Abstract
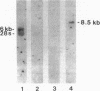
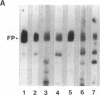
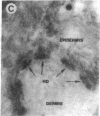
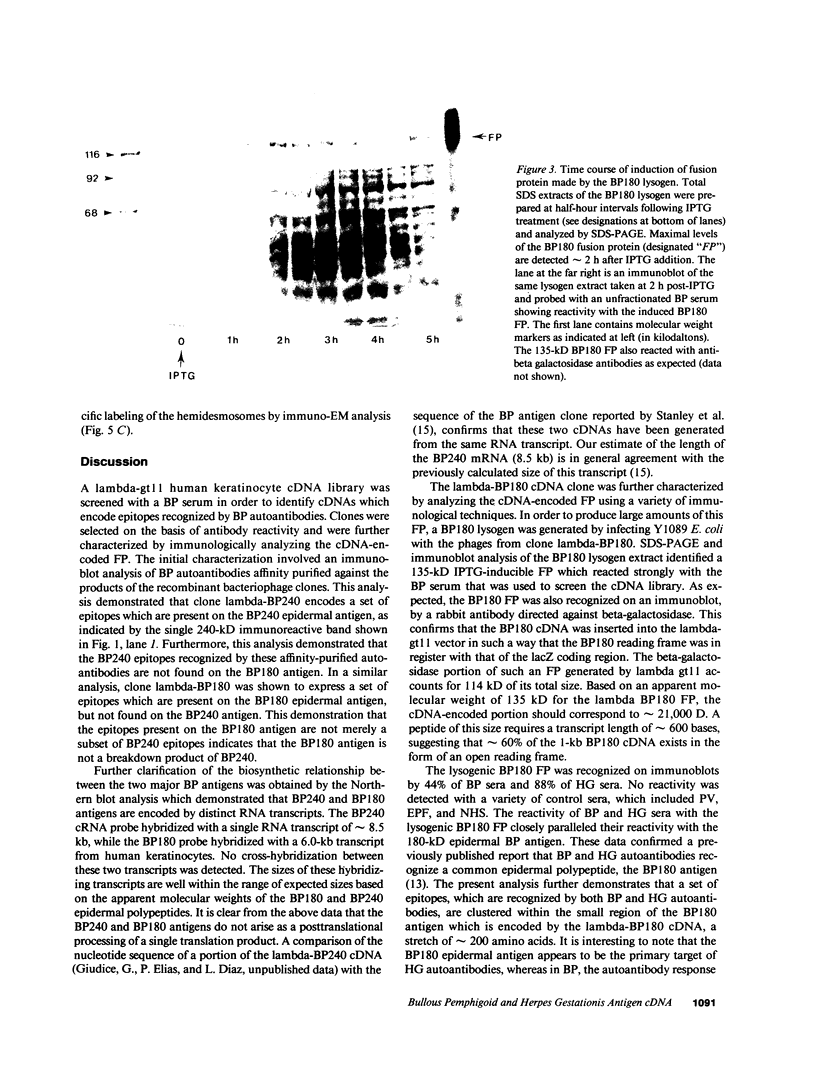
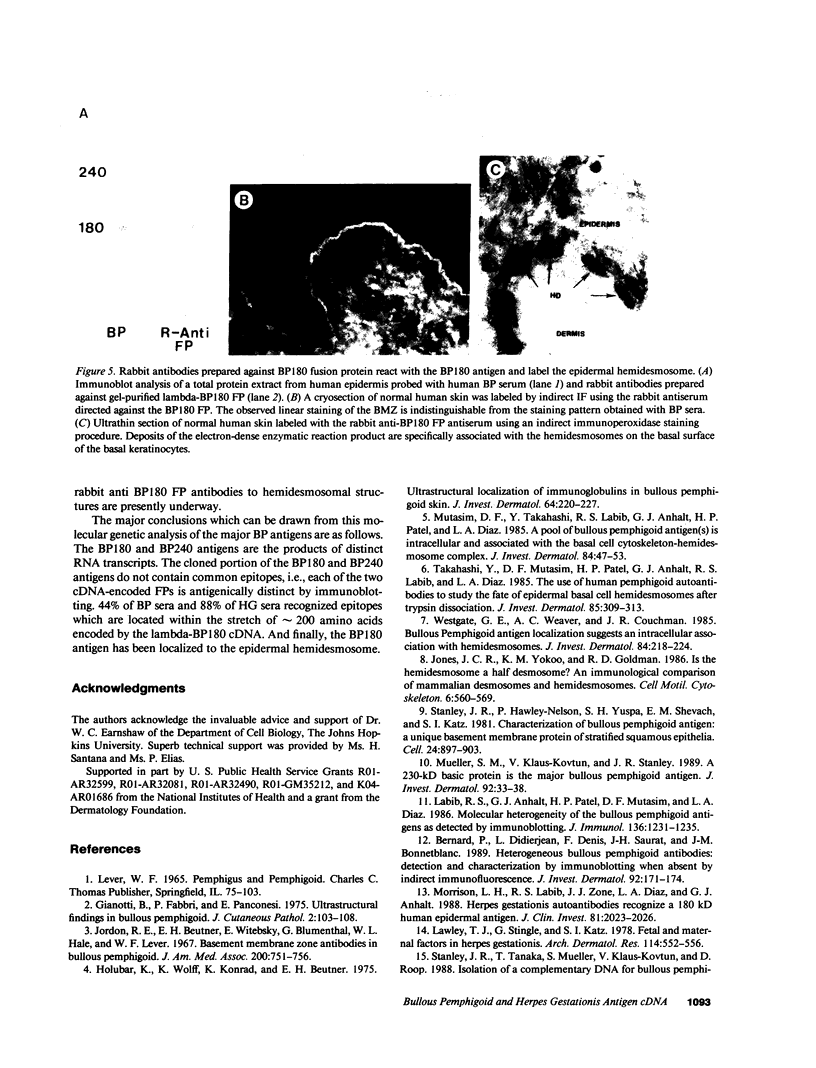
Autoantibodies present in the sera of patients with bullous pemphigoid (BP) bind to the basement membrane zone of normal human skin and commonly recognize two epidermal proteins, the BP240 and BP180 antigens. Two BP antigen cDNA clones from a lambda gt11 human keratinocyte library have been identified on the basis of reactivity with a BP serum. The fusion protein (FP) produced by one clone immunoadsorbed autoantibodies, which specifically recognized the BP180 by antigen, showing no cross-reactivity with BP240 by immunoblot analysis. The FP produced by the second clone immunoadsorbed autoantibodies which specifically reacted with the BP240 epidermal antigen. Northern blot analysis demonstrated that the BP180 and BP240 antigens are encoded by distinct RNA transcripts with lengths of 6.0 and 8.5 kb, respectively. Immunoblot analysis of the BP180 lysogen extract identified a 135-kD FP which was recognized by 7 of 16 BP sera and 7 of 8 herpes gestationis sera. A rabbit antiserum prepared against the lysogenic BP180 FP specifically recognized the BP180 antigen from human epidermal extracts by immunoblotting, labeled the BMZ by indirect immunofluorescence, and bound to human epidermal hemidesmosomes by immuno-electron microscopy. These results indicate that the BP180 antigen recognized by BP and herpes gestationis autoantibodies is a unique hemidesmosomal polypeptide, distinguishable from the BP240 antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard P., Didierjean L., Denis F., Saurat J. H., Bonnetblanc J. M. Heterogeneous bullous pemphigoid antibodies: detection and characterization by immunoblotting when absent by indirect immunofluorescence. J Invest Dermatol. 1989 Feb;92(2):171–174. doi: 10.1111/1523-1747.ep12276689. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti B., Fabbri P., Panconesi E. Ultrastructural findings in bullous pemphigoid. J Cutan Pathol. 1975;2(2):103–108. doi: 10.1111/j.1600-0560.1975.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Giudice G. J., Fuchs E. The transfection of epidermal keratin genes into fibroblasts and simple epithelial cells: evidence for inducing a type I keratin by a type II gene. Cell. 1987 Feb 13;48(3):453–463. doi: 10.1016/0092-8674(87)90196-6. [DOI] [PubMed] [Google Scholar]
- Holubar K., Wolff K., Konrad K., Beutner E. H. Ultrastructural localization of immunoglobulins in bullous pemphigoid skin. Employment of a new peroxidase-antiperoxidase multistep method. J Invest Dermatol. 1975 Apr;64(4):220–227. doi: 10.1111/1523-1747.ep12510658. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Yokoo K. M., Goldman R. D. Is the hemidesmosome a half desmosome? An immunological comparison of mammalian desmosomes and hemidesmosomes. Cell Motil Cytoskeleton. 1986;6(6):560–569. doi: 10.1002/cm.970060604. [DOI] [PubMed] [Google Scholar]
- Jordon R. E., Beutner E. H., Witebsky E., Blumental G., Hale W. L., Lever W. F. Basement zone antibodies in bullous pemphigoid. JAMA. 1967 May 29;200(9):751–756. [PubMed] [Google Scholar]
- Labib R. S., Anhalt G. J., Patel H. P., Mutasim D. F., Diaz L. A. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986 Feb 15;136(4):1231–1235. [PubMed] [Google Scholar]
- Lawley T. J., Stingl G., Katz S. I. Fetal and maternal risk factors in herpes gestationis. Arch Dermatol. 1978 Apr;114(4):552–555. [PubMed] [Google Scholar]
- Miller H. Practical aspects of preparing phage and plasmid DNA: growth, maintenance, and storage of bacteria and bacteriophage. Methods Enzymol. 1987;152:145–170. doi: 10.1016/0076-6879(87)52016-x. [DOI] [PubMed] [Google Scholar]
- Morrison L. H., Labib R. S., Zone J. J., Diaz L. A., Anhalt G. J. Herpes gestationis autoantibodies recognize a 180-kD human epidermal antigen. J Clin Invest. 1988 Jun;81(6):2023–2026. doi: 10.1172/JCI113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Klaus-Kovtun V., Stanley J. R. A 230-kD basic protein is the major bullous pemphigoid antigen. J Invest Dermatol. 1989 Jan;92(1):33–38. doi: 10.1111/1523-1747.ep13070476. [DOI] [PubMed] [Google Scholar]
- Mutasim D. F., Morrison L. H., Takahashi Y., Labib R. S., Skouge J., Diaz L. A., Anhalt G. J. Definition of bullous pemphigoid antibody binding to intracellular and extracellular antigen associated with hemidesmosomes. J Invest Dermatol. 1989 Feb;92(2):225–230. doi: 10.1111/1523-1747.ep12276753. [DOI] [PubMed] [Google Scholar]
- Mutasim D. F., Takahashi Y., Labib R. S., Anhalt G. J., Patel H. P., Diaz L. A. A pool of bullous pemphigoid antigen(s) is intracellular and associated with the basal cell cytoskeleton-hemidesmosome complex. J Invest Dermatol. 1985 Jan;84(1):47–53. doi: 10.1111/1523-1747.ep12274684. [DOI] [PubMed] [Google Scholar]
- Sheng H. Z., Hoogenraad J., Carnegie P. R., Bernard C. C. Use of protein-bearing nitrocellulose as immunogen for in vitro production of monoclonal antibodies: application to myelin basic protein electrophoretically separated from a complex brain protein mixture. Immunol Lett. 1987 Oct;16(1):75–81. doi: 10.1016/0165-2478(87)90065-4. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Yuspa S. H., Shevach E. M., Katz S. I. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981 Jun;24(3):897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Tanaka T., Mueller S., Klaus-Kovtun V., Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients' autoantibodies. J Clin Invest. 1988 Dec;82(6):1864–1870. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Mutasim D. F., Patel H. P., Anhalt G. J., Labib R. S., Diaz L. A. The use of human pemphigoid autoantibodies to study the fate of epidermal basal cell hemidesmosomes after trypsin dissociation. J Invest Dermatol. 1985 Oct;85(4):309–313. doi: 10.1111/1523-1747.ep12276893. [DOI] [PubMed] [Google Scholar]
- Westgate G. E., Weaver A. C., Couchman J. R. Bullous pemphigoid antigen localization suggests an intracellular association with hemidesmosomes. J Invest Dermatol. 1985 Mar;84(3):218–224. doi: 10.1111/1523-1747.ep12265229. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]