Abstract
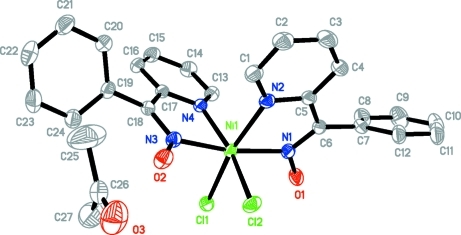
The Ni atom in the title compound, [NiCl2(C12H10N2O)2]·C3H6O, adopts a distorted octahedral geometry, being ligated by four N atoms from two different phenyl 2-pyridyl ketone oxime ligands and two Cl atoms. In the crystal structure, intermolecular O—H⋯Cl hydrogen bonds link the molecules into a chain structure along [010]. There is a π–π contact between the pyridine rings [centroid–centroid distance = 3.824 (5) Å].
Related literature
For related structures, see: Korpi et al. (2005 ▶); Pearse et al. (1989 ▶); Afrati et al. (2005 ▶); Stamatatos et al. (2006 ▶); Papatriantafyllopoulou et al. (2007 ▶).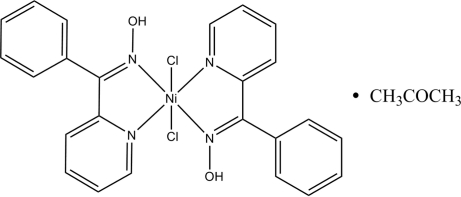
Experimental
Crystal data
[NiCl2(C12H10N2O)2]·C3H6O
M r = 584.13
Triclinic,
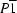
a = 9.0367 (11) Å
b = 12.9142 (16) Å
c = 13.0664 (16) Å
α = 105.4390 (10)°
β = 92.232 (2)°
γ = 108.183 (2)°
V = 1384.0 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.93 mm−1
T = 296 (2) K
0.22 × 0.18 × 0.16 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.822, T max = 0.866
6839 measured reflections
4761 independent reflections
4002 reflections with I > 2σ(I)
R int = 0.021
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.089
S = 1.05
4761 reflections
338 parameters
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.39 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808043961/at2700sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808043961/at2700Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Ni1—N3 | 2.0344 (18) |
| Ni1—N1 | 2.0418 (18) |
| Ni1—N4 | 2.0879 (17) |
| Ni1—N2 | 2.1188 (17) |
| Ni1—Cl1 | 2.3944 (6) |
| Ni1—Cl2 | 2.4153 (7) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯Cl2 | 0.82 | 2.27 | 2.9582 (18) | 142 |
| O1—H1⋯Cl1i | 0.82 | 2.91 | 3.4612 (16) | 127 |
| O1—H1⋯Cl1 | 0.82 | 2.37 | 3.0542 (16) | 141 |
Symmetry code: (i) 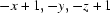 .
.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant No. 20731004), the Natural Science Foundation for Outstanding Scholars of Anhui Province, China (grant No. 044-J-04011) and the Natural Science Foundation of the Education Commission of Anhui Province, China (grant Nos. KJ2007B092 and KJ2008B004).
supplementary crystallographic information
Comment
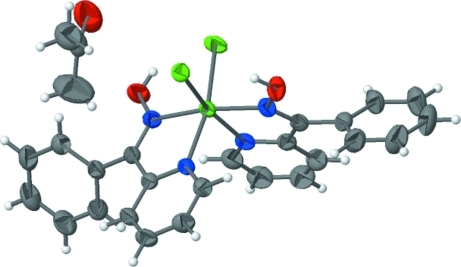
Pyridine-2-carbaldehyde oxime ligands usually bind to metals in a bidentate fashion, either chelating one metal center or bridging two metals. Their complexes find application in diverse areas such as functional supramolecular design, magnetic materials and catalysis (Korpi et al., 2005; Pearse et al., 1989; Afrati et al., 2005; Stamatatos et al., 2006). The title compound is a new nickel complex from the reaction of NiCl2 with phenyl-2-pyridyl ketone oxime (ppo). The compound consists of two N,N-chelating ligands and two chloride anion. The two ppo ligands are coordinated to Ni to form two five-membered NiC2N2 rings. The central Ni atom adopts a distorted octahedral geometry (Fig. 1), which are ligated by four N atoms from two different phenyl-2-pyridyl ketone oxime ligand and two Cl atoms. The bond distances Ni—N and Ni—Cl are in the expected ranges of 2.0344 (18)–2.1188 (17) and 2.3944 (6)–2.4153 (7) Å, respectively, and the coordination angles around Ni atom are in the range 76.84 (7)–170.18 (7)°, which are in agreement with the literature values (Papatriantafyllopoulou et al., 2007). In the crystal structure, intermolecular O—H···Cl hydrogen bonds link the molecules into one-dimensional chain structure (Table 2). There is a π–π contact between the pyridine rings, and the distance of centroid to centroid is 3.824 (5) Å.
Experimental
A colourless solution of phenyl-2-pyridyl ketone oxime (0.197 g, 1.00 mmol) in acetone (10 ml) was slowly added to a slurry of LiOH.H2O (0.042 g, 1.00 mmol) in MeOH (5 ml); the hydroxide soon dissolved. The solution was then added to a slurry of NiCl2.6H2O (0.297 g, 1.00 mmol) in MeOH (10 ml) and the resulting green solution was stirred for 1 h at room temperature. A small quantity of undissolved material was removed by filtration. The filtrate was allowed to stand undisturbed in a closed flask for a period of 4–5 d. Dark cyan crystals appeared which were collected by filtration, washed with cold MeOH (1 ml) and ice-cold Et2O (2 ml), and dried in air [Yield: 52%].
Refinement
All H atoms were placed in calculated positions, with O—H = 0.82 Å, Uiso(H) = 1.5Ueq(O), C—H = 0.93 Å, Uiso(H) = 1.2Ueq(C) for aromatic and C—H = 0.96 Å, Uiso(H) = 1.5Ueq(C) for CH3 atoms.
Figures
Fig. 1.
The molecular structure of the title compound, with atom labels and 30% probability displacement ellipsoids. The solvent molecule and H atoms have been omitted for clarity.
Fig. 2.
A packing diagram of the title compound. Hydrogen bonds are shown as dashed lines.
Crystal data
| [NiCl2(C12H10N2O)2]·C3H6O | Z = 2 |
| Mr = 584.13 | F(000) = 604 |
| Triclinic, P1 | Dx = 1.402 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.0367 (11) Å | Cell parameters from 3432 reflections |
| b = 12.9142 (16) Å | θ = 2.4–27.6° |
| c = 13.0664 (16) Å | µ = 0.93 mm−1 |
| α = 105.439 (1)° | T = 296 K |
| β = 92.232 (2)° | Block, dark cyan |
| γ = 108.183 (2)° | 0.22 × 0.18 × 0.16 mm |
| V = 1384.0 (3) Å3 |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 4761 independent reflections |
| Radiation source: sealed tube | 4002 reflections with I > 2σ(I) |
| graphite | Rint = 0.021 |
| φ and ω scans | θmax = 25.0°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −10→6 |
| Tmin = 0.822, Tmax = 0.866 | k = −10→15 |
| 6839 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.089 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0472P)2] where P = (Fo2 + 2Fc2)/3 |
| 4761 reflections | (Δ/σ)max < 0.001 |
| 338 parameters | Δρmax = 0.26 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. H atoms were positioned geometrically, with C—H = 0.93 and 0.96 Å for aromatic and methyl H atoms, respectively, and constrained to ride on their parent atoms, with Uiso(H) = xUeq(C), where x = 1.5 for methyl H and x = 1.2 for aromatic H atoms. The highest peak is located 1.10 Å from atom Cl1. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ni1 | 0.34978 (3) | 0.11803 (2) | 0.295182 (19) | 0.03252 (11) | |
| Cl1 | 0.43421 (6) | 0.11649 (4) | 0.47059 (4) | 0.03838 (15) | |
| Cl2 | 0.61388 (7) | 0.19661 (5) | 0.25662 (5) | 0.04710 (16) | |
| O1 | 0.3728 (2) | −0.11019 (13) | 0.29824 (12) | 0.0508 (4) | |
| H1 | 0.4028 | −0.0694 | 0.3602 | 0.076* | |
| O2 | 0.45576 (19) | 0.36884 (13) | 0.31744 (15) | 0.0519 (4) | |
| H2 | 0.5345 | 0.3509 | 0.3069 | 0.078* | |
| O3 | 0.8381 (3) | 0.7143 (2) | 0.2311 (2) | 0.0984 (8) | |
| N1 | 0.3351 (2) | −0.04856 (15) | 0.23669 (13) | 0.0373 (4) | |
| N2 | 0.2672 (2) | 0.07731 (16) | 0.13055 (14) | 0.0380 (4) | |
| N3 | 0.3367 (2) | 0.27794 (14) | 0.33148 (14) | 0.0367 (4) | |
| N4 | 0.1224 (2) | 0.09447 (14) | 0.33492 (13) | 0.0327 (4) | |
| C1 | 0.2320 (3) | 0.1443 (2) | 0.07928 (19) | 0.0488 (6) | |
| H1A | 0.2365 | 0.2169 | 0.1190 | 0.059* | |
| C2 | 0.1888 (3) | 0.1112 (2) | −0.0308 (2) | 0.0579 (7) | |
| H2A | 0.1652 | 0.1604 | −0.0643 | 0.070* | |
| C3 | 0.1820 (3) | 0.0046 (3) | −0.0884 (2) | 0.0629 (8) | |
| H3 | 0.1555 | −0.0193 | −0.1624 | 0.076* | |
| C4 | 0.2143 (3) | −0.0681 (2) | −0.03703 (18) | 0.0527 (6) | |
| H4 | 0.2073 | −0.1416 | −0.0756 | 0.063* | |
| C5 | 0.2575 (3) | −0.02919 (19) | 0.07348 (17) | 0.0395 (5) | |
| C6 | 0.2956 (2) | −0.10087 (18) | 0.13567 (16) | 0.0368 (5) | |
| C7 | 0.2878 (3) | −0.21931 (19) | 0.08362 (17) | 0.0416 (5) | |
| C8 | 0.1900 (3) | −0.3086 (2) | 0.1158 (2) | 0.0586 (7) | |
| H8 | 0.1293 | −0.2939 | 0.1701 | 0.070* | |
| C9 | 0.1835 (4) | −0.4189 (2) | 0.0668 (2) | 0.0736 (9) | |
| H9 | 0.1187 | −0.4784 | 0.0887 | 0.088* | |
| C10 | 0.2724 (4) | −0.4419 (2) | −0.0146 (2) | 0.0737 (9) | |
| H10 | 0.2667 | −0.5166 | −0.0476 | 0.088* | |
| C11 | 0.3686 (4) | −0.3545 (2) | −0.0462 (2) | 0.0672 (8) | |
| H11 | 0.4291 | −0.3699 | −0.1006 | 0.081* | |
| C12 | 0.3768 (3) | −0.2433 (2) | 0.00211 (18) | 0.0501 (6) | |
| H12 | 0.4424 | −0.1843 | −0.0202 | 0.060* | |
| C13 | 0.0213 (3) | 0.00010 (18) | 0.34823 (17) | 0.0396 (5) | |
| H13 | 0.0474 | −0.0661 | 0.3316 | 0.047* | |
| C14 | −0.1203 (3) | −0.0034 (2) | 0.38561 (19) | 0.0464 (6) | |
| H14 | −0.1876 | −0.0707 | 0.3942 | 0.056* | |
| C15 | −0.1606 (3) | 0.0934 (2) | 0.40997 (18) | 0.0463 (6) | |
| H15 | −0.2558 | 0.0926 | 0.4349 | 0.056* | |
| C16 | −0.0576 (3) | 0.1920 (2) | 0.39698 (17) | 0.0413 (5) | |
| H16 | −0.0826 | 0.2587 | 0.4129 | 0.050* | |
| C17 | 0.0833 (2) | 0.19055 (17) | 0.36005 (15) | 0.0322 (5) | |
| C18 | 0.2045 (2) | 0.29384 (17) | 0.34868 (16) | 0.0348 (5) | |
| C19 | 0.1721 (3) | 0.40061 (18) | 0.35581 (17) | 0.0387 (5) | |
| C20 | 0.0436 (3) | 0.3987 (2) | 0.2929 (2) | 0.0516 (6) | |
| H20 | −0.0242 | 0.3298 | 0.2481 | 0.062* | |
| C21 | 0.0160 (4) | 0.4993 (2) | 0.2966 (2) | 0.0644 (8) | |
| H21 | −0.0702 | 0.4977 | 0.2541 | 0.077* | |
| C22 | 0.1154 (4) | 0.6011 (2) | 0.3627 (2) | 0.0652 (8) | |
| H22 | 0.0971 | 0.6684 | 0.3641 | 0.078* | |
| C23 | 0.2412 (4) | 0.6044 (2) | 0.4266 (2) | 0.0614 (7) | |
| H23 | 0.3073 | 0.6736 | 0.4719 | 0.074* | |
| C24 | 0.2703 (3) | 0.50456 (19) | 0.42393 (19) | 0.0496 (6) | |
| H24 | 0.3557 | 0.5070 | 0.4678 | 0.060* | |
| C25 | 0.5679 (5) | 0.6853 (5) | 0.2243 (3) | 0.145 (2) | |
| H25A | 0.5795 | 0.7050 | 0.1586 | 0.217* | |
| H25B | 0.5336 | 0.7397 | 0.2742 | 0.217* | |
| H25C | 0.4915 | 0.6106 | 0.2105 | 0.217* | |
| C26 | 0.7191 (4) | 0.6861 (2) | 0.2698 (2) | 0.0583 (7) | |
| C27 | 0.7140 (4) | 0.6509 (3) | 0.3687 (3) | 0.0836 (10) | |
| H27A | 0.8184 | 0.6597 | 0.3968 | 0.125* | |
| H27B | 0.6489 | 0.5724 | 0.3527 | 0.125* | |
| H27C | 0.6714 | 0.6975 | 0.4208 | 0.125* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.03109 (17) | 0.04030 (18) | 0.02937 (16) | 0.01737 (13) | 0.00755 (12) | 0.00847 (12) |
| Cl1 | 0.0403 (3) | 0.0461 (3) | 0.0298 (3) | 0.0176 (2) | 0.0057 (2) | 0.0091 (2) |
| Cl2 | 0.0343 (3) | 0.0692 (4) | 0.0458 (3) | 0.0239 (3) | 0.0149 (3) | 0.0207 (3) |
| O1 | 0.0746 (13) | 0.0499 (10) | 0.0325 (8) | 0.0323 (9) | −0.0012 (8) | 0.0069 (7) |
| O2 | 0.0356 (10) | 0.0444 (9) | 0.0774 (12) | 0.0109 (8) | 0.0200 (9) | 0.0219 (8) |
| O3 | 0.0952 (19) | 0.0966 (17) | 0.1112 (19) | 0.0373 (14) | 0.0510 (16) | 0.0313 (14) |
| N1 | 0.0432 (11) | 0.0461 (10) | 0.0289 (10) | 0.0238 (9) | 0.0058 (8) | 0.0106 (8) |
| N2 | 0.0323 (10) | 0.0534 (11) | 0.0329 (10) | 0.0193 (9) | 0.0071 (8) | 0.0139 (8) |
| N3 | 0.0315 (10) | 0.0380 (10) | 0.0407 (10) | 0.0110 (8) | 0.0092 (8) | 0.0119 (8) |
| N4 | 0.0311 (10) | 0.0380 (10) | 0.0303 (9) | 0.0151 (8) | 0.0043 (7) | 0.0081 (7) |
| C1 | 0.0470 (15) | 0.0607 (15) | 0.0455 (14) | 0.0250 (12) | 0.0089 (11) | 0.0179 (12) |
| C2 | 0.0610 (18) | 0.0815 (19) | 0.0443 (15) | 0.0328 (15) | 0.0056 (13) | 0.0294 (14) |
| C3 | 0.0678 (19) | 0.093 (2) | 0.0307 (13) | 0.0324 (16) | 0.0005 (12) | 0.0163 (14) |
| C4 | 0.0563 (17) | 0.0718 (17) | 0.0315 (12) | 0.0300 (14) | 0.0017 (11) | 0.0081 (12) |
| C5 | 0.0318 (12) | 0.0545 (14) | 0.0316 (12) | 0.0171 (11) | 0.0052 (9) | 0.0085 (10) |
| C6 | 0.0321 (12) | 0.0482 (13) | 0.0306 (11) | 0.0163 (10) | 0.0076 (9) | 0.0081 (10) |
| C7 | 0.0412 (14) | 0.0467 (13) | 0.0305 (12) | 0.0147 (11) | −0.0003 (10) | 0.0016 (10) |
| C8 | 0.0563 (17) | 0.0578 (17) | 0.0501 (15) | 0.0099 (13) | 0.0131 (13) | 0.0068 (12) |
| C9 | 0.084 (2) | 0.0482 (17) | 0.069 (2) | 0.0041 (15) | 0.0085 (17) | 0.0073 (14) |
| C10 | 0.109 (3) | 0.0476 (16) | 0.0538 (17) | 0.0278 (17) | 0.0028 (17) | −0.0041 (13) |
| C11 | 0.094 (2) | 0.0696 (19) | 0.0435 (15) | 0.0437 (18) | 0.0186 (15) | 0.0057 (13) |
| C12 | 0.0602 (17) | 0.0531 (15) | 0.0385 (13) | 0.0252 (13) | 0.0129 (12) | 0.0076 (11) |
| C13 | 0.0404 (13) | 0.0410 (12) | 0.0396 (12) | 0.0156 (11) | 0.0067 (10) | 0.0130 (10) |
| C14 | 0.0387 (14) | 0.0510 (14) | 0.0494 (14) | 0.0098 (11) | 0.0110 (11) | 0.0198 (11) |
| C15 | 0.0319 (13) | 0.0618 (15) | 0.0475 (14) | 0.0174 (11) | 0.0137 (11) | 0.0165 (12) |
| C16 | 0.0348 (13) | 0.0499 (14) | 0.0420 (13) | 0.0215 (11) | 0.0093 (10) | 0.0088 (10) |
| C17 | 0.0311 (12) | 0.0379 (11) | 0.0268 (10) | 0.0131 (9) | 0.0026 (9) | 0.0064 (8) |
| C18 | 0.0328 (12) | 0.0382 (11) | 0.0319 (11) | 0.0141 (10) | 0.0039 (9) | 0.0053 (9) |
| C19 | 0.0379 (13) | 0.0387 (12) | 0.0421 (12) | 0.0174 (10) | 0.0123 (10) | 0.0098 (10) |
| C20 | 0.0562 (16) | 0.0506 (14) | 0.0491 (14) | 0.0266 (13) | −0.0020 (12) | 0.0073 (11) |
| C21 | 0.077 (2) | 0.0723 (19) | 0.0619 (18) | 0.0486 (17) | 0.0068 (15) | 0.0216 (15) |
| C22 | 0.086 (2) | 0.0531 (17) | 0.0743 (19) | 0.0423 (16) | 0.0262 (17) | 0.0230 (15) |
| C23 | 0.0629 (19) | 0.0396 (14) | 0.0752 (19) | 0.0170 (13) | 0.0205 (15) | 0.0050 (13) |
| C24 | 0.0458 (15) | 0.0444 (14) | 0.0553 (15) | 0.0162 (11) | 0.0096 (12) | 0.0077 (11) |
| C25 | 0.119 (4) | 0.261 (6) | 0.089 (3) | 0.113 (4) | 0.001 (3) | 0.051 (3) |
| C26 | 0.0649 (19) | 0.0529 (16) | 0.0536 (16) | 0.0272 (14) | 0.0089 (15) | 0.0010 (12) |
| C27 | 0.090 (3) | 0.101 (2) | 0.073 (2) | 0.049 (2) | 0.0144 (18) | 0.0263 (18) |
Geometric parameters (Å, °)
| Ni1—N3 | 2.0344 (18) | C10—H10 | 0.9300 |
| Ni1—N1 | 2.0418 (18) | C11—C12 | 1.384 (3) |
| Ni1—N4 | 2.0879 (17) | C11—H11 | 0.9300 |
| Ni1—N2 | 2.1188 (17) | C12—H12 | 0.9300 |
| Ni1—Cl1 | 2.3944 (6) | C13—C14 | 1.379 (3) |
| Ni1—Cl2 | 2.4153 (7) | C13—H13 | 0.9300 |
| O1—N1 | 1.373 (2) | C14—C15 | 1.370 (3) |
| O1—H1 | 0.8200 | C14—H14 | 0.9300 |
| O2—N3 | 1.383 (2) | C15—C16 | 1.381 (3) |
| O2—H2 | 0.8200 | C15—H15 | 0.9300 |
| O3—C26 | 1.200 (3) | C16—C17 | 1.382 (3) |
| N1—C6 | 1.290 (3) | C16—H16 | 0.9300 |
| N2—C1 | 1.328 (3) | C17—C18 | 1.486 (3) |
| N2—C5 | 1.352 (3) | C18—C19 | 1.477 (3) |
| N3—C18 | 1.292 (3) | C19—C20 | 1.386 (3) |
| N4—C13 | 1.335 (3) | C19—C24 | 1.393 (3) |
| N4—C17 | 1.356 (3) | C20—C21 | 1.386 (3) |
| C1—C2 | 1.389 (3) | C20—H20 | 0.9300 |
| C1—H1A | 0.9300 | C21—C22 | 1.371 (4) |
| C2—C3 | 1.363 (4) | C21—H21 | 0.9300 |
| C2—H2A | 0.9300 | C22—C23 | 1.367 (4) |
| C3—C4 | 1.381 (4) | C22—H22 | 0.9300 |
| C3—H3 | 0.9300 | C23—C24 | 1.386 (3) |
| C4—C5 | 1.392 (3) | C23—H23 | 0.9300 |
| C4—H4 | 0.9300 | C24—H24 | 0.9300 |
| C5—C6 | 1.488 (3) | C25—C26 | 1.464 (5) |
| C6—C7 | 1.477 (3) | C25—H25A | 0.9600 |
| C7—C12 | 1.387 (3) | C25—H25B | 0.9600 |
| C7—C8 | 1.392 (3) | C25—H25C | 0.9600 |
| C8—C9 | 1.380 (4) | C26—C27 | 1.477 (4) |
| C8—H8 | 0.9300 | C27—H27A | 0.9600 |
| C9—C10 | 1.382 (4) | C27—H27B | 0.9600 |
| C9—H9 | 0.9300 | C27—H27C | 0.9600 |
| C10—C11 | 1.365 (4) | ||
| N3—Ni1—N1 | 170.18 (7) | C9—C10—H10 | 120.1 |
| N3—Ni1—N4 | 76.84 (7) | C10—C11—C12 | 120.4 (3) |
| N1—Ni1—N4 | 99.25 (7) | C10—C11—H11 | 119.8 |
| N3—Ni1—N2 | 94.03 (7) | C12—C11—H11 | 119.8 |
| N1—Ni1—N2 | 76.92 (7) | C11—C12—C7 | 120.3 (2) |
| N4—Ni1—N2 | 91.16 (7) | C11—C12—H12 | 119.9 |
| N3—Ni1—Cl1 | 99.43 (5) | C7—C12—H12 | 119.9 |
| N1—Ni1—Cl1 | 89.47 (5) | N4—C13—C14 | 122.8 (2) |
| N4—Ni1—Cl1 | 89.49 (5) | N4—C13—H13 | 118.6 |
| N2—Ni1—Cl1 | 166.31 (5) | C14—C13—H13 | 118.6 |
| N3—Ni1—Cl2 | 87.94 (5) | C15—C14—C13 | 119.2 (2) |
| N1—Ni1—Cl2 | 95.62 (6) | C15—C14—H14 | 120.4 |
| N4—Ni1—Cl2 | 164.76 (5) | C13—C14—H14 | 120.4 |
| N2—Ni1—Cl2 | 88.97 (5) | C14—C15—C16 | 119.0 (2) |
| Cl1—Ni1—Cl2 | 93.97 (2) | C14—C15—H15 | 120.5 |
| N1—O1—H1 | 109.5 | C16—C15—H15 | 120.5 |
| N3—O2—H2 | 109.5 | C15—C16—C17 | 119.2 (2) |
| C6—N1—O1 | 115.73 (17) | C15—C16—H16 | 120.4 |
| C6—N1—Ni1 | 120.35 (15) | C17—C16—H16 | 120.4 |
| O1—N1—Ni1 | 123.85 (12) | N4—C17—C16 | 121.86 (19) |
| C1—N2—C5 | 118.66 (19) | N4—C17—C18 | 115.20 (18) |
| C1—N2—Ni1 | 127.91 (16) | C16—C17—C18 | 122.9 (2) |
| C5—N2—Ni1 | 113.37 (14) | N3—C18—C19 | 125.41 (19) |
| C18—N3—O2 | 116.43 (18) | N3—C18—C17 | 112.33 (19) |
| C18—N3—Ni1 | 120.22 (14) | C19—C18—C17 | 122.25 (18) |
| O2—N3—Ni1 | 122.03 (13) | C20—C19—C24 | 118.9 (2) |
| C13—N4—C17 | 117.97 (18) | C20—C19—C18 | 120.0 (2) |
| C13—N4—Ni1 | 127.23 (15) | C24—C19—C18 | 121.1 (2) |
| C17—N4—Ni1 | 114.43 (13) | C19—C20—C21 | 120.2 (2) |
| N2—C1—C2 | 123.1 (2) | C19—C20—H20 | 119.9 |
| N2—C1—H1A | 118.4 | C21—C20—H20 | 119.9 |
| C2—C1—H1A | 118.4 | C22—C21—C20 | 120.2 (3) |
| C3—C2—C1 | 118.1 (2) | C22—C21—H21 | 119.9 |
| C3—C2—H2A | 121.0 | C20—C21—H21 | 119.9 |
| C1—C2—H2A | 121.0 | C23—C22—C21 | 120.4 (3) |
| C2—C3—C4 | 120.1 (2) | C23—C22—H22 | 119.8 |
| C2—C3—H3 | 119.9 | C21—C22—H22 | 119.8 |
| C4—C3—H3 | 119.9 | C22—C23—C24 | 120.1 (3) |
| C3—C4—C5 | 118.7 (2) | C22—C23—H23 | 119.9 |
| C3—C4—H4 | 120.6 | C24—C23—H23 | 119.9 |
| C5—C4—H4 | 120.6 | C23—C24—C19 | 120.2 (2) |
| N2—C5—C4 | 121.2 (2) | C23—C24—H24 | 119.9 |
| N2—C5—C6 | 116.14 (18) | C19—C24—H24 | 119.9 |
| C4—C5—C6 | 122.6 (2) | C26—C25—H25A | 109.5 |
| N1—C6—C7 | 125.2 (2) | C26—C25—H25B | 109.5 |
| N1—C6—C5 | 113.03 (19) | H25A—C25—H25B | 109.5 |
| C7—C6—C5 | 121.78 (18) | C26—C25—H25C | 109.5 |
| C12—C7—C8 | 119.1 (2) | H25A—C25—H25C | 109.5 |
| C12—C7—C6 | 120.7 (2) | H25B—C25—H25C | 109.5 |
| C8—C7—C6 | 120.2 (2) | O3—C26—C25 | 123.1 (3) |
| C9—C8—C7 | 119.8 (3) | O3—C26—C27 | 122.5 (3) |
| C9—C8—H8 | 120.1 | C25—C26—C27 | 114.4 (3) |
| C7—C8—H8 | 120.1 | C26—C27—H27A | 109.5 |
| C8—C9—C10 | 120.7 (3) | C26—C27—H27B | 109.5 |
| C8—C9—H9 | 119.7 | H27A—C27—H27B | 109.5 |
| C10—C9—H9 | 119.7 | C26—C27—H27C | 109.5 |
| C11—C10—C9 | 119.7 (3) | H27A—C27—H27C | 109.5 |
| C11—C10—H10 | 120.1 | H27B—C27—H27C | 109.5 |
| N3—Ni1—N1—C6 | 26.9 (5) | O1—N1—C6—C7 | 0.3 (3) |
| N4—Ni1—N1—C6 | 92.66 (17) | Ni1—N1—C6—C7 | 177.24 (16) |
| N2—Ni1—N1—C6 | 3.62 (17) | O1—N1—C6—C5 | −179.56 (17) |
| Cl1—Ni1—N1—C6 | −177.94 (17) | Ni1—N1—C6—C5 | −2.7 (3) |
| Cl2—Ni1—N1—C6 | −84.00 (17) | N2—C5—C6—N1 | −0.9 (3) |
| N3—Ni1—N1—O1 | −156.4 (4) | C4—C5—C6—N1 | 179.0 (2) |
| N4—Ni1—N1—O1 | −90.70 (17) | N2—C5—C6—C7 | 179.2 (2) |
| N2—Ni1—N1—O1 | −179.74 (18) | C4—C5—C6—C7 | −0.9 (3) |
| Cl1—Ni1—N1—O1 | −1.30 (16) | N1—C6—C7—C12 | −121.8 (3) |
| Cl2—Ni1—N1—O1 | 92.64 (16) | C5—C6—C7—C12 | 58.1 (3) |
| N3—Ni1—N2—C1 | 3.12 (19) | N1—C6—C7—C8 | 58.4 (3) |
| N1—Ni1—N2—C1 | 179.2 (2) | C5—C6—C7—C8 | −121.7 (2) |
| N4—Ni1—N2—C1 | 80.01 (19) | C12—C7—C8—C9 | 0.2 (4) |
| Cl1—Ni1—N2—C1 | 172.62 (17) | C6—C7—C8—C9 | −179.9 (2) |
| Cl2—Ni1—N2—C1 | −84.74 (18) | C7—C8—C9—C10 | −0.4 (5) |
| N3—Ni1—N2—C5 | −179.93 (15) | C8—C9—C10—C11 | 0.6 (5) |
| N1—Ni1—N2—C5 | −3.81 (14) | C9—C10—C11—C12 | −0.5 (5) |
| N4—Ni1—N2—C5 | −103.04 (15) | C10—C11—C12—C7 | 0.3 (4) |
| Cl1—Ni1—N2—C5 | −10.4 (3) | C8—C7—C12—C11 | −0.2 (4) |
| Cl2—Ni1—N2—C5 | 92.21 (14) | C6—C7—C12—C11 | −180.0 (2) |
| N1—Ni1—N3—C18 | 59.8 (5) | C17—N4—C13—C14 | 0.4 (3) |
| N4—Ni1—N3—C18 | −7.67 (16) | Ni1—N4—C13—C14 | 172.90 (16) |
| N2—Ni1—N3—C18 | 82.58 (17) | N4—C13—C14—C15 | 0.3 (3) |
| Cl1—Ni1—N3—C18 | −94.92 (16) | C13—C14—C15—C16 | −0.4 (3) |
| Cl2—Ni1—N3—C18 | 171.40 (16) | C14—C15—C16—C17 | −0.1 (3) |
| N1—Ni1—N3—O2 | −106.6 (4) | C13—N4—C17—C16 | −0.9 (3) |
| N4—Ni1—N3—O2 | −174.12 (17) | Ni1—N4—C17—C16 | −174.40 (16) |
| N2—Ni1—N3—O2 | −83.87 (16) | C13—N4—C17—C18 | 177.00 (17) |
| Cl1—Ni1—N3—O2 | 98.64 (15) | Ni1—N4—C17—C18 | 3.5 (2) |
| Cl2—Ni1—N3—O2 | 4.95 (15) | C15—C16—C17—N4 | 0.8 (3) |
| N3—Ni1—N4—C13 | −171.15 (18) | C15—C16—C17—C18 | −176.94 (19) |
| N1—Ni1—N4—C13 | 18.03 (18) | O2—N3—C18—C19 | −0.9 (3) |
| N2—Ni1—N4—C13 | 94.97 (17) | Ni1—N3—C18—C19 | −168.04 (16) |
| Cl1—Ni1—N4—C13 | −71.35 (16) | O2—N3—C18—C17 | 178.61 (16) |
| Cl2—Ni1—N4—C13 | −174.69 (13) | Ni1—N3—C18—C17 | 11.4 (2) |
| N3—Ni1—N4—C17 | 1.61 (13) | N4—C17—C18—N3 | −9.5 (3) |
| N1—Ni1—N4—C17 | −169.20 (13) | C16—C17—C18—N3 | 168.4 (2) |
| N2—Ni1—N4—C17 | −92.26 (14) | N4—C17—C18—C19 | 170.02 (18) |
| Cl1—Ni1—N4—C17 | 101.42 (13) | C16—C17—C18—C19 | −12.1 (3) |
| Cl2—Ni1—N4—C17 | −1.9 (3) | N3—C18—C19—C20 | 127.1 (3) |
| C5—N2—C1—C2 | −1.4 (3) | C17—C18—C19—C20 | −52.4 (3) |
| Ni1—N2—C1—C2 | 175.42 (19) | N3—C18—C19—C24 | −51.9 (3) |
| N2—C1—C2—C3 | 0.2 (4) | C17—C18—C19—C24 | 128.6 (2) |
| C1—C2—C3—C4 | 1.3 (4) | C24—C19—C20—C21 | 1.3 (4) |
| C2—C3—C4—C5 | −1.6 (4) | C18—C19—C20—C21 | −177.7 (2) |
| C1—N2—C5—C4 | 1.1 (3) | C19—C20—C21—C22 | −0.2 (4) |
| Ni1—N2—C5—C4 | −176.19 (18) | C20—C21—C22—C23 | −0.9 (4) |
| C1—N2—C5—C6 | −178.99 (19) | C21—C22—C23—C24 | 0.8 (4) |
| Ni1—N2—C5—C6 | 3.7 (2) | C22—C23—C24—C19 | 0.3 (4) |
| C3—C4—C5—N2 | 0.4 (4) | C20—C19—C24—C23 | −1.4 (4) |
| C3—C4—C5—C6 | −179.5 (2) | C18—C19—C24—C23 | 177.6 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···Cl2 | 0.82 | 2.27 | 2.9582 (18) | 142 |
| O1—H1···Cl1i | 0.82 | 2.91 | 3.4612 (16) | 127 |
| O1—H1···Cl1 | 0.82 | 2.37 | 3.0542 (16) | 141 |
Symmetry codes: (i) −x+1, −y, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AT2700).
References
- Afrati, T., Dendrinou-Samara, C., Zaleski, C. M., Kampf, J. W., Pecoraro, V. L. & Kessissoglou, D. P. (2005). Inorg. Chem. Commun.8, 1173–1176.
- Bruker (2000). SADABS, SAINT and SMART Bruker AXS Inc., Madison, Wisconsin, USA.
- Korpi, H., Polamo, M., Leskela, M. & Repo, T. (2005). Inorg. Chem. Commun.8, 1181–1184.
- Papatriantafyllopoulou, C., Aromi, G., Tasiopoulos, A. J., Nastopoulos, V., Raptopoulou, C. P., Teat, S. J., Escuer, A. & Perlepes, S. P. (2007). Eur. J. Inorg. Chem. pp. 2761–2774.
- Pearse, G. A., Raithby, P. R. & Lewis, J. (1989). Polyhedron, 8, 301–304.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stamatatos, T. C., Vlahopoulou, J. C., Sanakis, Y., Raptopoulou, C. P., Psycharis, V., Boudalis, A. K. & Perlepes, S. P. (2006). Inorg. Chem. Commun.9, 814–818.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808043961/at2700sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808043961/at2700Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report