Abstract
In the title compound, [Co(C2H2N3)(C7H4NO4)]n, the CoII atom is five-coordinated by three triazolate ligands and one bidentate 3-nitrobenzoate anion in a distorted trigonal-bipyramidal geometry. The triazolate ligand bridges the CoII atoms, generating a two-dimensional net parallel to the ab plane, in which both the CoII atom and the triazolate ligand act as three-connected nodes. Two weak intermolecular C—H⋯O hydrogen bonds connect the nets.
Related literature
For metal–triazole complexes, see: Park et al. (2006 ▶); Yang et al. (2008 ▶); Zhai et al. (2007 ▶). For Co—O and Co—N bond lengths, see: Zhang et al. (2008 ▶).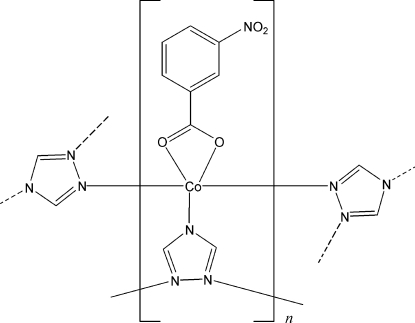
Experimental
Crystal data
[Co(C2H2N3)(C7H4NO4)]
M r = 293.11
Orthorhombic,

a = 9.2419 (18) Å
b = 10.377 (2) Å
c = 22.597 (5) Å
V = 2167.1 (8) Å3
Z = 8
Mo Kα radiation
μ = 1.60 mm−1
T = 296 (2) K
0.14 × 0.12 × 0.12 mm
Data collection
Bruker SMART 1K CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.802, T max = 0.826
19233 measured reflections
2477 independent reflections
2245 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.023
wR(F 2) = 0.059
S = 1.04
2477 reflections
163 parameters
H-atom parameters constrained
Δρmax = 0.33 e Å−3
Δρmin = −0.28 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808043596/is2370sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808043596/is2370Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Co1—O1 | 2.3314 (12) |
| Co1—O2 | 2.0008 (12) |
| Co1—N1 | 2.0232 (12) |
| Co1—N2i | 2.0118 (12) |
| Co1—N3ii | 2.0385 (12) |
Symmetry codes: (i)  ; (ii)
; (ii) 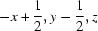 .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O2iii | 0.93 | 2.54 | 3.250 (3) | 134 |
| C8—H8⋯O4iv | 0.93 | 2.46 | 3.372 (2) | 169 |
Symmetry codes: (iii)  ; (iv)
; (iv) 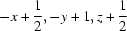 .
.
supplementary crystallographic information
Comment
Recently, more and more attention is paid on the coordination chemistry about trz ligand or analogy ligand (Park et al., 2006; Yang et al., 2008; Zhai et al., 2007), driven by their intriguing topological matrix and potential applications.
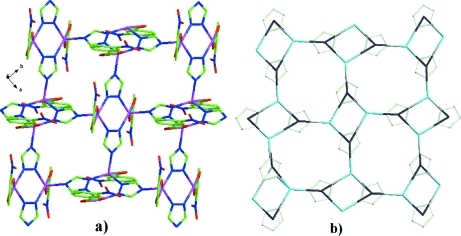
The asymmetric unit of I is shown in Fig. 1. The CoII atom is five-coordinated by two L (3-nitrobenzoate anion) O atoms, three trz N atoms to give rise to a distorted trigonal-bipyramidal geometry. The Co—O/N bond lengths of 2.0008 (12)–2.3314 (12)Å (Table 1) are in the normal range (Zhang et al., 2008). The trz and L ligand adopt bridging and bidentate coordinated modes, respectively. As shown in Fig. 2a, the CoII atoms are combined together by trz ligands to generate a two-dimensional net parallel to the ab plane with the L ligands ligated on the two-dimensional net up and down. From a topological point of view, if considering the trz ligands and cobalt ions as three-connected nodes. Moreover, besides the presence of two weak intermolecular C—H···O hydrogen bonds, see Table 2, there is not other obvious supramolecular interactions between two-dimensional nets,
Experimental
CoCl2 (1.0 mmol), 3-nitrobenzoic acid (1 mmol) and triazole (1 mmol) were dissolved in water (10 ml). The solution was heated in a 25 ml Teflonlined reaction vessel at 433 K for ca 3 days and then cooled to room temperature. Purple crystals of the title compound were obtained in a yield of 78%.
Refinement
All H atoms were positioned geometrically and refined using a riding model with C—H = 0.93 Å and with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
An ORTEP view of the asymmetric unit with 50% thermal ellipsoids for non-H atoms [symmetry codes: (A) -x + 1/2, y - 1/2, z; (B) x + 1/2, -y + 3/2, -z + 1].
Fig. 2.
a) View of the two-dimensional net onto the ab plane, formed by cobalt ions and trz ligands; b) View of the two-dimensional net built on three-connected trz and cobalt nodes.
Crystal data
| [Co(C2H2N3)(C7H4NO4)] | F(000) = 1176 |
| Mr = 293.11 | Dx = 1.797 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 15896 reflections |
| a = 9.2419 (18) Å | θ = 3.1–27.5° |
| b = 10.377 (2) Å | µ = 1.60 mm−1 |
| c = 22.597 (5) Å | T = 296 K |
| V = 2167.1 (8) Å3 | Block, purple |
| Z = 8 | 0.14 × 0.12 × 0.12 mm |
Data collection
| Bruker SMART 1K CCD area-detector diffractometer | 2477 independent reflections |
| Radiation source: sealed tube | 2245 reflections with I > 2σ(I) |
| graphite | Rint = 0.029 |
| Detector resolution: 8.192 pixels mm-1 | θmax = 27.5°, θmin = 3.1° |
| ω scans | h = −12→11 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | k = −13→12 |
| Tmin = 0.802, Tmax = 0.826 | l = −29→29 |
| 19233 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.023 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.059 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0278P)2 + 1.1998P] where P = (Fo2 + 2Fc2)/3 |
| 2477 reflections | (Δ/σ)max = 0.001 |
| 163 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.0918 (2) | 0.62096 (17) | 0.17212 (8) | 0.0358 (4) | |
| C2 | −0.0058 (2) | 0.7078 (2) | 0.14920 (8) | 0.0470 (5) | |
| H2 | −0.0198 | 0.7141 | 0.1085 | 0.056* | |
| C3 | −0.0823 (3) | 0.7853 (2) | 0.18746 (9) | 0.0522 (6) | |
| H3 | −0.1473 | 0.8457 | 0.1728 | 0.063* | |
| C4 | −0.0620 (2) | 0.77306 (18) | 0.24798 (8) | 0.0406 (4) | |
| H4 | −0.1157 | 0.8240 | 0.2738 | 0.049* | |
| C5 | 0.1165 (2) | 0.60861 (16) | 0.23241 (7) | 0.0322 (4) | |
| H5 | 0.1843 | 0.5503 | 0.2468 | 0.039* | |
| C6 | 0.03721 (19) | 0.68583 (16) | 0.27043 (7) | 0.0295 (3) | |
| C7 | 0.05354 (18) | 0.67429 (16) | 0.33614 (7) | 0.0290 (3) | |
| C8 | 0.32376 (16) | 0.75394 (14) | 0.51070 (7) | 0.0253 (3) | |
| H8 | 0.3425 | 0.6826 | 0.5344 | 0.030* | |
| C9 | 0.22994 (17) | 0.87843 (13) | 0.44808 (6) | 0.0231 (3) | |
| H9 | 0.1693 | 0.9118 | 0.4190 | 0.028* | |
| Co1 | 0.08538 (2) | 0.615646 (17) | 0.442261 (8) | 0.01829 (7) | |
| N1 | 0.21652 (13) | 0.75890 (11) | 0.47077 (5) | 0.0227 (2) | |
| N2 | 0.39953 (13) | 0.86141 (12) | 0.51260 (5) | 0.0225 (3) | |
| N3 | 0.33828 (13) | 0.94272 (11) | 0.47154 (5) | 0.0209 (2) | |
| N4 | 0.1706 (2) | 0.53740 (17) | 0.13114 (7) | 0.0459 (4) | |
| O1 | −0.00457 (13) | 0.75383 (12) | 0.36970 (5) | 0.0367 (3) | |
| O2 | 0.12664 (15) | 0.58069 (12) | 0.35684 (5) | 0.0351 (3) | |
| O3 | 0.2746 (2) | 0.47839 (18) | 0.14938 (7) | 0.0683 (5) | |
| O4 | 0.1257 (2) | 0.53020 (16) | 0.08006 (6) | 0.0627 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0517 (11) | 0.0329 (9) | 0.0228 (8) | −0.0083 (7) | 0.0032 (7) | −0.0016 (6) |
| C2 | 0.0745 (14) | 0.0453 (11) | 0.0211 (8) | −0.0027 (10) | −0.0091 (8) | 0.0050 (8) |
| C3 | 0.0778 (16) | 0.0444 (12) | 0.0345 (10) | 0.0148 (10) | −0.0150 (9) | 0.0058 (9) |
| C4 | 0.0592 (11) | 0.0331 (9) | 0.0295 (9) | 0.0079 (8) | −0.0040 (8) | 0.0000 (7) |
| C5 | 0.0422 (9) | 0.0299 (8) | 0.0246 (8) | −0.0020 (7) | −0.0011 (7) | 0.0013 (6) |
| C6 | 0.0418 (9) | 0.0261 (8) | 0.0208 (7) | −0.0037 (7) | −0.0026 (6) | 0.0022 (6) |
| C7 | 0.0371 (8) | 0.0284 (8) | 0.0214 (7) | −0.0058 (6) | −0.0020 (6) | 0.0014 (6) |
| C8 | 0.0290 (7) | 0.0196 (7) | 0.0274 (7) | −0.0033 (6) | −0.0042 (6) | 0.0061 (6) |
| C9 | 0.0269 (7) | 0.0190 (7) | 0.0234 (7) | −0.0015 (5) | −0.0031 (5) | 0.0034 (5) |
| Co1 | 0.02203 (12) | 0.01445 (11) | 0.01838 (11) | −0.00039 (6) | 0.00086 (7) | 0.00145 (7) |
| N1 | 0.0263 (6) | 0.0181 (6) | 0.0238 (6) | −0.0032 (5) | −0.0026 (5) | 0.0025 (5) |
| N2 | 0.0247 (6) | 0.0185 (6) | 0.0243 (6) | −0.0015 (5) | −0.0038 (5) | 0.0050 (5) |
| N3 | 0.0246 (6) | 0.0163 (6) | 0.0217 (6) | −0.0005 (4) | −0.0008 (4) | 0.0039 (5) |
| N4 | 0.0611 (11) | 0.0437 (9) | 0.0330 (8) | −0.0104 (8) | 0.0139 (7) | −0.0058 (7) |
| O1 | 0.0453 (7) | 0.0413 (7) | 0.0234 (6) | 0.0066 (6) | −0.0001 (5) | −0.0033 (5) |
| O2 | 0.0541 (7) | 0.0304 (6) | 0.0207 (5) | 0.0052 (6) | −0.0027 (5) | 0.0020 (5) |
| O3 | 0.0667 (11) | 0.0791 (12) | 0.0590 (10) | 0.0140 (10) | 0.0135 (8) | −0.0168 (9) |
| O4 | 0.1046 (13) | 0.0585 (10) | 0.0249 (7) | −0.0100 (9) | 0.0117 (8) | −0.0095 (7) |
Geometric parameters (Å, °)
| C1—C2 | 1.376 (3) | C8—N2 | 1.3175 (19) |
| C1—C5 | 1.388 (2) | C8—N1 | 1.3414 (19) |
| C1—N4 | 1.463 (2) | C8—H8 | 0.9300 |
| C2—C3 | 1.376 (3) | C9—N3 | 1.3149 (19) |
| C2—H2 | 0.9300 | C9—N1 | 1.3478 (18) |
| C3—C4 | 1.386 (3) | C9—H9 | 0.9300 |
| C3—H3 | 0.9300 | Co1—O1 | 2.3314 (12) |
| C4—C6 | 1.385 (2) | Co1—O2 | 2.0008 (12) |
| C4—H4 | 0.9300 | Co1—N1 | 2.0232 (12) |
| C5—C6 | 1.385 (2) | Co1—N2i | 2.0118 (12) |
| C5—H5 | 0.9300 | Co1—N3ii | 2.0385 (12) |
| C6—C7 | 1.497 (2) | N2—N3 | 1.3759 (16) |
| C7—O1 | 1.243 (2) | N4—O3 | 1.212 (2) |
| C7—O2 | 1.272 (2) | N4—O4 | 1.229 (2) |
| C2—C1—C5 | 122.56 (17) | N1—C9—H9 | 123.7 |
| C2—C1—N4 | 118.45 (17) | O2—Co1—N2i | 132.33 (5) |
| C5—C1—N4 | 118.98 (17) | O2—Co1—N1 | 109.04 (5) |
| C1—C2—C3 | 118.87 (17) | N2i—Co1—N1 | 105.25 (5) |
| C1—C2—H2 | 120.6 | O2—Co1—N3ii | 95.03 (5) |
| C3—C2—H2 | 120.6 | N2i—Co1—N3ii | 103.60 (5) |
| C2—C3—C4 | 119.79 (19) | N1—Co1—N3ii | 109.64 (5) |
| C2—C3—H3 | 120.1 | O2—Co1—O1 | 60.06 (5) |
| C4—C3—H3 | 120.1 | N2i—Co1—O1 | 88.82 (5) |
| C6—C4—C3 | 120.72 (18) | N1—Co1—O1 | 89.18 (5) |
| C6—C4—H4 | 119.6 | N3ii—Co1—O1 | 153.26 (5) |
| C3—C4—H4 | 119.6 | C8—N1—C9 | 102.90 (12) |
| C6—C5—C1 | 117.93 (16) | C8—N1—Co1 | 128.95 (10) |
| C6—C5—H5 | 121.0 | C9—N1—Co1 | 127.60 (10) |
| C1—C5—H5 | 121.0 | C8—N2—N3 | 106.17 (12) |
| C4—C6—C5 | 120.10 (15) | C8—N2—Co1iii | 124.78 (10) |
| C4—C6—C7 | 118.83 (15) | N3—N2—Co1iii | 128.36 (9) |
| C5—C6—C7 | 121.04 (15) | C9—N3—N2 | 105.91 (11) |
| O1—C7—O2 | 120.80 (14) | C9—N3—Co1iv | 125.40 (10) |
| O1—C7—C6 | 120.56 (15) | N2—N3—Co1iv | 128.05 (9) |
| O2—C7—C6 | 118.63 (15) | O3—N4—O4 | 123.81 (19) |
| N2—C8—N1 | 112.47 (13) | O3—N4—C1 | 118.64 (17) |
| N2—C8—H8 | 123.8 | O4—N4—C1 | 117.55 (19) |
| N1—C8—H8 | 123.8 | C7—O1—Co1 | 82.34 (10) |
| N3—C9—N1 | 112.56 (13) | C7—O2—Co1 | 96.61 (10) |
| N3—C9—H9 | 123.7 | ||
| C5—C1—C2—C3 | −0.2 (3) | C7—Co1—N1—C9 | 25.56 (14) |
| N4—C1—C2—C3 | 178.90 (19) | N1—C8—N2—N3 | 0.24 (17) |
| C1—C2—C3—C4 | −1.2 (3) | N1—C8—N2—Co1iii | −170.88 (10) |
| C2—C3—C4—C6 | 1.7 (3) | N1—C9—N3—N2 | −0.56 (17) |
| C2—C1—C5—C6 | 1.2 (3) | N1—C9—N3—Co1iv | 170.84 (10) |
| N4—C1—C5—C6 | −177.94 (16) | C8—N2—N3—C9 | 0.19 (16) |
| C3—C4—C6—C5 | −0.7 (3) | Co1iii—N2—N3—C9 | 170.89 (10) |
| C3—C4—C6—C7 | −178.94 (19) | C8—N2—N3—Co1iv | −170.91 (10) |
| C1—C5—C6—C4 | −0.7 (3) | Co1iii—N2—N3—Co1iv | −0.21 (18) |
| C1—C5—C6—C7 | 177.51 (16) | C2—C1—N4—O3 | 166.47 (19) |
| C4—C6—C7—O1 | −10.4 (2) | C5—C1—N4—O3 | −14.4 (3) |
| C5—C6—C7—O1 | 171.35 (16) | C2—C1—N4—O4 | −14.4 (3) |
| C4—C6—C7—O2 | 168.80 (16) | C5—C1—N4—O4 | 164.78 (17) |
| C5—C6—C7—O2 | −9.4 (2) | O2—C7—O1—Co1 | −4.03 (15) |
| N2—C8—N1—C9 | −0.55 (17) | C6—C7—O1—Co1 | 175.16 (15) |
| N2—C8—N1—Co1 | 171.29 (10) | O2—Co1—O1—C7 | 2.54 (10) |
| N3—C9—N1—C8 | 0.68 (17) | N2i—Co1—O1—C7 | −139.56 (10) |
| N3—C9—N1—Co1 | −171.31 (10) | N1—Co1—O1—C7 | 115.17 (10) |
| O2—Co1—N1—C8 | −113.76 (13) | N3ii—Co1—O1—C7 | −20.77 (16) |
| N2i—Co1—N1—C8 | 99.90 (13) | O1—C7—O2—Co1 | 4.68 (18) |
| N3ii—Co1—N1—C8 | −10.96 (14) | C6—C7—O2—Co1 | −174.52 (13) |
| O1—Co1—N1—C8 | −171.55 (13) | N2i—Co1—O2—C7 | 53.70 (13) |
| O2—Co1—N1—C9 | 56.19 (14) | N1—Co1—O2—C7 | −79.98 (11) |
| N2i—Co1—N1—C9 | −90.15 (13) | N3ii—Co1—O2—C7 | 167.23 (10) |
| N3ii—Co1—N1—C9 | 158.99 (12) | O1—Co1—O2—C7 | −2.47 (9) |
| O1—Co1—N1—C9 | −1.60 (13) |
Symmetry codes: (i) x−1/2, −y+3/2, −z+1; (ii) −x+1/2, y−1/2, z; (iii) x+1/2, −y+3/2, −z+1; (iv) −x+1/2, y+1/2, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O2v | 0.93 | 2.54 | 3.250 (3) | 134 |
| C8—H8···O4vi | 0.93 | 2.46 | 3.372 (2) | 169 |
Symmetry codes: (v) −x, y+1/2, −z+1/2; (vi) −x+1/2, −y+1, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2370).
References
- Bruker (2001). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Park, H., Moureau, D. M. & Parise, J. B. (2006). Chem. Mater.18, 525–531.
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Yang, E.-C., Liu, Z.-Y., Wang, X.-G., Batten, S. R. & Zhao, X.-J. (2008). CrystEngComm, 10, 1140–1143.
- Zhai, Q.-G., Lu, C.-Z., Wu, X.-Y. & Batten, S. R. (2007). Cryst. Growth Des.7, 2332–2342.
- Zhang, J., Chew, E., Chen, S.-M., Pham, J. T. H. & Bu, X.-H. (2008). Inorg. Chem.47, 3495–3497. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808043596/is2370sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808043596/is2370Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report