Abstract
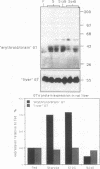
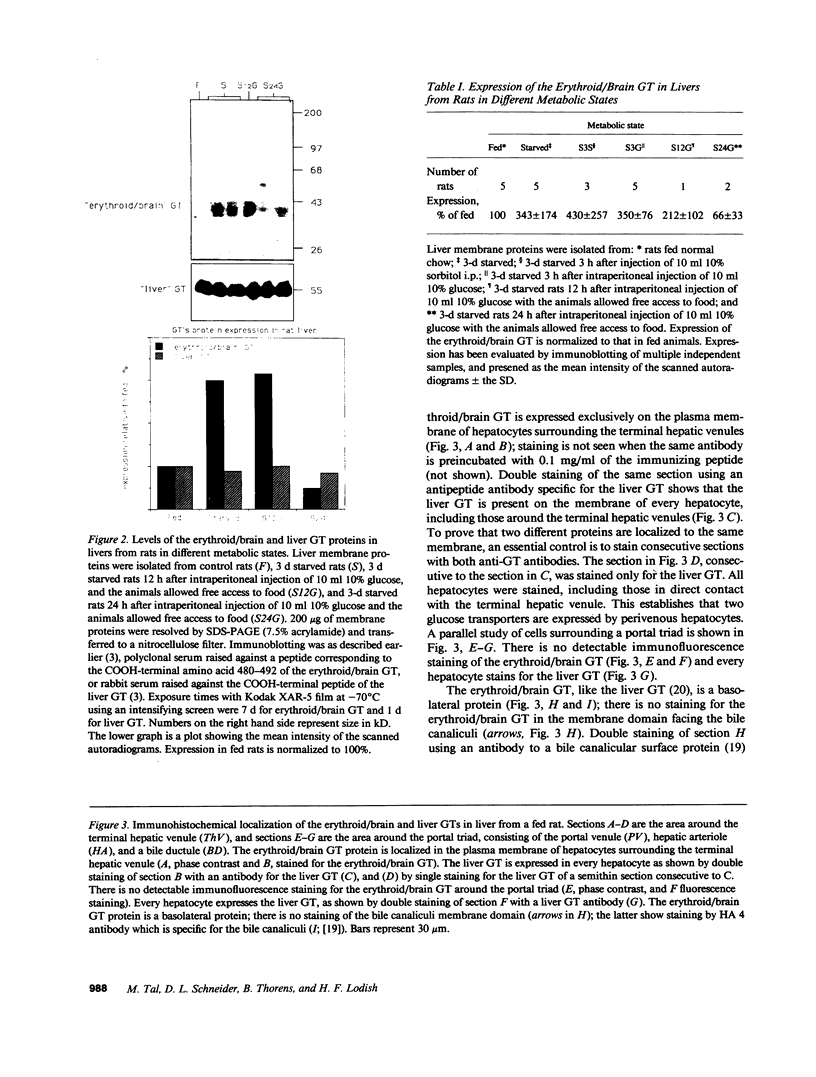
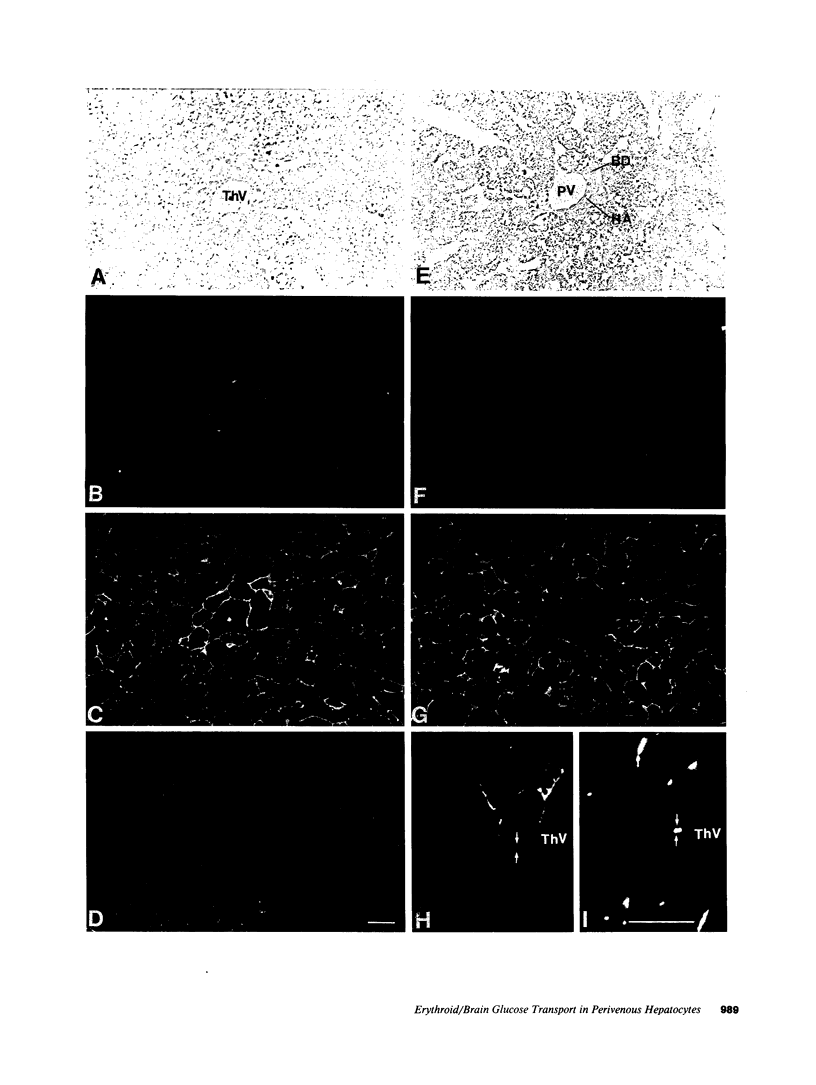
The "erythroid/brain" glucose transporter (GT) isoform is expressed only in a subset of hepatocytes, those forming the first row around the terminal hepatic venules, while the "liver" GT is expressed in all hepatocytes. After 3 d of starvation, a three- to fourfold elevation of expression of the erythroid/brain GT mRNA and protein is detected in the liver as a whole; this correlates with the expression of this GT in more hepatocytes, those forming the first three to four rows around the hepatic venules. Starvation-dependent expression of the erythroid/brain GT on the plasma membrane of these additional hepatocytes is lost within 3 h of glucose refeeding; however, by immunoblotting we show that the protein is still present. Its loss from the surface is possibly explained by internalization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bartels H., Vogt B., Jungermann K. Glycogen synthesis via the indirect gluconeogenic pathway in the periportal and via the direct glucose utilizing pathway in the perivenous zone of perfused rat liver. Histochemistry. 1988;89(3):253–260. doi: 10.1007/BF00493149. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Brown D., Gluck S., Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol. 1987 Oct;105(4):1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik J. D., Elliott K. R. Kinetics of 3-O-methyl-D-glucose transport in isolated rat hepatocytes. Biochem J. 1979 Aug 15;182(2):503–508. doi: 10.1042/bj1820503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Ick M., Katz N. R. Reciprocal distribution of hexokinase and glucokinase in the periportal and perivenous zone of the rat liver acinus. Hoppe Seylers Z Physiol Chem. 1982 Apr;363(4):375–380. doi: 10.1515/bchm2.1982.363.1.375. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Schmidt U. Liver cell heterogeneity. The distribution of pyruvate kinase and phosphoenolpyruvate carboxykinase (GTP) in the liver lobule of fed and starved rats. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(12):1793–1800. doi: 10.1515/bchm2.1976.357.2.1793. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Bartles J. R., Braiterman L. T. Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol. 1985 Apr;100(4):1115–1125. doi: 10.1083/jcb.100.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Katz N., Teutsch H. F., Jungermann K., Sasse D. Heterogeneous reciprocal localization of fructose-1,6-bisphosphatase and of glucokinase in microdissected periportal and perivenous rat liver tissue. FEBS Lett. 1977 Nov 15;83(2):272–276. doi: 10.1016/0014-5793(77)81021-1. [DOI] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Lawrence G. M., Jepson M. A., Trayer I. P., Walker D. G. The compartmentation of glycolytic and gluconeogenic enzymes in rat kidney and liver and its significance to renal and hepatic metabolism. Histochem J. 1986 Jan;18(1):45–53. doi: 10.1007/BF01676198. [DOI] [PubMed] [Google Scholar]
- Lawrence G. M., Trayer I. P., Walker D. G. Histochemical and immunohistochemical localization of hexokinase isoenzymes in normal rat liver. Histochem J. 1984 Oct;16(10):1099–1111. doi: 10.1007/BF01002897. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Probst I., Schwartz P., Jungermann K. Induction in primary culture of 'gluconeogenic' and 'glycolytic' hepatocytes resembling periportal and perivenous cells. Eur J Biochem. 1982 Aug;126(2):271–278. doi: 10.1111/j.1432-1033.1982.tb06775.x. [DOI] [PubMed] [Google Scholar]
- Quistorff B. Gluconeogenesis in periportal and perivenous hepatocytes of rat liver, isolated by a new high-yield digitonin/collagenase perfusion technique. Biochem J. 1985 Jul 1;229(1):221–226. doi: 10.1042/bj2290221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPAPORT A. M., BOROWY Z. J., LOUGHEED W. M., LOTTO W. N. Subdivision of hexagonal liver lobules into a structural and functional unit; role in hepatic physiology and pathology. Anat Rec. 1954 May;119(1):11–33. doi: 10.1002/ar.1091190103. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Yang M., Bonner J. Nucleotide sequence of cloned rat serum albumin messenger RNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):243–246. doi: 10.1073/pnas.78.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse D., Katz N., Jungermann K. Functional heterogeneity of rat liver parenchyma and of isolated hepatocytes. FEBS Lett. 1975 Sep 1;57(1):83–88. doi: 10.1016/0014-5793(75)80157-8. [DOI] [PubMed] [Google Scholar]
- Thorens B., Flier J. S., Lodish H. F., Kahn B. B. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. doi: 10.2337/diab.39.6.712. [DOI] [PubMed] [Google Scholar]
- Thorens B., Lodish H. F., Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990 Dec;259(6 Pt 1):C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus M., Zawalich K., Gaynor D., Matschinsky F. Hexokinase and glucokinase distribution in the liver lobule. J Histochem Cytochem. 1980 Jun;28(6):579–581. doi: 10.1177/28.6.7391551. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J Biol Chem. 1981 Sep 10;256(17):8907–8914. [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Williams T. F., Exton J. H., Park C. R., Regen D. M. Stereospecific transport of glucose in the perfused rat liver. Am J Physiol. 1968 Nov;215(5):1200–1209. doi: 10.1152/ajplegacy.1968.215.5.1200. [DOI] [PubMed] [Google Scholar]