Abstract
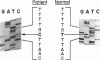
Preproparathyroid hormone (preproPTH) gene mutation has been proposed as a cause of familial isolated hypoparathyroidism (FIH). We cloned the preproPTH alleles of a patient with autosomal dominant FIH and sequenced the coding regions, 5' flanking regions, and splice junctions. The putatively abnormal (based on previous linkage studies) allele differed from the other allele's normal sequence at only one nucleotide. This T to C point mutation changes the codon for position 18 of the 31 amino acid prepro sequence from cysteine to arginine, disrupting the hydrophobic core of the signal sequence. Because the hydrophobic core is required by secreted proteins for efficient translocation across the endoplasmic reticulum, the mutant protein is likely to be inefficiently processed. Indeed, in vitro studies demonstrated dramatically impaired processing of the mutant preproPTH to proPTH. In summary, we observed a point mutation in the signal peptide-encoding region of a preproPTH gene in one FIH kindred and demonstrated a functional defect caused by the mutation. Mutation of the signal sequence constitutes a novel pathophysiologic mechanism in man, and further study may yield important insights both into this form of hormone deficiency and into the role of signal sequences in human physiology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn T. G., Antonarakis S. E., Kronenberg H. M., Igarashi T., Levine M. A. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore) 1986 Mar;65(2):73–81. [PubMed] [Google Scholar]
- Arnold A., Kim H. G., Gaz R. D., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Kronenberg H. M. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest. 1989 Jun;83(6):2034–2040. doi: 10.1172/JCI114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi J. A., Allen K. L., Lively M. O., Kemper B. Parallel effects of signal peptide hydrophobic core modifications on co-translational translocation and post-translational cleavage by purified signal peptidase. J Biol Chem. 1989 Sep 5;264(25):15052–15058. [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. W., Wiren K. M., Rapoport A., Lazar M., Potts J. T., Jr, Kronenberg H. M. Consequences of amino-terminal deletions of preproparathyroid hormone signal sequence. Mol Endocrinol. 1987 Sep;1(9):628–638. doi: 10.1210/mend-1-9-628. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hendy G. N., Kronenberg H. M., Potts J. T., Jr, Rich A. Nucleotide sequence of cloned cDNAs encoding human preproparathyroid hormone. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7365–7369. doi: 10.1073/pnas.78.12.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Botstein D. Secretion-defective mutations in the signal sequence for Saccharomyces cerevisiae invertase. Mol Cell Biol. 1986 Jul;6(7):2382–2391. doi: 10.1128/mcb.6.7.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. New bacteriophage lambda vectors with positive selection for cloned inserts. Methods Enzymol. 1983;101:3–19. doi: 10.1016/0076-6879(83)01004-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Rubenstein A. H., Tager H. S. Familial hyperproinsulinemia. Two cohorts secreting indistinguishable type II intermediates of proinsulin conversion. J Clin Invest. 1984 Mar;73(3):714–719. doi: 10.1172/JCI111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasicek T. J., McDevitt B. E., Freeman M. W., Fennick B. J., Hendy G. N., Potts J. T., Jr, Rich A., Kronenberg H. M. Nucleotide sequence of the human parathyroid hormone gene. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2127–2131. doi: 10.1073/pnas.80.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]
- Whyte M. P., Weldon V. V. Idiopathic hypoparathyroidism presenting with seizures during infancy: X-linked recessive inheritance in a large Missouri kindred. J Pediatr. 1981 Oct;99(4):608–611. doi: 10.1016/s0022-3476(81)80272-7. [DOI] [PubMed] [Google Scholar]
- Winter W. E., Silverstein J. H., Maclaren N. K., Riley W. J., Chiaro J. J. Autosomal dominant hypoparathyroidism with variable, age-dependent severity. J Pediatr. 1983 Sep;103(3):387–390. doi: 10.1016/s0022-3476(83)80408-9. [DOI] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]