Biol Chem. 1987 Oct 15;262(29):14080–14085. [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Vaudaux P. E., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984 Sep;45(3):768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P. E., Zulian G., Huggler E., Waldvogel F. A. Attachment of Staphylococcus aureus to polymethylmethacrylate increases its resistance to phagocytosis in foreign body infection. Infect Immun. 1985 Nov;50(2):472–477. doi: 10.1128/iai.50.2.472-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P., Suzuki R., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984 Oct;150(4):546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Smith D. E., Nguyen B. Y., Hoidal J. R., Wilkinson B. J., Verhoef J., Furcht L. T. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981 Sep;33(3):811–819. doi: 10.1128/iai.33.3.811-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Fibronectin receptor of human macrophages recognizes the sequence Arg-Gly-Asp-Ser. J Exp Med. 1985 Aug 1;162(2):762–767. doi: 10.1084/jem.162.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Weitz J. I., Huang A. J., Levin S. M., Silverstein S. C., Loike J. D. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. D., Augustine N. H., Gonzalez L. A., Bohnsack J. F., Hill H. R. Effects of fibronectin on the interaction of polymorphonuclear leukocytes with unopsonized and antibody-opsonized bacteria. J Infect Dis. 1988 Oct;158(4):823–830. doi: 10.1093/infdis/158.4.823. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Sasaki T., Hashimoto H., Kashiba S. Opsonic effect of fibronectin on staphylococcal phagocytosis by human polymorphonuclear leukocytes: its relative inefficiency in post-phagocytic metabolic activities and in intracellular killing. Microbiol Immunol. 1988;32(8):795–805. doi: 10.1111/j.1348-0421.1988.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977 Nov;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]