Abstract
In the title molecule, C13H15N3O2, the dihedral angle between the mean plane of the 1,3-dioxolane group and the 2-hydrazino-7-methylisoquinoline unit is 85.21 (5)°. The conformation of the molecule is influenced by bifurcated N—H⋯(O,O) and N—H⋯N intramolecular hydrogen bonds. In the crystal structure, molecules are linked via intermolecular N—H⋯O hydrogen bonds, forming extended chains along [001].
Related literature
For general background to hydrazine compounds, see: Broadhurst et al. (2001 ▶); Behrens (1999 ▶); Broadhurst (1991 ▶); Chao et al. (1999 ▶); Kametani (1968 ▶). For related crystal structures, see: Yang et al. (2008 ▶); Choudhury & Guru Row (2006 ▶); Choudhury et al. (2002 ▶); Hathwar et al. (2008 ▶); Cho et al. (2002 ▶); Manivel et al. (2009 ▶), and references therein. For bond-length data, see: Allen et al., 1987 ▶)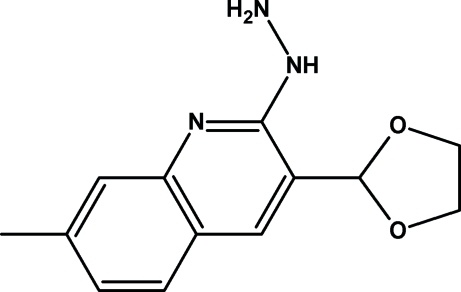
Experimental
Crystal data
C13H15N3O2
M r = 245.28
Monoclinic,

a = 13.1909 (17) Å
b = 10.1165 (13) Å
c = 9.7805 (13) Å
β = 109.956 (2)°
V = 1226.8 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 290 (2) K
0.30 × 0.21 × 0.14 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.942, T max = 0.987
8929 measured reflections
2279 independent reflections
1699 reflections with I > 2σ(I)
R int = 0.018
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.129
S = 1.06
2279 reflections
176 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.19 e Å−3
Δρmin = −0.14 e Å−3
Data collection: SMART (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and PLATON (Spek, 2003 ▶); software used to prepare material for publication: PLATON.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809003031/lh2748sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809003031/lh2748Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2N⋯O1 | 0.843 (19) | 2.372 (18) | 2.9329 (17) | 124.5 (15) |
| N2—H2N⋯O2 | 0.843 (19) | 2.653 (18) | 3.0968 (19) | 114.3 (14) |
| N3—H3NA⋯N1 | 0.94 (2) | 2.35 (2) | 2.691 (2) | 100.9 (15) |
| N3—H3NB⋯O2i | 0.92 (2) | 2.44 (2) | 3.207 (2) | 141.2 (19) |
Symmetry code: (i)  .
.
Acknowledgments
We thank the Department of Science and Technology, India, for use of the CCD facility setup under the IRHPA-DST program at IISc. We thank Professor T. N. Guru Row, IISc, Bangalore, for useful crystallographic discussions. FNK thanks the DST for Fast Track Proposal funding.
supplementary crystallographic information
Comment
The title compound (I), belongs to the quinoline class. Quinolines and quinolinones are an integral part of many naturally occurring fused heterocycles and find application in synthetic and pharmaceutical chemistry (Kametani, 1968). Isoquinolinones and isoquinolineamines have been reported as cancer chemotherapeutic agents (Behrens, 1999) whereas quinolyl and isoquinolyl derivatives have been reported as insecticidal compounds (Broadhurst, 1991). 3-substituted isoquinolines have potent use in medicine (Chao et al., 1999) and in general, hydrazine derivatives can be used as medicaments (Broadhurst et al., 2001; Choudhury, et al., 2002; Choudhury & Guru Row, 2006; Yang, et al., 2008). Due to the importance of quinoline derivates (Cho et al., 2002) and in continuous of our research on quinolines and isoquinoline derivatives (Hathwar et al., 2008; Manivel et al., 2009) we present here crystal structure of the title compound.
In (I) the dihedral angle between 1,3-dioxolane moiety and 2 hyrazino-7-methyl isoquinoline unit is 85.21 (5)°. All bond lengths (Allen et al., 1987) and angles are within normal ranges. The conformation of the molecule is influenced by N—H···O and N—H···N intramolecular hydrogen bonds whereas the crystal structure is stabilized by intermolecular N—H···O hydrogen bonds forming exteded chains along [001].
Experimental
A solution of 2-chloro (3-(1,3-dioxolan-2-yl)-7-methylquinoline in ethanol was treated with hydrazine hydrate and stirred at 323 K for 3hr. The product was filtered. The solid was washed with water and diethyl ether and dried under vacuum. Single crystals were obtained by recrystalization of (I) from DMSO.
Refinement
All H atoms positioned geometrically and refined using a riding model with bond lengths C—H = 0.93 Å (for aromatic), 0.97 Å (for methylene) and 0.96 Å (for methyl). The Uiso(H) = 1.5Ueq(C) for methyl and Uiso(H) = 1.2Ueq(C) for all other carbon bound H atoms. H atoms bonded to N atoms were located in difference Fourier maps and refined isotropically.
Figures
Fig. 1.
The molecular structure of (I) with 50% probability displacement ellipsoids. Dashed lines indicate hydrogen bonds.
Fig. 2.
Part of the crystal structure of (I) showing hydrogen bonds as dashed lines. H atoms not involved in hydrogen bonds have been omitted.
Crystal data
| C13H15N3O2 | F(000) = 520 |
| Mr = 245.28 | Dx = 1.328 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 948 reflections |
| a = 13.1909 (17) Å | θ = 1.8–24.6° |
| b = 10.1165 (13) Å | µ = 0.09 mm−1 |
| c = 9.7805 (13) Å | T = 290 K |
| β = 109.956 (2)° | Block, brown |
| V = 1226.8 (3) Å3 | 0.30 × 0.21 × 0.14 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 2279 independent reflections |
| Radiation source: fine-focus sealed tube | 1699 reflections with I > 2σ(I) |
| graphite | Rint = 0.018 |
| φ and ω scans | θmax = 25.5°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −15→15 |
| Tmin = 0.942, Tmax = 0.987 | k = −10→12 |
| 8929 measured reflections | l = −11→11 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.129 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.072P)2 + 0.1104P] where P = (Fo2 + 2Fc2)/3 |
| 2279 reflections | (Δ/σ)max < 0.001 |
| 176 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.14 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.10652 (9) | 0.42297 (12) | −0.28334 (11) | 0.0621 (4) | |
| O2 | 0.09919 (10) | 0.22186 (13) | −0.18872 (12) | 0.0701 (4) | |
| N1 | 0.32415 (10) | 0.45872 (13) | 0.15262 (13) | 0.0524 (4) | |
| N2 | 0.14264 (11) | 0.45599 (16) | 0.02767 (15) | 0.0597 (4) | |
| N3 | 0.12501 (13) | 0.53613 (19) | 0.13548 (18) | 0.0664 (4) | |
| C1 | 0.24495 (12) | 0.42351 (15) | 0.03448 (15) | 0.0464 (4) | |
| C2 | 0.26133 (12) | 0.35152 (15) | −0.08326 (15) | 0.0480 (4) | |
| C3 | 0.36404 (13) | 0.31857 (15) | −0.06896 (17) | 0.0550 (4) | |
| H3A | 0.3772 | 0.2726 | −0.1435 | 0.066* | |
| C4 | 0.56033 (15) | 0.32091 (18) | 0.0793 (2) | 0.0688 (5) | |
| H4A | 0.5778 | 0.2750 | 0.0080 | 0.083* | |
| C5 | 0.64009 (14) | 0.35734 (19) | 0.2051 (2) | 0.0732 (6) | |
| H5A | 0.7111 | 0.3344 | 0.2186 | 0.088* | |
| C6 | 0.61719 (14) | 0.4286 (2) | 0.3145 (2) | 0.0662 (5) | |
| C7 | 0.51244 (13) | 0.46184 (19) | 0.29207 (17) | 0.0615 (5) | |
| H7A | 0.4966 | 0.5107 | 0.3628 | 0.074* | |
| C8 | 0.42773 (12) | 0.42464 (15) | 0.16556 (16) | 0.0496 (4) | |
| C9 | 0.45173 (12) | 0.35270 (15) | 0.05728 (17) | 0.0533 (4) | |
| C10 | 0.70689 (16) | 0.4661 (3) | 0.4525 (2) | 0.0949 (8) | |
| H10A | 0.6767 | 0.4936 | 0.5244 | 0.142* | |
| H10B | 0.7481 | 0.5372 | 0.4329 | 0.142* | |
| H10C | 0.7530 | 0.3911 | 0.4882 | 0.142* | |
| C11 | 0.17109 (13) | 0.31376 (16) | −0.21833 (17) | 0.0540 (4) | |
| H11A | 0.2010 | 0.2745 | −0.2879 | 0.065* | |
| C12 | −0.00343 (14) | 0.2421 (2) | −0.2979 (2) | 0.0782 (6) | |
| H12A | −0.0588 | 0.2540 | −0.2544 | 0.094* | |
| H12B | −0.0228 | 0.1675 | −0.3640 | 0.094* | |
| C13 | 0.00918 (15) | 0.3653 (2) | −0.37670 (19) | 0.0764 (6) | |
| H13A | 0.0146 | 0.3440 | −0.4706 | 0.092* | |
| H13B | −0.0513 | 0.4247 | −0.3910 | 0.092* | |
| H2N | 0.0904 (15) | 0.4447 (17) | −0.050 (2) | 0.064 (5)* | |
| H3NB | 0.1453 (18) | 0.484 (2) | 0.218 (2) | 0.095 (7)* | |
| H3NA | 0.1821 (17) | 0.597 (2) | 0.158 (2) | 0.078 (6)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0577 (7) | 0.0724 (8) | 0.0494 (6) | 0.0026 (6) | 0.0095 (5) | 0.0034 (5) |
| O2 | 0.0667 (8) | 0.0722 (8) | 0.0642 (7) | −0.0201 (6) | 0.0130 (6) | −0.0040 (6) |
| N1 | 0.0475 (8) | 0.0647 (9) | 0.0438 (7) | −0.0047 (6) | 0.0141 (6) | −0.0015 (6) |
| N2 | 0.0476 (8) | 0.0838 (11) | 0.0443 (7) | 0.0051 (7) | 0.0114 (6) | −0.0113 (7) |
| N3 | 0.0626 (10) | 0.0782 (11) | 0.0586 (9) | 0.0087 (9) | 0.0211 (7) | −0.0131 (9) |
| C1 | 0.0467 (8) | 0.0495 (9) | 0.0426 (8) | 0.0001 (7) | 0.0145 (7) | 0.0037 (6) |
| C2 | 0.0497 (9) | 0.0465 (8) | 0.0470 (8) | 0.0006 (7) | 0.0153 (7) | 0.0012 (6) |
| C3 | 0.0580 (10) | 0.0492 (9) | 0.0589 (9) | 0.0019 (7) | 0.0214 (8) | −0.0077 (7) |
| C4 | 0.0566 (10) | 0.0607 (11) | 0.0890 (13) | 0.0055 (8) | 0.0245 (9) | −0.0053 (10) |
| C5 | 0.0424 (9) | 0.0707 (12) | 0.0974 (14) | 0.0022 (8) | 0.0122 (9) | 0.0105 (11) |
| C6 | 0.0518 (10) | 0.0774 (13) | 0.0628 (11) | −0.0136 (9) | 0.0108 (8) | 0.0122 (9) |
| C7 | 0.0526 (10) | 0.0785 (12) | 0.0512 (9) | −0.0134 (8) | 0.0148 (8) | 0.0024 (8) |
| C8 | 0.0480 (9) | 0.0526 (9) | 0.0470 (8) | −0.0057 (7) | 0.0147 (7) | 0.0065 (7) |
| C9 | 0.0474 (9) | 0.0470 (9) | 0.0629 (10) | 0.0001 (7) | 0.0156 (7) | 0.0035 (7) |
| C10 | 0.0550 (11) | 0.138 (2) | 0.0771 (13) | −0.0265 (12) | 0.0040 (10) | 0.0078 (13) |
| C11 | 0.0531 (9) | 0.0597 (10) | 0.0492 (9) | −0.0007 (7) | 0.0175 (7) | −0.0083 (7) |
| C12 | 0.0554 (11) | 0.0901 (15) | 0.0852 (13) | −0.0112 (10) | 0.0188 (10) | −0.0274 (12) |
| C13 | 0.0565 (11) | 0.1135 (17) | 0.0500 (9) | 0.0012 (11) | 0.0065 (8) | −0.0119 (11) |
Geometric parameters (Å, °)
| O1—C11 | 1.4074 (19) | C4—H4A | 0.9300 |
| O1—C13 | 1.422 (2) | C5—C6 | 1.406 (3) |
| O2—C12 | 1.424 (2) | C5—H5A | 0.9300 |
| O2—C11 | 1.427 (2) | C6—C7 | 1.365 (3) |
| N1—C1 | 1.3151 (18) | C6—C10 | 1.509 (2) |
| N1—C8 | 1.372 (2) | C7—C8 | 1.406 (2) |
| N2—C1 | 1.3683 (19) | C7—H7A | 0.9300 |
| N2—N3 | 1.411 (2) | C8—C9 | 1.407 (2) |
| N2—H2N | 0.843 (19) | C10—H10A | 0.9600 |
| N3—H3NB | 0.92 (2) | C10—H10B | 0.9600 |
| N3—H3NA | 0.94 (2) | C10—H10C | 0.9600 |
| C1—C2 | 1.440 (2) | C11—H11A | 0.9800 |
| C2—C3 | 1.355 (2) | C12—C13 | 1.504 (3) |
| C2—C11 | 1.495 (2) | C12—H12A | 0.9700 |
| C3—C9 | 1.417 (2) | C12—H12B | 0.9700 |
| C3—H3A | 0.9300 | C13—H13A | 0.9700 |
| C4—C5 | 1.368 (3) | C13—H13B | 0.9700 |
| C4—C9 | 1.411 (2) | ||
| C11—O1—C13 | 104.05 (14) | N1—C8—C7 | 118.75 (15) |
| C12—O2—C11 | 106.35 (14) | N1—C8—C9 | 122.22 (14) |
| C1—N1—C8 | 118.70 (13) | C7—C8—C9 | 119.03 (15) |
| C1—N2—N3 | 120.87 (13) | C8—C9—C4 | 118.70 (15) |
| C1—N2—H2N | 120.1 (12) | C8—C9—C3 | 117.09 (14) |
| N3—N2—H2N | 117.4 (12) | C4—C9—C3 | 124.20 (16) |
| N2—N3—H3NB | 104.8 (14) | C6—C10—H10A | 109.5 |
| N2—N3—H3NA | 102.9 (12) | C6—C10—H10B | 109.5 |
| H3NB—N3—H3NA | 101.6 (18) | H10A—C10—H10B | 109.5 |
| N1—C1—N2 | 116.89 (14) | C6—C10—H10C | 109.5 |
| N1—C1—C2 | 123.28 (14) | H10A—C10—H10C | 109.5 |
| N2—C1—C2 | 119.82 (13) | H10B—C10—H10C | 109.5 |
| C3—C2—C1 | 117.35 (13) | O1—C11—O2 | 105.16 (13) |
| C3—C2—C11 | 119.66 (14) | O1—C11—C2 | 112.04 (13) |
| C1—C2—C11 | 122.99 (13) | O2—C11—C2 | 111.79 (13) |
| C2—C3—C9 | 121.34 (15) | O1—C11—H11A | 109.2 |
| C2—C3—H3A | 119.3 | O2—C11—H11A | 109.2 |
| C9—C3—H3A | 119.3 | C2—C11—H11A | 109.2 |
| C5—C4—C9 | 120.33 (18) | O2—C12—C13 | 105.10 (14) |
| C5—C4—H4A | 119.8 | O2—C12—H12A | 110.7 |
| C9—C4—H4A | 119.8 | C13—C12—H12A | 110.7 |
| C4—C5—C6 | 121.55 (17) | O2—C12—H12B | 110.7 |
| C4—C5—H5A | 119.2 | C13—C12—H12B | 110.7 |
| C6—C5—H5A | 119.2 | H12A—C12—H12B | 108.8 |
| C7—C6—C5 | 118.21 (16) | O1—C13—C12 | 104.14 (14) |
| C7—C6—C10 | 121.57 (19) | O1—C13—H13A | 110.9 |
| C5—C6—C10 | 120.22 (17) | C12—C13—H13A | 110.9 |
| C6—C7—C8 | 122.15 (17) | O1—C13—H13B | 110.9 |
| C6—C7—H7A | 118.9 | C12—C13—H13B | 110.9 |
| C8—C7—H7A | 118.9 | H13A—C13—H13B | 108.9 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2N···O1 | 0.843 (19) | 2.372 (18) | 2.9329 (17) | 124.5 (15) |
| N2—H2N···O2 | 0.843 (19) | 2.653 (18) | 3.0968 (19) | 114.3 (14) |
| N3—H3NA···N1 | 0.94 (2) | 2.35 (2) | 2.691 (2) | 100.9 (15) |
| N3—H3NB···O2i | 0.92 (2) | 2.44 (2) | 3.207 (2) | 141.2 (19) |
Symmetry codes: (i) x, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH2748).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Behrens, C. H. (1999). US Patent No. 4 942 163.
- Broadhurst, M. D. (1991). US Patent No. 5 070 097.
- Broadhurst, M. D., Michael, J. J., William, H. W. & Daryl, S. (2001). US Patent No. 6 235 787.
- Bruker (2004). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chao, Q., Deng, L., Shih, H., Leoni, L. M., Genini, D., Carson, D. A. & Cottam, H. B. (1999). J. Med. Chem.2, 3860–3873. [DOI] [PubMed]
- Cho, W., Kim, E., Park, I. Y., Jeong, E. Y., Kim, T. S., Le, T. N., Kim, D. & Leed, E. (2002). Bioorg. Med. Chem.10, 2953–2961. [DOI] [PubMed]
- Choudhury, A. R. & Guru Row, T. N. (2006). CrystEngComm, 8, 265–274.
- Choudhury, A. R., Urs, U. K., Guru Row, T. N. & Nagarajan, K. (2002). J. Mol. Struct.605, 71–77.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Hathwar, V. R., Prabakaran, K., Subashini, R., Manivel, P. & Khan, F. N. (2008). Acta Cryst. E64, o2295. [DOI] [PMC free article] [PubMed]
- Kametani, T. (1968). The Chemistry of the Isoquinoline Alkaloids Tokyo, Amsterdam: Hirokawa, Elsevier.
- Manivel, P., Hathwar, V. R., Nithya, P., Prabakaran, K. & Khan, F. N. (2009). Acta Cryst. E65, o137–o138. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Yang, Y., Yang, P., Zhang, C. & Wu, B. (2008). Anal. Sci.24, x97–x98.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809003031/lh2748sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809003031/lh2748Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




