Abstract
The title compound, 2C21H34N4O3S2+·4I−·5H2O, was prepared exclusively as the E isomer by methylation of the corresponding N-phenylpyridin-4-amine. There are two symmetry-independent molecules in the asymmetric unit with no significant differences in bond lengths and angles. The aromatic rings are not coplanar with the pyridin-4-imine groups, as indicated by the C—N—C—C torsion angles of 47.7 (7) and 132.6 (5)°.
Related literature
For background information see: Bjorkman & Bhattarai (2005 ▶); Yeates et al. (2008 ▶). For related literature structures, see: Lopes et al. (2004 ▶); Wang et al. (2008 ▶); Djedouani et al. (2008 ▶).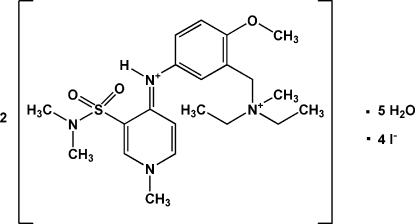
Experimental
Crystal data
2C21H34N4O3S2+·4I−·5H2O
M r = 1442.86
Triclinic,
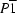
a = 12.7930 (5) Å
b = 13.5539 (6) Å
c = 16.8386 (7) Å
α = 96.670 (2)°
β = 97.667 (2)°
γ = 98.224 (1)°
V = 2836.5 (2) Å3
Z = 2
Mo Kα radiation
μ = 2.33 mm−1
T = 100 (2) K
0.35 × 0.2 × 0.08 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.575, T max = 0.830
49577 measured reflections
11468 independent reflections
8753 reflections with I > 2σ(I)
R int = 0.050
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.116
S = 1.00
11468 reflections
654 parameters
17 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 2.07 e Å−3
Δρmin = −1.42 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809000324/bg2227sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809000324/bg2227Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1W—H1WA⋯I4 | 0.91 (3) | 2.60 (4) | 3.489 (4) | 170 (3) |
| O1W—H1WB⋯I2 | 0.90 (2) | 2.663 (17) | 3.561 (4) | 176 (4) |
| O2W—H2WA⋯O5wi | 0.90 (4) | 1.98 (5) | 2.799 (7) | 147 (5) |
| O2W—H2WB⋯I3 | 0.90 (4) | 2.69 (4) | 3.580 (5) | 175 (4) |
| O3W—H3WA⋯O1Wi | 0.90 (4) | 1.91 (4) | 2.781 (6) | 161 (18) |
| O3W—H3WB⋯I1 | 0.90 (4) | 2.64 (4) | 3.544 (4) | 178 (5) |
| O4W—H4WA⋯I4 | 0.90 (3) | 2.75 (3) | 3.613 (5) | 160 (4) |
| O4W—H4WB⋯O3Wii | 0.89 (5) | 1.92 (4) | 2.795 (7) | 162 (2) |
| O5W—H5WA⋯I2 | 0.90 (4) | 2.75 (5) | 3.614 (5) | 165 (4) |
| O5W—H5WB⋯O4wiii | 0.90 (4) | 1.94 (4) | 2.809 (7) | 161 (3) |
| N14—H14⋯O9 | 0.90 (3) | 1.93 (4) | 2.733 (5) | 149 (4) |
| N54—H54⋯O49 | 0.90 (5) | 2.08 (4) | 2.767 (5) | 133 (4) |
Symmetry codes: (i) 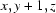 ; (ii)
; (ii) 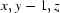 ; (iii)
; (iii) 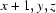 .
.
Acknowledgments
Intensity measurements were performed at the Unidade de Raios X, RIAIDT, University of Santiago de Compostela, SPAIN. This work was supported by Fundação para a Ciência e Tecnologia (FCT, Portugal); TR acknowledges the FCT for the PhD grant SFRH/BD/30689/2006.
supplementary crystallographic information
Comment
Malaria is accounted as one of the major diseases worldwide, and for which few efficient drugs are known today [Bjorkman and Bhattarai, 2005]. 4(1H)-Pyridones are currently being developed as important and potential antimalarial agents, capable of inhibiting the bc1 complex, at the oxidation site (Qo site) level in the Plasmodium falciparum mitochondrion [Yeates et al., 2008]. As part of our project towards the synthesis of 4(1H)-pyridone bioisosteric scaffolds, the (1H-pyridin-4-ylidene)amine scaffold was studied.
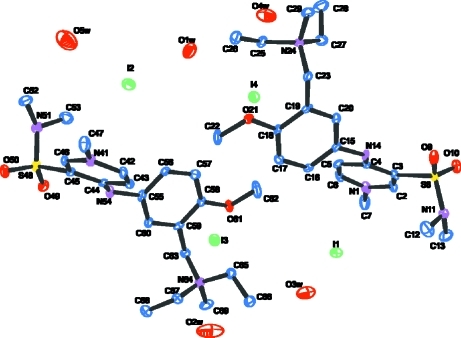
The title compound was prepared by reaction of the corresponding N-phenylpyridin-4-amine with methyl iodide. Interestingly, only the E isomer of the compound was obtained, as it was previously observed for amodiaquine analogues [Lopes et al., 2004]. There are two symmetry-independent molecules in the asymmetric unit with no significant differences in bond lengths and angles. The observed imine bond distances C4—N14 and C44—N54 are longer than the expected by ca 0.035 Å [Wang et al., 2008 and Djedouani et al., 2008], a consequence of the imine group being protonated. The aromatic rings are not coplanar relatively to the pyridin-4-imine moieties, as indicated by the C4—N14—C15—C16 and C44—N54—C55—C56 dihedral angles of 47.7 (7)° and 132.6 (5)°, respectively. The molecules are hydrogen-bonded through the imine nitrogen atoms at N14 and N54, acting as donors towards the sulfonyl oxygen atoms O9 and O19 of each sulfonamide moiety, respectively. The (1H-pyridin-4-ylidene)amine scaffold is nearly planar and the C5—C4—N14—C15 dihedral angle is 7.9 (7)° for one of the molecules, whereas the C43—C44—N54—C55 dihedral angle on the other molecule is -14.1 (7)°.
Experimental
The title compound was prepared at room temperature by reacting 2-[(diethylamino)methyl]-4-(pyridin-4-ylamino)phenol with methyl iodide in the presence of NaH in DMF. Crystals were grown from water.
Refinement
The hydroxy H atoms for the water solvent molecules were initially located in a difference Fourier map, but their distances were constrained with DFIX at 0.9 Å from the O atom and with DANG at 2.5 Å from the other H water atom. The hydrogen atoms linked to the charged N14 and N54 atoms were located in a difference Fourier map, but the distances N—H were constrained at 0.9 Å, in order to get the refinement stabilization. The rest of the H atoms were positioned geometrically and included as riding atoms with C—H = 0.95 or 0.98 Å and Uiso(H)= 1.2 or 1.5 times Ueq(C).
Figures
Fig. 1.
An ORTEPIII (Farrugia, 1997) view of the molecular structure of the title compound, showing the labelling of all non-H atoms. Displacement ellipsoids for non-H atoms are shown at the 50% probability level. H atoms have been omitted for clarity.
Crystal data
| 2C21H34N4O3S2+·4I−·5H2O | Z = 2 |
| Mr = 1442.86 | F(000) = 1436.0 |
| Triclinic, P1 | Dx = 1.687 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.7107 Å |
| a = 12.7930 (5) Å | Cell parameters from 9916 reflections |
| b = 13.5539 (6) Å | θ = 2.4–25.8° |
| c = 16.8386 (7) Å | µ = 2.33 mm−1 |
| α = 96.670 (2)° | T = 100 K |
| β = 97.667 (2)° | Prism, colourless |
| γ = 98.224 (1)° | 0.35 × 0.2 × 0.08 mm |
| V = 2836.5 (2) Å3 |
Data collection
| Bruker APEXII CCD diffractometer | 8753 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.050 |
| ω and φ scans | θmax = 26.4°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | h = −15→15 |
| Tmin = 0.575, Tmax = 0.830 | k = −16→16 |
| 49577 measured reflections | l = 0→21 |
| 11468 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.043 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.116 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0655P)2] where P = (Fo2 + 2Fc2)/3 |
| 11468 reflections | (Δ/σ)max = 0.002 |
| 654 parameters | Δρmax = 2.07 e Å−3 |
| 17 restraints | Δρmin = −1.42 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1W | 0.4474 (3) | 0.1184 (3) | 0.6544 (2) | 0.0347 (10) | |
| H1WA | 0.414 (3) | 0.172 (2) | 0.665 (3) | 0.042* | |
| H1WB | 0.5190 (9) | 0.135 (3) | 0.665 (3) | 0.042* | |
| O2W | 0.8769 (5) | 0.9278 (3) | 0.8610 (3) | 0.0587 (14) | |
| H2WA | 0.913 (5) | 0.940 (4) | 0.820 (3) | 0.070* | |
| H2WB | 0.836 (5) | 0.867 (2) | 0.852 (3) | 0.070* | |
| O3W | 0.2931 (4) | 0.9651 (3) | 0.6823 (2) | 0.0428 (11) | |
| H3WA | 0.352 (3) | 1.010 (3) | 0.681 (3) | 0.051* | |
| H3WB | 0.299 (4) | 0.931 (4) | 0.725 (2) | 0.051* | |
| O4W | 0.1157 (4) | 0.0625 (4) | 0.6565 (3) | 0.0485 (12) | |
| H4WA | 0.145 (4) | 0.1267 (15) | 0.655 (4) | 0.058* | |
| H4WB | 0.166 (3) | 0.026 (3) | 0.674 (4) | 0.058* | |
| O5W | 0.9315 (4) | 0.0196 (4) | 0.7284 (3) | 0.0555 (13) | |
| H5WA | 0.892 (4) | 0.065 (4) | 0.713 (4) | 0.067* | |
| H5WB | 0.989 (3) | 0.018 (4) | 0.703 (3) | 0.067* | |
| I1 | 0.32351 (3) | 0.83284 (3) | 0.85134 (2) | 0.02456 (10) | |
| I2 | 0.73146 (3) | 0.17740 (3) | 0.68619 (2) | 0.02986 (11) | |
| I3 | 0.71949 (3) | 0.68226 (3) | 0.811724 (17) | 0.01851 (10) | |
| I4 | 0.28842 (3) | 0.30343 (3) | 0.687434 (18) | 0.02097 (10) | |
| N1 | 0.0320 (3) | 0.6774 (3) | 0.7703 (2) | 0.0179 (9) | |
| C2 | 0.0149 (4) | 0.6876 (4) | 0.8477 (2) | 0.0171 (11) | |
| H2 | −0.0368 | 0.7251 | 0.8623 | 0.021* | |
| C3 | 0.0710 (4) | 0.6446 (4) | 0.9058 (2) | 0.0147 (10) | |
| C4 | 0.1531 (4) | 0.5890 (3) | 0.8865 (3) | 0.0136 (10) | |
| C5 | 0.1607 (4) | 0.5714 (4) | 0.8024 (3) | 0.0155 (10) | |
| H5 | 0.2057 | 0.5282 | 0.7846 | 0.019* | |
| C6 | 0.1033 (4) | 0.6167 (4) | 0.7484 (2) | 0.0185 (11) | |
| H6 | 0.1126 | 0.6064 | 0.6943 | 0.022* | |
| C7 | −0.0207 (4) | 0.7323 (4) | 0.7111 (3) | 0.0278 (13) | |
| H7A | −0.0628 | 0.7754 | 0.7374 | 0.042* | |
| H7B | −0.0662 | 0.6853 | 0.6693 | 0.042* | |
| H7C | 0.0323 | 0.7722 | 0.6878 | 0.042* | |
| S8 | 0.03896 (9) | 0.67067 (9) | 1.00503 (6) | 0.0135 (3) | |
| O9 | 0.0913 (3) | 0.6067 (3) | 1.05430 (18) | 0.0209 (8) | |
| O10 | −0.0746 (3) | 0.6639 (3) | 0.99700 (18) | 0.0204 (8) | |
| N11 | 0.0902 (3) | 0.7864 (3) | 1.0408 (2) | 0.0175 (9) | |
| C12 | 0.2061 (4) | 0.8092 (5) | 1.0648 (3) | 0.0321 (14) | |
| H12A | 0.2401 | 0.8128 | 1.0175 | 0.048* | |
| H12B | 0.2299 | 0.7573 | 1.0934 | 0.048* | |
| H12C | 0.2245 | 0.8726 | 1.0993 | 0.048* | |
| C13 | 0.0461 (5) | 0.8645 (4) | 0.9992 (3) | 0.0293 (13) | |
| H13A | 0.0769 | 0.8710 | 0.9508 | 0.044* | |
| H13B | 0.0625 | 0.9276 | 1.0343 | 0.044* | |
| H13C | −0.0301 | 0.8460 | 0.9855 | 0.044* | |
| N14 | 0.2167 (3) | 0.5534 (3) | 0.9424 (2) | 0.0146 (9) | |
| H14 | 0.194 (4) | 0.561 (3) | 0.9901 (14) | 0.017* | |
| C15 | 0.3067 (4) | 0.5053 (3) | 0.9282 (2) | 0.0134 (10) | |
| C16 | 0.3822 (4) | 0.5466 (3) | 0.8849 (2) | 0.0116 (10) | |
| H16 | 0.3753 | 0.6065 | 0.8643 | 0.014* | |
| C17 | 0.4682 (4) | 0.4985 (4) | 0.8721 (2) | 0.0141 (10) | |
| H17 | 0.5191 | 0.5262 | 0.8429 | 0.017* | |
| C18 | 0.4789 (4) | 0.4094 (4) | 0.9026 (2) | 0.0134 (10) | |
| C19 | 0.4046 (4) | 0.3688 (3) | 0.9500 (2) | 0.0131 (10) | |
| C20 | 0.3190 (4) | 0.4191 (4) | 0.9622 (2) | 0.0135 (10) | |
| H20 | 0.2696 | 0.3941 | 0.9936 | 0.016* | |
| O21 | 0.5609 (3) | 0.3568 (2) | 0.89193 (18) | 0.0156 (7) | |
| C22 | 0.6274 (4) | 0.3884 (4) | 0.8348 (3) | 0.0284 (13) | |
| H22A | 0.5836 | 0.3918 | 0.7845 | 0.043* | |
| H22B | 0.6751 | 0.3410 | 0.8261 | 0.043* | |
| H22C | 0.6681 | 0.4536 | 0.8553 | 0.043* | |
| C23 | 0.4197 (4) | 0.2776 (3) | 0.9912 (2) | 0.0140 (10) | |
| H23A | 0.3960 | 0.2871 | 1.0436 | 0.017* | |
| H23B | 0.4956 | 0.2745 | 1.0010 | 0.017* | |
| N24 | 0.3617 (3) | 0.1764 (3) | 0.9456 (2) | 0.0142 (9) | |
| C25 | 0.4097 (4) | 0.1478 (4) | 0.8698 (3) | 0.0172 (11) | |
| H25A | 0.3627 | 0.0909 | 0.8369 | 0.021* | |
| H25B | 0.4130 | 0.2037 | 0.8386 | 0.021* | |
| C26 | 0.5201 (4) | 0.1207 (4) | 0.8870 (3) | 0.0238 (12) | |
| H26A | 0.5641 | 0.1716 | 0.9267 | 0.036* | |
| H26B | 0.5511 | 0.1162 | 0.8380 | 0.036* | |
| H26C | 0.5154 | 0.0570 | 0.9071 | 0.036* | |
| C27 | 0.2446 (4) | 0.1837 (4) | 0.9202 (3) | 0.0199 (11) | |
| H27A | 0.2177 | 0.2154 | 0.9663 | 0.024* | |
| H27B | 0.2399 | 0.2271 | 0.8784 | 0.024* | |
| C28 | 0.1728 (4) | 0.0841 (4) | 0.8887 (3) | 0.0295 (13) | |
| H28A | 0.2054 | 0.0462 | 0.8499 | 0.044* | |
| H28B | 0.1049 | 0.0963 | 0.8635 | 0.044* | |
| H28C | 0.1626 | 0.0468 | 0.9329 | 0.044* | |
| C29 | 0.3720 (4) | 0.1004 (4) | 1.0034 (3) | 0.0193 (11) | |
| H29A | 0.3495 | 0.0338 | 0.9745 | 0.029* | |
| H29B | 0.3280 | 0.1116 | 1.0445 | 0.029* | |
| H29C | 0.4452 | 0.1073 | 1.0283 | 0.029* | |
| N41 | 0.9817 (3) | 0.3632 (3) | 0.7359 (2) | 0.0143 (9) | |
| C42 | 0.9073 (4) | 0.4212 (4) | 0.7574 (2) | 0.0150 (11) | |
| H42 | 0.8988 | 0.4330 | 0.8115 | 0.018* | |
| C43 | 0.8464 (4) | 0.4612 (4) | 0.7019 (3) | 0.0140 (10) | |
| H43 | 0.7975 | 0.5008 | 0.7185 | 0.017* | |
| C44 | 0.8558 (4) | 0.4437 (3) | 0.6181 (2) | 0.0118 (10) | |
| C45 | 0.9372 (4) | 0.3870 (3) | 0.5991 (2) | 0.0113 (10) | |
| C46 | 0.9962 (4) | 0.3490 (3) | 0.6584 (3) | 0.0135 (10) | |
| H46 | 1.0484 | 0.3119 | 0.6445 | 0.016* | |
| C47 | 1.0393 (4) | 0.3134 (4) | 0.7974 (3) | 0.0226 (12) | |
| H47A | 0.9904 | 0.2617 | 0.8136 | 0.034* | |
| H47B | 1.0703 | 0.3621 | 0.8436 | 0.034* | |
| H47C | 1.0948 | 0.2840 | 0.7751 | 0.034* | |
| S48 | 0.95911 (10) | 0.34819 (9) | 0.49898 (6) | 0.0144 (3) | |
| O49 | 0.9141 (3) | 0.4153 (2) | 0.44787 (17) | 0.0172 (7) | |
| O50 | 1.0700 (3) | 0.3398 (3) | 0.50195 (18) | 0.0179 (8) | |
| N51 | 0.8905 (3) | 0.2370 (3) | 0.4701 (2) | 0.0191 (9) | |
| C52 | 0.9303 (5) | 0.1519 (4) | 0.5057 (3) | 0.0262 (13) | |
| H52A | 0.9004 | 0.1429 | 0.5542 | 0.039* | |
| H52B | 1.0068 | 0.1660 | 0.5182 | 0.039* | |
| H52C | 0.9093 | 0.0915 | 0.4676 | 0.039* | |
| C53 | 0.7730 (4) | 0.2294 (4) | 0.4602 (3) | 0.0265 (13) | |
| H53A | 0.7516 | 0.2823 | 0.4316 | 0.040* | |
| H53B | 0.7505 | 0.2356 | 0.5125 | 0.040* | |
| H53C | 0.7405 | 0.1653 | 0.4301 | 0.040* | |
| N54 | 0.7923 (3) | 0.4792 (3) | 0.5618 (2) | 0.0145 (9) | |
| H54 | 0.813 (4) | 0.486 (3) | 0.5136 (14) | 0.017* | |
| C55 | 0.6969 (4) | 0.5173 (4) | 0.5752 (2) | 0.0142 (10) | |
| C56 | 0.6208 (4) | 0.4609 (4) | 0.6102 (2) | 0.0129 (10) | |
| H56 | 0.6335 | 0.3998 | 0.6263 | 0.015* | |
| C57 | 0.5259 (4) | 0.4951 (4) | 0.6215 (2) | 0.0156 (10) | |
| H57 | 0.4751 | 0.4571 | 0.6451 | 0.019* | |
| C58 | 0.5075 (4) | 0.5860 (4) | 0.5973 (2) | 0.0123 (10) | |
| C59 | 0.5836 (4) | 0.6442 (3) | 0.5615 (2) | 0.0120 (10) | |
| C60 | 0.6781 (4) | 0.6071 (4) | 0.5503 (2) | 0.0148 (10) | |
| H60 | 0.7287 | 0.6438 | 0.5257 | 0.018* | |
| O61 | 0.4154 (3) | 0.6249 (3) | 0.60549 (18) | 0.0172 (8) | |
| C62 | 0.3569 (5) | 0.5914 (5) | 0.6669 (3) | 0.0381 (16) | |
| H62A | 0.3151 | 0.5264 | 0.6473 | 0.057* | |
| H62B | 0.3105 | 0.6385 | 0.6803 | 0.057* | |
| H62C | 0.4060 | 0.5869 | 0.7143 | 0.057* | |
| C63 | 0.5599 (4) | 0.7401 (3) | 0.5315 (2) | 0.0154 (10) | |
| H63A | 0.4832 | 0.7341 | 0.5159 | 0.019* | |
| H63B | 0.5925 | 0.7473 | 0.4833 | 0.019* | |
| N64 | 0.5981 (3) | 0.8360 (3) | 0.5915 (2) | 0.0162 (9) | |
| C65 | 0.5493 (4) | 0.8250 (4) | 0.6690 (3) | 0.0182 (11) | |
| H65A | 0.4721 | 0.8108 | 0.6546 | 0.022* | |
| H65B | 0.5718 | 0.7672 | 0.6912 | 0.022* | |
| C66 | 0.5774 (4) | 0.9148 (4) | 0.7346 (3) | 0.0221 (12) | |
| H66A | 0.6522 | 0.9236 | 0.7556 | 0.033* | |
| H66B | 0.5363 | 0.9037 | 0.7774 | 0.033* | |
| H66C | 0.5615 | 0.9740 | 0.7124 | 0.033* | |
| C67 | 0.7185 (4) | 0.8567 (4) | 0.6140 (3) | 0.0192 (11) | |
| H67A | 0.7416 | 0.7993 | 0.6366 | 0.023* | |
| H67B | 0.7377 | 0.9144 | 0.6559 | 0.023* | |
| C68 | 0.7793 (4) | 0.8771 (4) | 0.5438 (3) | 0.0298 (14) | |
| H68A | 0.7636 | 0.8192 | 0.5030 | 0.045* | |
| H68B | 0.8547 | 0.8907 | 0.5632 | 0.045* | |
| H68C | 0.7578 | 0.9342 | 0.5212 | 0.045* | |
| C69 | 0.5592 (4) | 0.9205 (4) | 0.5531 (3) | 0.0244 (12) | |
| H69A | 0.5949 | 0.9835 | 0.5833 | 0.037* | |
| H69B | 0.4835 | 0.9158 | 0.5528 | 0.037* | |
| H69C | 0.5742 | 0.9167 | 0.4986 | 0.037* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1W | 0.039 (3) | 0.033 (3) | 0.037 (2) | 0.018 (2) | 0.011 (2) | 0.0050 (19) |
| O2W | 0.083 (4) | 0.032 (3) | 0.046 (3) | −0.016 (3) | −0.019 (3) | 0.008 (2) |
| O3W | 0.059 (3) | 0.027 (3) | 0.037 (2) | 0.000 (2) | −0.003 (2) | 0.0035 (19) |
| O4W | 0.044 (3) | 0.034 (3) | 0.061 (3) | −0.010 (2) | 0.001 (2) | 0.008 (2) |
| O5W | 0.034 (3) | 0.063 (4) | 0.072 (3) | 0.003 (2) | 0.006 (2) | 0.028 (3) |
| I1 | 0.0291 (2) | 0.0148 (2) | 0.03152 (18) | 0.00230 (15) | 0.01180 (15) | 0.00385 (14) |
| I2 | 0.0374 (2) | 0.0207 (2) | 0.03183 (19) | −0.00250 (17) | 0.01728 (17) | 0.00016 (15) |
| I3 | 0.02080 (19) | 0.0211 (2) | 0.01620 (15) | 0.00576 (15) | 0.00688 (13) | 0.00553 (13) |
| I4 | 0.02065 (19) | 0.0215 (2) | 0.02325 (16) | 0.00578 (15) | 0.00691 (14) | 0.00628 (14) |
| N1 | 0.021 (2) | 0.026 (3) | 0.0111 (17) | 0.009 (2) | 0.0055 (16) | 0.0093 (17) |
| C2 | 0.016 (3) | 0.024 (3) | 0.013 (2) | 0.005 (2) | 0.0072 (19) | 0.005 (2) |
| C3 | 0.020 (3) | 0.016 (3) | 0.010 (2) | 0.002 (2) | 0.0079 (19) | 0.0060 (19) |
| C4 | 0.015 (3) | 0.009 (3) | 0.017 (2) | −0.001 (2) | 0.0066 (19) | 0.0013 (19) |
| C5 | 0.013 (3) | 0.010 (3) | 0.020 (2) | −0.001 (2) | 0.001 (2) | −0.0069 (19) |
| C6 | 0.020 (3) | 0.028 (3) | 0.008 (2) | 0.001 (2) | 0.0085 (19) | 0.003 (2) |
| C7 | 0.033 (3) | 0.043 (4) | 0.014 (2) | 0.019 (3) | 0.008 (2) | 0.011 (2) |
| S8 | 0.0166 (6) | 0.0169 (7) | 0.0104 (5) | 0.0073 (5) | 0.0068 (5) | 0.0052 (5) |
| O9 | 0.027 (2) | 0.030 (2) | 0.0143 (15) | 0.0194 (17) | 0.0100 (14) | 0.0111 (14) |
| O10 | 0.0186 (19) | 0.029 (2) | 0.0169 (15) | 0.0085 (16) | 0.0073 (14) | 0.0054 (15) |
| N11 | 0.020 (2) | 0.016 (2) | 0.0162 (18) | 0.0019 (19) | 0.0064 (17) | −0.0004 (17) |
| C12 | 0.027 (3) | 0.045 (4) | 0.019 (2) | −0.007 (3) | 0.002 (2) | 0.000 (2) |
| C13 | 0.045 (4) | 0.017 (3) | 0.032 (3) | 0.014 (3) | 0.012 (3) | 0.007 (2) |
| N14 | 0.020 (2) | 0.017 (2) | 0.0110 (17) | 0.0109 (19) | 0.0099 (16) | 0.0027 (16) |
| C15 | 0.017 (3) | 0.010 (3) | 0.013 (2) | 0.004 (2) | 0.0037 (19) | −0.0021 (18) |
| C16 | 0.014 (2) | 0.009 (3) | 0.0121 (19) | 0.003 (2) | 0.0003 (18) | 0.0009 (18) |
| C17 | 0.013 (2) | 0.015 (3) | 0.013 (2) | −0.001 (2) | 0.0028 (18) | 0.0013 (19) |
| C18 | 0.017 (3) | 0.013 (3) | 0.012 (2) | 0.005 (2) | 0.0078 (19) | −0.0003 (18) |
| C19 | 0.018 (3) | 0.009 (3) | 0.012 (2) | 0.006 (2) | −0.0009 (19) | −0.0010 (18) |
| C20 | 0.018 (3) | 0.016 (3) | 0.0069 (19) | 0.003 (2) | 0.0046 (18) | −0.0002 (18) |
| O21 | 0.0182 (18) | 0.0119 (19) | 0.0200 (15) | 0.0068 (15) | 0.0084 (14) | 0.0044 (14) |
| C22 | 0.031 (3) | 0.033 (4) | 0.033 (3) | 0.020 (3) | 0.025 (3) | 0.013 (2) |
| C23 | 0.019 (3) | 0.013 (3) | 0.013 (2) | 0.006 (2) | 0.0072 (19) | 0.0026 (18) |
| N24 | 0.022 (2) | 0.009 (2) | 0.0134 (17) | 0.0030 (18) | 0.0061 (16) | 0.0032 (16) |
| C25 | 0.021 (3) | 0.015 (3) | 0.015 (2) | 0.006 (2) | 0.006 (2) | −0.0019 (19) |
| C26 | 0.026 (3) | 0.023 (3) | 0.026 (2) | 0.010 (2) | 0.010 (2) | 0.004 (2) |
| C27 | 0.017 (3) | 0.016 (3) | 0.027 (2) | 0.004 (2) | 0.003 (2) | 0.005 (2) |
| C28 | 0.027 (3) | 0.018 (3) | 0.040 (3) | 0.001 (3) | 0.002 (3) | −0.006 (2) |
| C29 | 0.027 (3) | 0.012 (3) | 0.020 (2) | 0.005 (2) | 0.005 (2) | 0.004 (2) |
| N41 | 0.015 (2) | 0.018 (2) | 0.0118 (17) | 0.0056 (18) | 0.0040 (16) | 0.0040 (16) |
| C42 | 0.018 (3) | 0.019 (3) | 0.011 (2) | 0.006 (2) | 0.0092 (19) | 0.0050 (19) |
| C43 | 0.011 (2) | 0.013 (3) | 0.019 (2) | 0.003 (2) | 0.0057 (19) | 0.0019 (19) |
| C44 | 0.016 (3) | 0.008 (2) | 0.013 (2) | 0.003 (2) | 0.0039 (19) | 0.0030 (18) |
| C45 | 0.017 (3) | 0.010 (3) | 0.0083 (19) | 0.003 (2) | 0.0049 (18) | 0.0022 (17) |
| C46 | 0.012 (2) | 0.013 (3) | 0.019 (2) | 0.005 (2) | 0.0088 (19) | 0.0037 (19) |
| C47 | 0.024 (3) | 0.034 (3) | 0.016 (2) | 0.014 (3) | 0.008 (2) | 0.012 (2) |
| S48 | 0.0197 (7) | 0.0166 (7) | 0.0100 (5) | 0.0068 (5) | 0.0079 (5) | 0.0029 (5) |
| O49 | 0.024 (2) | 0.017 (2) | 0.0133 (14) | 0.0087 (16) | 0.0059 (14) | 0.0050 (14) |
| O50 | 0.0163 (19) | 0.024 (2) | 0.0172 (15) | 0.0073 (16) | 0.0084 (14) | 0.0061 (14) |
| N51 | 0.025 (2) | 0.016 (2) | 0.0178 (19) | 0.0041 (19) | 0.0074 (18) | 0.0003 (17) |
| C52 | 0.038 (3) | 0.014 (3) | 0.029 (3) | 0.005 (3) | 0.013 (2) | 0.003 (2) |
| C53 | 0.026 (3) | 0.025 (3) | 0.026 (3) | −0.002 (3) | 0.004 (2) | 0.000 (2) |
| N54 | 0.014 (2) | 0.021 (2) | 0.0113 (17) | 0.0080 (18) | 0.0051 (16) | 0.0044 (16) |
| C55 | 0.012 (2) | 0.020 (3) | 0.011 (2) | 0.007 (2) | 0.0008 (18) | −0.0023 (19) |
| C56 | 0.019 (3) | 0.010 (3) | 0.012 (2) | 0.006 (2) | 0.0043 (19) | 0.0023 (18) |
| C57 | 0.018 (3) | 0.016 (3) | 0.012 (2) | 0.001 (2) | 0.0027 (19) | 0.0011 (19) |
| C58 | 0.014 (3) | 0.015 (3) | 0.0090 (19) | 0.007 (2) | 0.0026 (18) | 0.0024 (18) |
| C59 | 0.017 (3) | 0.009 (3) | 0.0084 (19) | 0.003 (2) | −0.0013 (18) | −0.0018 (17) |
| C60 | 0.018 (3) | 0.015 (3) | 0.014 (2) | 0.006 (2) | 0.0068 (19) | 0.0022 (19) |
| O61 | 0.0165 (18) | 0.023 (2) | 0.0173 (15) | 0.0080 (16) | 0.0107 (14) | 0.0070 (14) |
| C62 | 0.039 (4) | 0.050 (4) | 0.045 (3) | 0.030 (3) | 0.033 (3) | 0.032 (3) |
| C63 | 0.017 (3) | 0.014 (3) | 0.015 (2) | 0.002 (2) | 0.0048 (19) | 0.0026 (19) |
| N64 | 0.023 (2) | 0.013 (2) | 0.0151 (18) | 0.0059 (19) | 0.0065 (17) | 0.0036 (16) |
| C65 | 0.022 (3) | 0.018 (3) | 0.018 (2) | 0.007 (2) | 0.009 (2) | 0.006 (2) |
| C66 | 0.029 (3) | 0.020 (3) | 0.020 (2) | 0.005 (2) | 0.010 (2) | 0.004 (2) |
| C67 | 0.018 (3) | 0.018 (3) | 0.022 (2) | 0.001 (2) | 0.006 (2) | 0.002 (2) |
| C68 | 0.033 (3) | 0.024 (3) | 0.036 (3) | 0.001 (3) | 0.021 (3) | 0.006 (2) |
| C69 | 0.039 (3) | 0.012 (3) | 0.025 (2) | 0.010 (3) | 0.005 (2) | 0.007 (2) |
Geometric parameters (Å, °)
| O1W—H1WA | 0.90 (6) | C28—H28B | 0.9600 |
| O1W—H1WB | 0.90 (2) | C28—H28C | 0.9600 |
| O2W—H2WA | 0.90 (6) | C29—H29A | 0.9600 |
| O2W—H2WB | 0.89 (5) | C29—H29B | 0.9600 |
| O3W—H3WA | 0.89 (4) | C29—H29C | 0.9600 |
| O3W—H3WB | 0.90 (3) | N41—C46 | 1.338 (5) |
| O4W—H4WA | 0.90 (4) | N41—C42 | 1.377 (6) |
| O4W—H4WB | 0.90 (3) | N41—C47 | 1.473 (6) |
| O5W—H5WA | 0.89 (4) | C42—C43 | 1.349 (7) |
| O5W—H5WB | 0.89 (5) | C42—H42 | 0.9300 |
| N1—C2 | 1.346 (5) | C43—C44 | 1.428 (6) |
| N1—C6 | 1.371 (6) | C43—H43 | 0.9300 |
| N1—C7 | 1.461 (6) | C44—N54 | 1.342 (6) |
| C2—C3 | 1.369 (6) | C44—C45 | 1.427 (6) |
| C2—H2 | 0.9300 | C45—C46 | 1.365 (6) |
| C3—C4 | 1.428 (6) | C45—S48 | 1.777 (4) |
| C3—S8 | 1.781 (4) | C46—H46 | 0.9300 |
| C4—N14 | 1.339 (6) | C47—H47A | 0.9600 |
| C4—C5 | 1.428 (6) | C47—H47B | 0.9600 |
| C5—C6 | 1.351 (7) | C47—H47C | 0.9600 |
| C5—H5 | 0.9300 | S48—O50 | 1.434 (3) |
| C6—H6 | 0.9300 | S48—O49 | 1.449 (3) |
| C7—H7A | 0.9600 | S48—N51 | 1.617 (4) |
| C7—H7B | 0.9600 | N51—C53 | 1.478 (6) |
| C7—H7C | 0.9600 | N51—C52 | 1.482 (6) |
| S8—O10 | 1.430 (3) | C52—H52A | 0.9600 |
| S8—O9 | 1.440 (3) | C52—H52B | 0.9600 |
| S8—N11 | 1.622 (4) | C52—H52C | 0.9600 |
| N11—C12 | 1.463 (6) | C53—H53A | 0.9600 |
| N11—C13 | 1.473 (6) | C53—H53B | 0.9600 |
| C12—H12A | 0.9600 | C53—H53C | 0.9600 |
| C12—H12B | 0.9600 | N54—C55 | 1.426 (6) |
| C12—H12C | 0.9600 | N54—H54 | 0.90 (6) |
| C13—H13A | 0.9600 | C55—C60 | 1.375 (6) |
| C13—H13B | 0.9600 | C55—C56 | 1.388 (6) |
| C13—H13C | 0.9600 | C56—C57 | 1.387 (6) |
| N14—C15 | 1.436 (6) | C56—H56 | 0.9300 |
| N14—H14 | 0.89 (6) | C57—C58 | 1.383 (6) |
| C15—C16 | 1.380 (6) | C57—H57 | 0.9300 |
| C15—C20 | 1.378 (6) | C58—O61 | 1.375 (5) |
| C16—C17 | 1.385 (6) | C58—C59 | 1.407 (6) |
| C16—H16 | 0.9300 | C59—C60 | 1.401 (6) |
| C17—C18 | 1.382 (6) | C59—C63 | 1.504 (6) |
| C17—H17 | 0.9300 | C60—H60 | 0.9300 |
| C18—O21 | 1.369 (5) | O61—C62 | 1.433 (5) |
| C18—C19 | 1.414 (6) | C62—H62A | 0.9600 |
| C19—C20 | 1.396 (6) | C62—H62B | 0.9600 |
| C19—C23 | 1.509 (6) | C62—H62C | 0.9600 |
| C20—H20 | 0.9300 | C63—N64 | 1.526 (6) |
| O21—C22 | 1.429 (5) | C63—H63A | 0.9700 |
| C22—H22A | 0.9600 | C63—H63B | 0.9700 |
| C22—H22B | 0.9600 | N64—C69 | 1.493 (6) |
| C22—H22C | 0.9600 | N64—C67 | 1.513 (6) |
| C23—N24 | 1.528 (6) | N64—C65 | 1.534 (5) |
| C23—H23A | 0.9700 | C65—C66 | 1.511 (6) |
| C23—H23B | 0.9700 | C65—H65A | 0.9700 |
| N24—C29 | 1.505 (6) | C65—H65B | 0.9700 |
| N24—C27 | 1.522 (6) | C66—H66A | 0.9600 |
| N24—C25 | 1.523 (5) | C66—H66B | 0.9600 |
| C25—C26 | 1.510 (7) | C66—H66C | 0.9600 |
| C25—H25A | 0.9700 | C67—C68 | 1.529 (6) |
| C25—H25B | 0.9700 | C67—H67A | 0.9700 |
| C26—H26A | 0.9600 | C67—H67B | 0.9700 |
| C26—H26B | 0.9600 | C68—H68A | 0.9600 |
| C26—H26C | 0.9600 | C68—H68B | 0.9600 |
| C27—C28 | 1.514 (7) | C68—H68C | 0.9600 |
| C27—H27A | 0.9700 | C69—H69A | 0.9600 |
| C27—H27B | 0.9700 | C69—H69B | 0.9600 |
| C28—H28A | 0.9600 | C69—H69C | 0.9600 |
| H1WA—O1W—H1WB | 112 (4) | H29A—C29—H29C | 109.5 |
| H2WA—O2W—H2WB | 113 (5) | H29B—C29—H29C | 109.5 |
| H3WA—O3W—H3WB | 111 (4) | C46—N41—C42 | 119.0 (4) |
| H4WA—O4W—H4WB | 111 (4) | C46—N41—C47 | 121.3 (4) |
| H5WA—O5W—H5WB | 113 (5) | C42—N41—C47 | 119.6 (3) |
| C2—N1—C6 | 118.4 (4) | C43—C42—N41 | 121.6 (4) |
| C2—N1—C7 | 121.3 (4) | C43—C42—H42 | 119.2 |
| C6—N1—C7 | 120.3 (4) | N41—C42—H42 | 119.2 |
| N1—C2—C3 | 122.2 (4) | C42—C43—C44 | 121.0 (4) |
| N1—C2—H2 | 118.9 | C42—C43—H43 | 119.5 |
| C3—C2—H2 | 118.9 | C44—C43—H43 | 119.5 |
| C2—C3—C4 | 120.7 (4) | N54—C44—C43 | 121.6 (4) |
| C2—C3—S8 | 115.0 (3) | N54—C44—C45 | 123.0 (4) |
| C4—C3—S8 | 124.2 (3) | C43—C44—C45 | 115.4 (4) |
| N14—C4—C5 | 122.1 (4) | C46—C45—C44 | 120.4 (4) |
| N14—C4—C3 | 123.0 (4) | C46—C45—S48 | 115.1 (3) |
| C5—C4—C3 | 114.9 (4) | C44—C45—S48 | 124.1 (3) |
| C6—C5—C4 | 120.7 (4) | N41—C46—C45 | 122.4 (4) |
| C6—C5—H5 | 119.7 | N41—C46—H46 | 118.8 |
| C4—C5—H5 | 119.7 | C45—C46—H46 | 118.8 |
| C5—C6—N1 | 122.4 (4) | N41—C47—H47A | 109.5 |
| C5—C6—H6 | 118.8 | N41—C47—H47B | 109.5 |
| N1—C6—H6 | 118.8 | H47A—C47—H47B | 109.5 |
| N1—C7—H7A | 109.5 | N41—C47—H47C | 109.5 |
| N1—C7—H7B | 109.5 | H47A—C47—H47C | 109.5 |
| H7A—C7—H7B | 109.5 | H47B—C47—H47C | 109.5 |
| N1—C7—H7C | 109.5 | O50—S48—O49 | 119.00 (19) |
| H7A—C7—H7C | 109.5 | O50—S48—N51 | 107.7 (2) |
| H7B—C7—H7C | 109.5 | O49—S48—N51 | 107.2 (2) |
| O10—S8—O9 | 119.4 (2) | O50—S48—C45 | 107.9 (2) |
| O10—S8—N11 | 107.1 (2) | O49—S48—C45 | 107.1 (2) |
| O9—S8—N11 | 107.6 (2) | N51—S48—C45 | 107.4 (2) |
| O10—S8—C3 | 107.0 (2) | C53—N51—C52 | 113.4 (4) |
| O9—S8—C3 | 107.0 (2) | C53—N51—S48 | 116.5 (3) |
| N11—S8—C3 | 108.3 (2) | C52—N51—S48 | 117.6 (3) |
| C12—N11—C13 | 113.2 (4) | N51—C52—H52A | 109.5 |
| C12—N11—S8 | 117.3 (4) | N51—C52—H52B | 109.5 |
| C13—N11—S8 | 116.3 (3) | H52A—C52—H52B | 109.5 |
| N11—C12—H12A | 109.5 | N51—C52—H52C | 109.5 |
| N11—C12—H12B | 109.5 | H52A—C52—H52C | 109.5 |
| H12A—C12—H12B | 109.5 | H52B—C52—H52C | 109.5 |
| N11—C12—H12C | 109.5 | N51—C53—H53A | 109.5 |
| H12A—C12—H12C | 109.5 | N51—C53—H53B | 109.5 |
| H12B—C12—H12C | 109.5 | H53A—C53—H53B | 109.5 |
| N11—C13—H13A | 109.5 | N51—C53—H53C | 109.5 |
| N11—C13—H13B | 109.5 | H53A—C53—H53C | 109.5 |
| H13A—C13—H13B | 109.5 | H53B—C53—H53C | 109.5 |
| N11—C13—H13C | 109.5 | C44—N54—C55 | 124.6 (4) |
| H13A—C13—H13C | 109.5 | C44—N54—H54 | 119 (3) |
| H13B—C13—H13C | 109.5 | C55—N54—H54 | 116 (3) |
| C4—N14—C15 | 125.7 (4) | C60—C55—C56 | 120.1 (4) |
| C4—N14—H14 | 110 (3) | C60—C55—N54 | 120.4 (4) |
| C15—N14—H14 | 124 (3) | C56—C55—N54 | 119.5 (4) |
| C16—C15—C20 | 120.5 (4) | C55—C56—C57 | 120.5 (4) |
| C16—C15—N14 | 121.1 (4) | C55—C56—H56 | 119.8 |
| C20—C15—N14 | 118.4 (4) | C57—C56—H56 | 119.8 |
| C15—C16—C17 | 119.8 (4) | C58—C57—C56 | 119.4 (4) |
| C15—C16—H16 | 120.1 | C58—C57—H57 | 120.3 |
| C17—C16—H16 | 120.1 | C56—C57—H57 | 120.3 |
| C16—C17—C18 | 120.3 (4) | O61—C58—C57 | 123.2 (4) |
| C16—C17—H17 | 119.8 | O61—C58—C59 | 115.8 (4) |
| C18—C17—H17 | 119.8 | C57—C58—C59 | 120.9 (4) |
| O21—C18—C17 | 123.9 (4) | C60—C59—C58 | 118.2 (4) |
| O21—C18—C19 | 115.8 (4) | C60—C59—C63 | 121.4 (4) |
| C17—C18—C19 | 120.3 (4) | C58—C59—C63 | 120.3 (4) |
| C20—C19—C18 | 118.0 (4) | C55—C60—C59 | 120.9 (4) |
| C20—C19—C23 | 120.0 (4) | C55—C60—H60 | 119.6 |
| C18—C19—C23 | 121.8 (4) | C59—C60—H60 | 119.6 |
| C15—C20—C19 | 120.9 (4) | C58—O61—C62 | 116.7 (4) |
| C15—C20—H20 | 119.5 | O61—C62—H62A | 109.5 |
| C19—C20—H20 | 119.5 | O61—C62—H62B | 109.5 |
| C18—O21—C22 | 116.3 (4) | H62A—C62—H62B | 109.5 |
| O21—C22—H22A | 109.5 | O61—C62—H62C | 109.5 |
| O21—C22—H22B | 109.5 | H62A—C62—H62C | 109.5 |
| H22A—C22—H22B | 109.5 | H62B—C62—H62C | 109.5 |
| O21—C22—H22C | 109.5 | C59—C63—N64 | 115.6 (4) |
| H22A—C22—H22C | 109.5 | C59—C63—H63A | 108.4 |
| H22B—C22—H22C | 109.5 | N64—C63—H63A | 108.4 |
| C19—C23—N24 | 116.0 (4) | C59—C63—H63B | 108.4 |
| C19—C23—H23A | 108.3 | N64—C63—H63B | 108.4 |
| N24—C23—H23A | 108.3 | H63A—C63—H63B | 107.4 |
| C19—C23—H23B | 108.3 | C69—N64—C67 | 110.6 (4) |
| N24—C23—H23B | 108.3 | C69—N64—C63 | 107.3 (4) |
| H23A—C23—H23B | 107.4 | C67—N64—C63 | 112.2 (3) |
| C29—N24—C27 | 110.4 (4) | C69—N64—C65 | 109.5 (4) |
| C29—N24—C25 | 111.2 (4) | C67—N64—C65 | 108.2 (3) |
| C27—N24—C25 | 108.3 (3) | C63—N64—C65 | 109.1 (3) |
| C29—N24—C23 | 106.6 (3) | C66—C65—N64 | 116.0 (4) |
| C27—N24—C23 | 109.3 (3) | C66—C65—H65A | 108.3 |
| C25—N24—C23 | 111.0 (3) | N64—C65—H65A | 108.3 |
| C26—C25—N24 | 113.8 (4) | C66—C65—H65B | 108.3 |
| C26—C25—H25A | 108.8 | N64—C65—H65B | 108.3 |
| N24—C25—H25A | 108.8 | H65A—C65—H65B | 107.4 |
| C26—C25—H25B | 108.8 | C65—C66—H66A | 109.5 |
| N24—C25—H25B | 108.8 | C65—C66—H66B | 109.5 |
| H25A—C25—H25B | 107.7 | H66A—C66—H66B | 109.5 |
| C25—C26—H26A | 109.5 | C65—C66—H66C | 109.5 |
| C25—C26—H26B | 109.5 | H66A—C66—H66C | 109.5 |
| H26A—C26—H26B | 109.5 | H66B—C66—H66C | 109.5 |
| C25—C26—H26C | 109.5 | N64—C67—C68 | 114.7 (4) |
| H26A—C26—H26C | 109.5 | N64—C67—H67A | 108.6 |
| H26B—C26—H26C | 109.5 | C68—C67—H67A | 108.6 |
| C28—C27—N24 | 115.0 (4) | N64—C67—H67B | 108.6 |
| C28—C27—H27A | 108.5 | C68—C67—H67B | 108.6 |
| N24—C27—H27A | 108.5 | H67A—C67—H67B | 107.6 |
| C28—C27—H27B | 108.5 | C67—C68—H68A | 109.5 |
| N24—C27—H27B | 108.5 | C67—C68—H68B | 109.5 |
| H27A—C27—H27B | 107.5 | H68A—C68—H68B | 109.5 |
| C27—C28—H28A | 109.5 | C67—C68—H68C | 109.5 |
| C27—C28—H28B | 109.5 | H68A—C68—H68C | 109.5 |
| H28A—C28—H28B | 109.5 | H68B—C68—H68C | 109.5 |
| C27—C28—H28C | 109.5 | N64—C69—H69A | 109.5 |
| H28A—C28—H28C | 109.5 | N64—C69—H69B | 109.5 |
| H28B—C28—H28C | 109.5 | H69A—C69—H69B | 109.5 |
| N24—C29—H29A | 109.5 | N64—C69—H69C | 109.5 |
| N24—C29—H29B | 109.5 | H69A—C69—H69C | 109.5 |
| H29A—C29—H29B | 109.5 | H69B—C69—H69C | 109.5 |
| N24—C29—H29C | 109.5 | ||
| C6—N1—C2—C3 | 4.1 (7) | C46—N41—C42—C43 | 2.2 (7) |
| C7—N1—C2—C3 | −173.6 (5) | C47—N41—C42—C43 | −174.4 (5) |
| N1—C2—C3—C4 | 1.9 (8) | N41—C42—C43—C44 | 0.9 (7) |
| N1—C2—C3—S8 | 178.1 (4) | C42—C43—C44—N54 | 176.9 (5) |
| C2—C3—C4—N14 | 173.7 (5) | C42—C43—C44—C45 | −3.6 (7) |
| S8—C3—C4—N14 | −2.2 (7) | N54—C44—C45—C46 | −177.1 (4) |
| C2—C3—C4—C5 | −8.0 (7) | C43—C44—C45—C46 | 3.4 (7) |
| S8—C3—C4—C5 | 176.2 (4) | N54—C44—C45—S48 | −4.7 (7) |
| N14—C4—C5—C6 | −173.1 (5) | C43—C44—C45—S48 | 175.7 (3) |
| C3—C4—C5—C6 | 8.5 (7) | C42—N41—C46—C45 | −2.4 (7) |
| C4—C5—C6—N1 | −3.1 (8) | C47—N41—C46—C45 | 174.2 (5) |
| C2—N1—C6—C5 | −3.5 (7) | C44—C45—C46—N41 | −0.5 (7) |
| C7—N1—C6—C5 | 174.2 (5) | S48—C45—C46—N41 | −173.5 (4) |
| C2—C3—S8—O10 | 41.5 (4) | C46—C45—S48—O50 | −35.4 (4) |
| C4—C3—S8—O10 | −142.5 (4) | C44—C45—S48—O50 | 151.9 (4) |
| C2—C3—S8—O9 | 170.5 (4) | C46—C45—S48—O49 | −164.7 (4) |
| C4—C3—S8—O9 | −13.4 (5) | C44—C45—S48—O49 | 22.6 (5) |
| C2—C3—S8—N11 | −73.7 (4) | C46—C45—S48—N51 | 80.4 (4) |
| C4—C3—S8—N11 | 102.4 (4) | C44—C45—S48—N51 | −92.3 (4) |
| O10—S8—N11—C12 | 171.9 (3) | O50—S48—N51—C53 | −177.9 (3) |
| O9—S8—N11—C12 | 42.4 (4) | O49—S48—N51—C53 | −48.7 (4) |
| C3—S8—N11—C12 | −73.1 (4) | C45—S48—N51—C53 | 66.1 (4) |
| O10—S8—N11—C13 | −49.7 (4) | O50—S48—N51—C52 | 42.5 (4) |
| O9—S8—N11—C13 | −179.2 (3) | O49—S48—N51—C52 | 171.7 (3) |
| C3—S8—N11—C13 | 65.4 (4) | C45—S48—N51—C52 | −73.4 (4) |
| C5—C4—N14—C15 | 7.9 (7) | C43—C44—N54—C55 | −14.1 (7) |
| C3—C4—N14—C15 | −173.8 (4) | C45—C44—N54—C55 | 166.3 (4) |
| C4—N14—C15—C16 | 47.5 (7) | C44—N54—C55—C60 | 132.6 (5) |
| C4—N14—C15—C20 | −135.6 (5) | C44—N54—C55—C56 | −50.3 (6) |
| C20—C15—C16—C17 | 3.1 (7) | C60—C55—C56—C57 | −0.9 (7) |
| N14—C15—C16—C17 | −180.0 (4) | N54—C55—C56—C57 | −178.0 (4) |
| C15—C16—C17—C18 | −0.1 (7) | C55—C56—C57—C58 | 0.1 (6) |
| C16—C17—C18—O21 | 179.3 (4) | C56—C57—C58—O61 | 179.3 (4) |
| C16—C17—C18—C19 | −2.7 (7) | C56—C57—C58—C59 | 0.0 (6) |
| O21—C18—C19—C20 | −179.5 (4) | O61—C58—C59—C60 | −178.8 (4) |
| C17—C18—C19—C20 | 2.4 (7) | C57—C58—C59—C60 | 0.6 (6) |
| O21—C18—C19—C23 | 4.4 (6) | O61—C58—C59—C63 | −2.7 (6) |
| C17—C18—C19—C23 | −173.7 (4) | C57—C58—C59—C63 | 176.7 (4) |
| C16—C15—C20—C19 | −3.4 (7) | C56—C55—C60—C59 | 1.5 (7) |
| N14—C15—C20—C19 | 179.6 (4) | N54—C55—C60—C59 | 178.6 (4) |
| C18—C19—C20—C15 | 0.7 (7) | C58—C59—C60—C55 | −1.3 (6) |
| C23—C19—C20—C15 | 176.8 (4) | C63—C59—C60—C55 | −177.4 (4) |
| C17—C18—O21—C22 | −10.8 (6) | C57—C58—O61—C62 | 24.1 (6) |
| C19—C18—O21—C22 | 171.1 (4) | C59—C58—O61—C62 | −156.6 (4) |
| C20—C19—C23—N24 | 88.3 (5) | C60—C59—C63—N64 | −90.8 (5) |
| C18—C19—C23—N24 | −95.7 (5) | C58—C59—C63—N64 | 93.3 (5) |
| C19—C23—N24—C29 | −170.2 (4) | C59—C63—N64—C69 | −175.4 (4) |
| C19—C23—N24—C27 | −50.9 (5) | C59—C63—N64—C67 | 63.0 (5) |
| C19—C23—N24—C25 | 68.5 (5) | C59—C63—N64—C65 | −56.9 (5) |
| C29—N24—C25—C26 | −48.6 (5) | C69—N64—C65—C66 | −61.7 (5) |
| C27—N24—C25—C26 | −170.1 (4) | C67—N64—C65—C66 | 58.9 (5) |
| C23—N24—C25—C26 | 69.9 (5) | C63—N64—C65—C66 | −178.8 (4) |
| C29—N24—C27—C28 | −51.8 (5) | C69—N64—C67—C68 | −56.0 (5) |
| C25—N24—C27—C28 | 70.2 (5) | C63—N64—C67—C68 | 63.7 (5) |
| C23—N24—C27—C28 | −168.7 (4) | C65—N64—C67—C68 | −175.8 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1W—H1WA···I4 | 0.91 (3) | 2.60 (4) | 3.489 (4) | 170 (3) |
| O1W—H1WB···I2 | 0.90 (2) | 2.66 (2) | 3.561 (4) | 176 (4) |
| O2W—H2WA···O5wi | 0.90 (4) | 1.98 (5) | 2.799 (7) | 147 (5) |
| O2W—H2WB···I3 | 0.90 (4) | 2.69 (4) | 3.580 (5) | 175 (4) |
| O3W—H3WA···O1Wi | 0.90 (4) | 1.91 (4) | 2.781 (6) | 161 (18) |
| O3W—H3WB···I1 | 0.90 (4) | 2.64 (4) | 3.544 (4) | 178 (5) |
| O4W—H4WA···I4 | 0.90 (3) | 2.75 (3) | 3.613 (5) | 160 (4) |
| O4W—H4WB···O3Wii | 0.89 (5) | 1.92 (4) | 2.795 (7) | 162 (2) |
| O5W—H5WA···I2 | 0.90 (4) | 2.75 (5) | 3.614 (5) | 165 (4) |
| O5W—H5WB···O4wiii | 0.90 (4) | 1.94 (4) | 2.809 (7) | 161 (3) |
| N14—H14···O9 | 0.90 (3) | 1.93 (4) | 2.733 (5) | 149 (4) |
| N54—H54···O49 | 0.90 (5) | 2.08 (4) | 2.767 (5) | 133 (4) |
Symmetry codes: (i) x, y+1, z; (ii) x, y−1, z; (iii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BG2227).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Bjorkman, A. & Bhattarai, A. (2005). Acta Trop.94, 163–169. [DOI] [PubMed]
- Bruker (2005). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Djedouani, A., Boufas, S., Allain, M., Bouet, G. & Khan, M. (2008). Acta Cryst. E64, o1785. [DOI] [PMC free article] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Lopes, F., Capela, R., Gonçalves, J. O., Horton, P. N., Hursthouse, M. B., Iley, J., Casimiro, C. M., Bom, J. & Moreira, R. (2004). Tetrahedron Lett.45, 7663–7666.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, Y., Zhang, J., Chen, H. & Luo, S. (2008). Acta Cryst. E64, o1025. [DOI] [PMC free article] [PubMed]
- Yeates, C. L., Batchelor, J. F., Capon, E. C., Cheesman, N. J., Fry, M., Hudson, A. T., Pudney, M., Trimming, H., Woolven, J., Bueno, J. M., Chicharro, J., Fernández, E., Fiandor, J. M., Gargallo-Viola, D., de las Heras, F. G., Herreros, E. & León, M. L. (2008). J. Med. Chem.51, 2845–2852. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809000324/bg2227sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809000324/bg2227Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report