Abstract
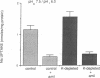
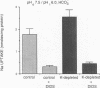
Most HCO3- reabsorption in proximal tubules occurs via electroneutral Na+/H+ exchange in brush border membranes (BBMS) and electrogenic Na+:CO3=:HCO3- cotransport in basolateral membranes (BLMS). Since potassium depletion (KD) increases HCO3- reabsorption in proximal tubules, we evaluated these transport systems using BBM and BLM vesicles, respectively, from control (C) and KD rats. Feeding rats a potassium deficient diet for 3-4 wk resulted in lower plasma [K+] (2.94 mEq/liter, KD vs. 4.47 C), and higher arterial pH (7.51 KD vs. 7.39 C). KD rats gained less weight than C but had higher renal cortical weight. Influx of 1 mM 22Na+ at 5 s (pHo 7.5, pHi 6.0, 10% CO2, 90% N2) into BLM vesicles was 44% higher in the KD group compared to C with no difference in equilibrium uptake. The increment in Na+ influx in the KD group was DIDS sensitive, suggesting that Na+:CO3=:HCO3- cotransport accounted for the observed differences. Kinetic analysis of Na+ influx showed a Km of 8.2 mM in KD vs. 7.6 mM in C and Vmax of 278 nmol/min/mg protein in KD vs. 177 nmol/min/mg protein in C. Influx of 1 mM 22Na+ at 5 s (pHo 7.5, pHi 6.0) into BBM vesicles was 34% higher in the KD group compared to C with no difference in equilibrium uptake. The increment in Na+ influx in the KD group was amiloride sensitive, suggesting that Na+/H+ exchange was responsible for the observed differences. Kinetic analysis of Na+ influx showed a Km of 6.2 mM in KD vs. 7.1 mM in C and Vmax of 209 nmol/min/mg protein in KD vs. 144 nmol/min/mg protein in C. Uptakes of Na(+)-dependent [3H]glucose into BBM and [14C]succinate into BLM vesicles were not different in KD and C groups, suggesting that the Na+/H+ exchanger and Na+:CO3=:HCO3- cotransporter activities were specifically altered in KD. We conclude that adaptive increases in basolateral Na+:CO3=:HCO3- cotransport and luminal Na+H+ exchange are likely responsible for increased HCO3- reabsorption in proximal tubules of KD animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W. R., Koretsky A. P., Weiner M. W. 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol. 1986 Nov;251(5 Pt 2):F904–F910. doi: 10.1152/ajprenal.1986.251.5.F904. [DOI] [PubMed] [Google Scholar]
- Akiba T., Alpern R. J., Eveloff J., Calamina J., Warnock D. G. Electrogenic sodium/bicarbonate cotransport in rabbit renal cortical basolateral membrane vesicles. J Clin Invest. 1986 Dec;78(6):1472–1478. doi: 10.1172/JCI112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba T., Rocco V. K., Warnock D. G. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987 Aug;80(2):308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest. 1986 Aug;78(2):502–510. doi: 10.1172/JCI112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Sacktor B. Transport of D-glucose by brush border membranes isolated from the renal cortex. Biochim Biophys Acta. 1974 Jul 31;356(2):231–243. doi: 10.1016/0005-2736(74)90286-7. [DOI] [PubMed] [Google Scholar]
- BROCH O. J. Low potassium alkalosis with acid urine in ulcerative colitis. Scand J Clin Lab Invest. 1950;2(2):113–119. doi: 10.3109/00365515009051845. [DOI] [PubMed] [Google Scholar]
- Biagi B. A., Sohtell M. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F267–F272. doi: 10.1152/ajprenal.1986.250.2.F267. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. M., Teubner E. J., Simpson D. P. Metabolic acidosis accompanying potassium deprivation. Am J Physiol. 1974 Aug;227(2):329–333. doi: 10.1152/ajplegacy.1974.227.2.329. [DOI] [PubMed] [Google Scholar]
- COOKE R. E., SEGAR W. E., CHEEK D. B., COVILLE F. E., DARROW D. C. The extrarenal correction of alkalosis associated with potassium deficiency. J Clin Invest. 1952 Aug;31(8):798–805. doi: 10.1172/JCI102665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G., Jaeger P., Giebisch G., Guckian V., Malnic G. Renal bicarbonate reabsorption in the rat. II. Distal tubule load dependence and effect of hypokalemia. J Clin Invest. 1987 Aug;80(2):409–414. doi: 10.1172/JCI113087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemerikić D., Wilcox C. S., Giebisch G. Intracellular potential and K+ activity in rat kidney proximal tubular cells in acidosis and K+ depletion. J Membr Biol. 1982;69(2):159–165. doi: 10.1007/BF01872275. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Biagi B., Giebisch G. Control mechanisms of bicarbonate transport across the rat proximal convoluted tubule. Am J Physiol. 1982 May;242(5):F532–F543. doi: 10.1152/ajprenal.1982.242.5.F532. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Liu F. Y. Metabolic alkalosis in the rat. Evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest. 1983 May;71(5):1141–1160. doi: 10.1172/JCI110864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers C., Haase W., Murer H., Kinne R. Properties of brush border vesicles isolated from rat kidney cortex by calcium precipitation. Membr Biochem. 1978;1(3-4):203–219. doi: 10.3109/09687687809063848. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Sterzel R. B., Boron W. F. Arginine vasopressin enhances pHi regulation in the presence of HCO3- by stimulating three acid-base transport systems. Nature. 1989 Feb 16;337(6208):648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- Garella S., Chang B., Kahn S. I. Alterations of hydrogen ion homeostasis in pure potassium depletion: studies in rats and dogs during the recovery phase. J Lab Clin Med. 1979 Feb;93(2):321–331. [PubMed] [Google Scholar]
- Grassl S. M., Aronson P. S. Na+/HCO3-co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem. 1986 Jul 5;261(19):8778–8783. [PubMed] [Google Scholar]
- Hernandez R. E., Schambelan M., Cogan M. G., Colman J., Morris R. C., Jr, Sebastian A. Dietary NaCl determines severity of potassium depletion-induced metabolic alkalosis. Kidney Int. 1987 Jun;31(6):1356–1367. doi: 10.1038/ki.1987.150. [DOI] [PubMed] [Google Scholar]
- Ives H. E. Proton/hydroxyl permeability of proximal tubule brush border vesicles. Am J Physiol. 1985 Jan;248(1 Pt 2):F78–F86. doi: 10.1152/ajprenal.1985.248.1.F78. [DOI] [PubMed] [Google Scholar]
- Jones B., Simpson D. P. Influence of alterations in acid-base conditions on intracellular pH of intact renal cortex. Ren Physiol. 1983;6(1):28–35. doi: 10.1159/000172878. [DOI] [PubMed] [Google Scholar]
- Jones J. W., Sebastian A., Hulter H. N., Schambelan M., Sutton J. M., Biglieri E. G. Systemic and renal acid-base effects of chronic dietary potassium depletion in humans. Kidney Int. 1982 Feb;21(2):402–410. doi: 10.1038/ki.1982.36. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- Kunau R. T., Jr, Frick A., Rector F. C., Jr, Seldin D. W. Micropuncture study of the proximal tubular factors responsible for the maintenance of alkalosis during potassium deficiency in the rat. Clin Sci. 1968 Apr;34(2):223–231. [PubMed] [Google Scholar]
- Levine D. Z., Walker T., Nash L. A. Effects of KCl infusions on proximal tubular function in normal and potassium-depleted rats. Kidney Int. 1973 Nov;4(5):318–325. doi: 10.1038/ki.1973.123. [DOI] [PubMed] [Google Scholar]
- Luke R. G., Levitin H. Impaired renal conservation of chloride and the acid-base changes associated with potassium depletion in the rat. Clin Sci. 1967 Jun;32(3):511–526. [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. Interrelationships among quinidine, amiloride, and lithium as inhibitors of the renal Na+-H+ exchanger. J Biol Chem. 1985 Oct 15;260(23):12586–12592. [PubMed] [Google Scholar]
- McKinney T. D., Davidson K. K. Effect of potassium depletion and protein intake in vivo on renal tubular bicarbonate transport in vitro. Am J Physiol. 1987 Mar;252(3 Pt 2):F509–F516. doi: 10.1152/ajprenal.1987.252.3.F509. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Davidson K. K. Effect of potassium depletion and protein intake in vivo on renal tubular bicarbonate transport in vitro. Am J Physiol. 1987 Mar;252(3 Pt 2):F509–F516. doi: 10.1152/ajprenal.1987.252.3.F509. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Kunnemann M. E. Procainamide transport in rabbit renal cortical brush border membrane vesicles. Am J Physiol. 1985 Oct;249(4 Pt 2):F532–F541. doi: 10.1152/ajprenal.1985.249.4.F532. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, BLOOMER H. A., SELDIN D. W. EFFECT OF POTASSIUM DEFICIENCY ON THE REABSORPTION OF BICARBONATE IN THE PROXIMAL TUBULE OF THE RAT KIDNEY. J Clin Invest. 1964 Oct;43:1976–1982. doi: 10.1172/JCI105071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra W. W., Warnock D. G., Yee V. J., Forte J. G. Proton gradients in renal cortex brush-border membrane vesicles. Demonstration of a rheogenic proton flux with acridine orange. J Biol Chem. 1981 Nov 25;256(22):11663–11666. [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Seldin D. W., Rector F. C., Jr Symposium on acid-base homeostasis. The generation and maintenance of metabolic alkalosis. Kidney Int. 1972 May;1(5):306–321. doi: 10.1038/ki.1972.43. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Aronson P. S. Effects of acetazolamide on Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1989 Mar;83(3):945–951. doi: 10.1172/JCI113980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M., Aronson P. S. Ionic mechanism of Na+-HCO3- cotransport in rabbit renal basolateral membrane vesicles. J Biol Chem. 1989 Nov 5;264(31):18302–18308. [PubMed] [Google Scholar]
- Soleimani M., Grassi S. M., Aronson P. S. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987 Apr;79(4):1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Slyke K. K., Evans E. I. The Paradox of Aciduria in the Presence of Alkalosis Caused by Hypochloremia. Ann Surg. 1947 Oct;126(4):545–567. doi: 10.1097/00000658-194710000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh-Reitz M. M., Toback F. G. Kidney epithelial cell growth is stimulated by lowering extracellular potassium concentration. Am J Physiol. 1983 May;244(5):C429–C432. doi: 10.1152/ajpcell.1983.244.5.C429. [DOI] [PubMed] [Google Scholar]
- Whinnery M. A., Kunau R. T., Jr Effect of potassium deficiency on papillary plasma flow in the rat. Am J Physiol. 1979 Sep;237(3):F226–F231. doi: 10.1152/ajprenal.1979.237.3.F226. [DOI] [PubMed] [Google Scholar]
- Wright S. H., Wunz T. M. Succinate and citrate transport in renal basolateral and brush-border membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F432–F439. doi: 10.1152/ajprenal.1987.253.3.F432. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]