Abstract
Accumulating evidence implicates a central role for synovial T cells in the pathogenesis of rheumatoid arthritis, but the activation pathways that drive proliferation and effector function of these cells are not known. We have recently generated a novel monoclonal antibody against a rheumatoid synovial T cell line that recognizes an antigen termed UM4D4 (CDw60). This antigen is expressed on a minority of peripheral blood T cells, and represents the surface component of a distinct pathway of human T cell activation. The current studies were performed to examine the expression and function of UM4D4 on T cells obtained from synovial fluid and synovial membranes of patients with rheumatoid arthritis and other forms of inflammatory joint disease. The UM4D4 antigen is expressed at high surface density on about three-fourths of synovial fluid T cells and on a small subset of synovial fluid natural killer cells; in synovial tissue it is present on more than 90% of T cells in lymphoid aggregates, and on approximately 50% of T cells in stromal infiltrates In addition, UM4D4 is expressed in synovial tissue on a previously undescribed population of HLA-DR/DP-negative non-T cells with a dendritic morphology. Anti-UM4D4 was co-mitogenic for both RA and non-RA synovial fluid mononuclear cells, and induced IL-2 receptor expression. The UM4D4/CDw60 antigen may represent a functional activation pathway for synovial compartment T cells, which could play an important role in the pathogenesis of inflammatory arthritis.
Full text
PDF



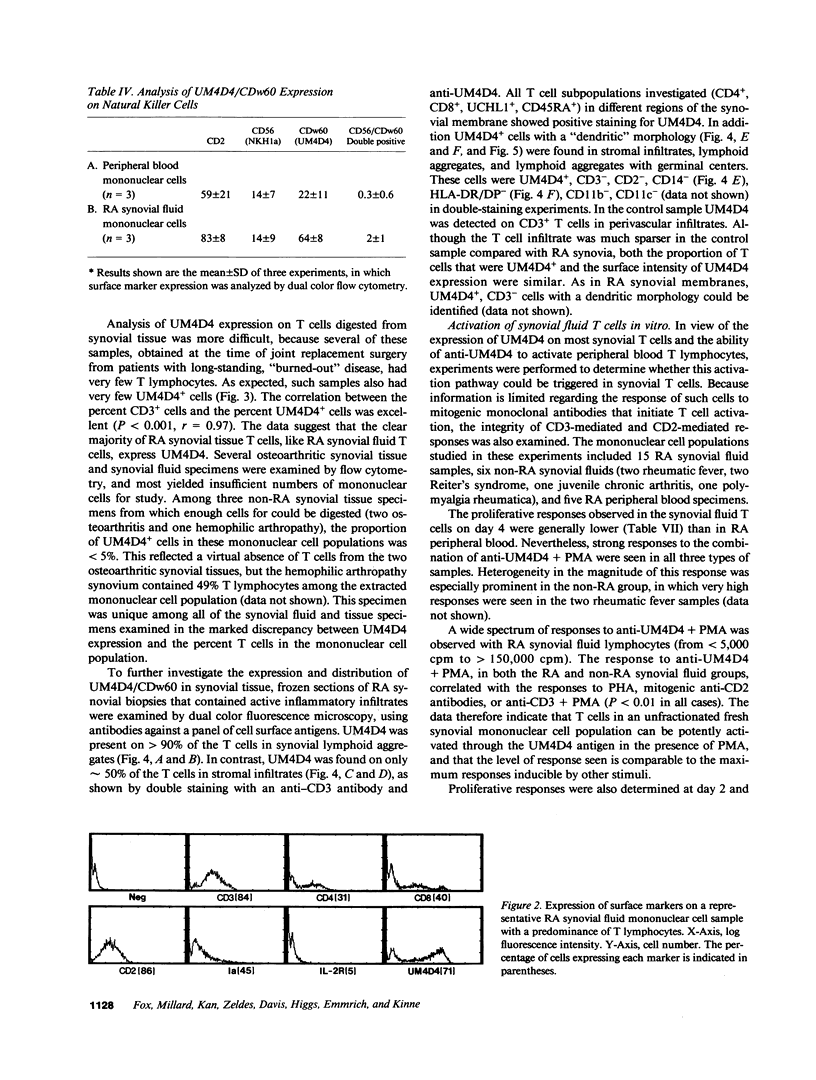
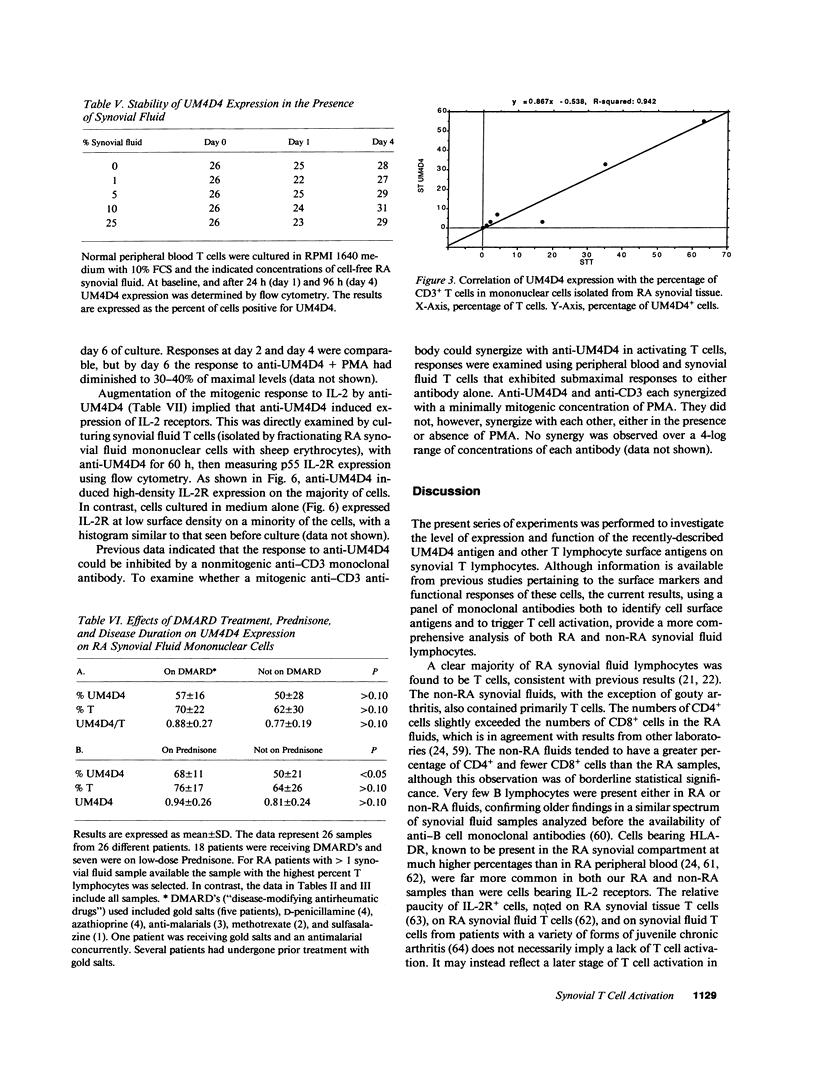







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Malone D. G., Wahl S. M., Calandra G. B., Wilder R. L. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985 Sep;76(3):1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amento E. P., Kurnick J. T., Epstein A., Krane S. M. Modulation of synovial cell products by a factor from a human cell line: T lymphocyte induction of a mononuclear cell factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5307–5311. doi: 10.1073/pnas.79.17.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianakos A. A., Sharp J. T., Person D. A., Lidsky M. D., Duffy J. Cell-mediated immunity in rheumatoid arthritis. Ann Rheum Dis. 1977 Feb;36(1):13–20. doi: 10.1136/ard.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Husby G., Williams R. C., Jr Predominance of T cells in the lymphocytic infiltrates of synovial tissues in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):555–562. doi: 10.1002/art.1780190307. [DOI] [PubMed] [Google Scholar]
- Bergroth V., Konttinen Y. T., Pelkonen P., Haapala M., Haapasaari J., Nordström D., Kunnamo I., Friman C. Synovial fluid lymphocytes in different subtypes of juvenile rheumatoid arthritis. Arthritis Rheum. 1988 Jun;31(6):780–783. doi: 10.1002/art.1780310613. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Brahn E., Trentham D. E. Antigen-specific suppression of collagen arthritis by adoptive transfer of spleen cells. Clin Immunol Immunopathol. 1984 Apr;31(1):124–131. doi: 10.1016/0090-1229(84)90197-1. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Guyre P. M. Increased proliferation of human synovial fibroblasts treated with recombinant immune interferon. J Immunol. 1985 May;134(5):3142–3146. [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L. T., Tilden A. B., Dunlap N. E. Analysis of the monocyte Fc receptors and antibody-mediated cellular interactions required for the induction of T cell proliferation by anti-T3 antibodies. J Immunol. 1985 Jul;135(1):165–171. [PubMed] [Google Scholar]
- Clements P. J., Yu D. T., Levy J., Paulus H. E., Barnett E. V. Effects of cyclophosphamide on B- and T-lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1974 Jul-Aug;17(4):347–353. doi: 10.1002/art.1780170403. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Holoshitz J., van Eden W., Frenkel A. T lymphocyte clones illuminate pathogenesis and affect therapy of experimental arthritis. Arthritis Rheum. 1985 Aug;28(8):841–845. doi: 10.1002/art.1780280802. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Denning S. M., Dustin M. L., Springer T. A., Singer K. H., Haynes B. F. Purified lymphocyte function-associated antigen-3 (LFA-3) activates human thymocytes via the CD2 pathway. J Immunol. 1988 Nov 1;141(9):2980–2985. [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Mackay I. R., Muirden K. D., Rowley M. Deficiency of the suppressor inducer subset of T lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1987 Aug;30(8):849–856. doi: 10.1002/art.1780300802. [DOI] [PubMed] [Google Scholar]
- Emery P., Wood N., Gentry K., Stockman A., Mackay I. R., Bernard O. High-affinity interleukin-2 receptors on blood lymphocytes are decreased during active rheumatoid arthritis. Arthritis Rheum. 1988 Sep;31(9):1176–1181. doi: 10.1002/art.1780310914. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Chan L. S., Kan L., Baadsgaard O., Cooper K. D. Expression and function of the UM4D4 antigen in human thymus. J Immunol. 1989 Oct 1;143(7):2166–2175. [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Acuto O., Poole C., Palley L., Daley J. F., Schlossman S. F., Reinherz E. L. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984 Sep;133(3):1250–1256. [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Fox D. A., Schlossman S. F., Reinherz E. L. Regulation of the alternative pathway of T cell activation by anti-T3 monoclonal antibody. J Immunol. 1986 Mar 15;136(6):1945–1950. [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Froebel K., Sturrock R. D., Reynolds P., Grennan A., Roxburgh A., MacSween R. N. In-vitro reactions of lymphocytes in rheumatoid arthritis and other rheumatic diseases. Ann Rheum Dis. 1979 Dec;38(6):535–543. doi: 10.1136/ard.38.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Shared T cell recognition sites on human histocompatibility leukocyte antigen class II molecules of patients with seropositive rheumatoid arthritis. J Clin Invest. 1986 Mar;77(3):1042–1049. doi: 10.1172/JCI112358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Fox D. A., Benjamin D., Weiner H. L. Antigen reactive memory T cells are defined by Ta1. J Immunol. 1986 Jul 15;137(2):414–418. [PubMed] [Google Scholar]
- Hara T., Fu S. M. Human T cell activation. I. Monocyte-independent activation and proliferation induced by anti-T3 monoclonal antibodies in the presence of tumor promoter 12-o-tetradecanoyl phorbol-13 acetate. J Exp Med. 1985 Apr 1;161(4):641–656. doi: 10.1084/jem.161.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Malone D. G., Allen J. B., Katona I. M., Wahl S. M. Immune function in severe, active rheumatoid arthritis: a relationship between peripheral blood mononuclear cell proliferation to soluble antigens and mononuclear cell subset profiles. J Immunol. 1984 Aug;133(2):697–701. [PubMed] [Google Scholar]
- Helfgott S. M., Dynesius-Trentham R., Brahn E., Trentham D. E. An arthritogenic lymphokine in the rat. J Exp Med. 1985 Nov 1;162(5):1531–1545. doi: 10.1084/jem.162.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Glass D., Coblyn J. S., Jacobson J. G. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J Clin Invest. 1986 Sep;78(3):696–702. doi: 10.1172/JCI112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn B., McDuffie F. C., Ritts R. E., Jr Impaired blastogenic response of lymphocytes from synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1976 Jun;3(2):118–123. [PubMed] [Google Scholar]
- Hercend T., Griffin J. D., Bensussan A., Schmidt R. E., Edson M. A., Brennan A., Murray C., Daley J. F., Schlossman S. F., Ritz J. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985 Mar;75(3):932–943. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs J. B., Zeldes W., Kozarsky K., Schteingart M., Kan L., Bohlke P., Krieger K., Davis W., Fox D. A. A novel pathway of human T lymphocyte activation. Identification by a monoclonal antibody generated against a rheumatoid synovial T cell line. J Immunol. 1988 Jun 1;140(11):3758–3765. [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Hurd E. R., Ziff M. Parameters of improvement in patients with rheumatoid arthritis treated with cyclophosphamide. Arthritis Rheum. 1974 Jan-Feb;17(1):72–78. doi: 10.1002/art.1780170111. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Kammer G. M., Sapolsky A. I., Malemud C. J. Secretion of an articular cartilage proteoglycan-degrading enzyme activity by murine T lymphocytes in vitro. J Clin Invest. 1985 Aug;76(2):395–402. doi: 10.1172/JCI111985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsh J., Wright D. G., Klippel J. H., Decker J. L., Deisseroth A. B., Flye M. W. Lymphocyte depletion by continuous flow cell centrifugation in rheumatoid arthritis: clinical effects. Arthritis Rheum. 1979 Oct;22(10):1055–1059. doi: 10.1002/art.1780221002. [DOI] [PubMed] [Google Scholar]
- Kashiwado T., Miossec P., Oppenheimer-Marks N., Ziff M. Inhibitor of interleukin-2 synthesis and response in rheumatoid synovial fluid. Arthritis Rheum. 1987 Dec;30(12):1339–1347. doi: 10.1002/art.1780301204. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Snow K. M., Bombardier C., Chang C. H., Nelson D. L., Rubin L. A. Elevated soluble interleukin-2 receptor levels in the sera and synovial fluids of patients with rheumatoid arthritis. Arthritis Rheum. 1988 Jul;31(7):844–849. doi: 10.1002/art.1780310704. [DOI] [PubMed] [Google Scholar]
- Kingsley G. H., Pitzalis C., Panayi G. S. Abnormal lymphocyte reactivity to self-major histocompatibility antigens in rheumatoid arthritis. J Rheumatol. 1987 Aug;14(4):667–673. [PubMed] [Google Scholar]
- Kotzin B. L., Kansas G. S., Engleman E. G., Hoppe R. T., Kaplan H. S., Strober S. Changes in T-cell subsets in patients with rheumatoid arthritis treated with total lymphoid irradiation. Clin Immunol Immunopathol. 1983 May;27(2):250–260. doi: 10.1016/0090-1229(83)90075-2. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Strober S., Engleman E. G., Calin A., Hoppe R. T., Kansas G. S., Terrell C. P., Kaplan H. S. Treatment of intractable rheumatoid arthritis with total lymphoid irradiation. N Engl J Med. 1981 Oct 22;305(17):969–976. doi: 10.1056/NEJM198110223051702. [DOI] [PubMed] [Google Scholar]
- Lance E. M., Knight S. C. Immunologic reactivity in rheumatoid arthritis. Response to mitogens. Arthritis Rheum. 1974 Sep-Oct;17(5):513–520. doi: 10.1002/art.1780170504. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E. Immunosuppression by D-penicillamine in vitro. Inhibition of human T lymphocyte proliferation by copper- or ceruloplasmin-dependent generation of hydrogen peroxide and protection by monocytes. J Clin Invest. 1984 Jan;73(1):53–65. doi: 10.1172/JCI111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of antigen- and mitogen-induced human lymphocyte proliferation by gold compounds. J Clin Invest. 1977 Mar;59(3):455–466. doi: 10.1172/JCI108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Robinson C. A., Dinarello C. A., Carson D. A., Vaughan J. H. Basis for defective responses of rheumatoid arthritis synovial fluid lymphocytes to anti-CD3 (T3) antibodies. J Clin Invest. 1986 Sep;78(3):713–721. doi: 10.1172/JCI112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleder H. I., Paulus H. Clinical and immunological alterations observed in patients undergoing long-term thoracic duct drainage. Surgery. 1978 Jul;84(1):157–165. [PubMed] [Google Scholar]
- Malone D. G., Wahl S. M., Tsokos M., Cattell H., Decker J. L., Wilder R. L. Immune function in severe, active rheumatoid arthritis. A relationship between peripheral blood mononuclear cell proliferation to soluble antigens and synovial tissue immunohistologic characteristics. J Clin Invest. 1984 Oct;74(4):1173–1185. doi: 10.1172/JCI111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard H. A., Dion J., Richard C. Antinuclear antibody: predictive of lymphocyte response in rheumatoid arthritis. J Rheumatol. 1977 Spring;4(1):21–26. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Kashiwado T., Ziff M. Inhibitor of interleukin-2 in rheumatoid synovial fluid. Arthritis Rheum. 1987 Feb;30(2):121–129. doi: 10.1002/art.1780300201. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Romain P. L., Fox D. A., Anderson P., DiMaggio M., Levine H., Schlossman S. F. Abnormalities in CD4+ T-lymphocyte subsets in inflammatory rheumatic diseases. Am J Med. 1988 May;84(5):817–825. doi: 10.1016/0002-9343(88)90058-7. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Paulus H. E., Machleder H. I., Levine S., Yu D. T., MacDonald N. S. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977 Jul-Aug;20(6):1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Lanchbury J. S., Murphy J., Panayi G. S. Expression of HLA-DR, DQ and DP antigens and interleukin-2 receptor on synovial fluid T lymphocyte subsets in rheumatoid arthritis: evidence for "frustrated" activation. J Rheumatol. 1987 Aug;14(4):662–666. [PubMed] [Google Scholar]
- Pope R. M., McChesney L., Talal N., Fischbach M. Characterization of the defective autologous mixed lymphocyte response in rheumatoid arthritis. Arthritis Rheum. 1984 Nov;27(11):1234–1244. doi: 10.1002/art.1780271105. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Romain P. L., Burmester G. R., Enlow R. W., Winchester R. J. Multiple abnormalities in immunoregulatory function of synovial compartment T cells in patients with rheumatoid arthritis. Recognition of a helper augmentation effect. Rheumatol Int. 1982;2(3):121–127. doi: 10.1007/BF00541164. [DOI] [PubMed] [Google Scholar]
- Runge L. A. In vitro lymphocyte response in early rheumatoid arthritis. J Rheumatol. 1981 May-Jun;8(3):468–475. [PubMed] [Google Scholar]
- Silver R. M., Redelman D., Zvaifler N. J. Studies of rheumatoid synovial fluid lymphocytes. II. A comparison of their behavior with blood mononuclear cells in the autologous mixed lymphocyte reaction and response to TCGF. Clin Immunol Immunopathol. 1983 Apr;27(1):15–27. doi: 10.1016/0090-1229(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- Singal D. P., D'Souza M., Reid B., Bensen W. G., Kassam Y. B., Adachi J. D. HLA-DQ beta-chain polymorphism in HLA-DR4 haplotypes associated with rheumatoid arthritis. Lancet. 1987 Nov 14;2(8568):1118–1120. doi: 10.1016/s0140-6736(87)91548-0. [DOI] [PubMed] [Google Scholar]
- Slavin S., Strober S. In-vitro T cell mediated function in patients with active rheumatoid arthritis. Ann Rheum Dis. 1981 Feb;40(1):60–63. doi: 10.1136/ard.40.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Stratton J. A., Peter J. B. The responses of peripheral blood and synovial fluid lymphocytes of patients with rheumatoid arthritis to in vitro stimulation with mitogens. Clin Immunol Immunopathol. 1978 Jun;10(2):233–241. doi: 10.1016/0090-1229(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Takagishi K., Kaibara N., Hotokebuchi T., Arita C., Morinaga M., Arai K. Effects of cyclosporin on collagen induced arthritis in mice. Ann Rheum Dis. 1986 Apr;45(4):339–344. doi: 10.1136/ard.45.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Trentham D. E., Belli J. A., Anderson R. J., Buckley J. A., Goetzl E. J., David J. R., Austen K. F. Clinical and immunologic effects of fractionated total lymphoid irradiation in refractory rheumatoid arthritis. N Engl J Med. 1981 Oct 22;305(17):976–982. doi: 10.1056/NEJM198110223051703. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Dynesius R. A., David J. R. Passive transfer by cells of type II collagen-induced arthritis in rats. J Clin Invest. 1978 Aug;62(2):359–366. doi: 10.1172/JCI109136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueo T., Tanaka S., Tominaga Y., Ogawa H., Sakurami T. The effect of thoracic duct drainage on lymphocyte dynamics and clinical symptoms in patients with rheumatoid arthritis. Arthritis Rheum. 1979 Dec;22(12):1405–1412. doi: 10.1002/art.1780221217. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Verbruggen G., Schindler J., Goldstein G. Evaluation of T cell subsets with monoclonal antibodies in synovial fluid in rheumatoid arthritis. J Rheumatol. 1982 Nov-Dec;9(6):821–826. [PubMed] [Google Scholar]
- Waalen K., Førre O., Natvig J. B. Dendritic cells in rheumatoid inflammation. Springer Semin Immunopathol. 1988;10(2-3):141–156. doi: 10.1007/BF01857220. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Chouaib S., Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 sheep erythrocyte receptor determinants. J Immunol. 1986 Aug 15;137(4):1097–1100. [PubMed] [Google Scholar]
- Yocum D. E., Klippel J. H., Wilder R. L., Gerber N. L., Austin H. A., 3rd, Wahl S. M., Lesko L., Minor J. R., Preuss H. G., Yarboro C. Cyclosporin A in severe, treatment-refractory rheumatoid arthritis. A randomized study. Ann Intern Med. 1988 Dec 1;109(11):863–869. doi: 10.7326/0003-4819-109-11-863. [DOI] [PubMed] [Google Scholar]
- Yy D. T., Clements P. J., Peter J. B., Levy J., Paulus H. E., Barnett E. V. Lymphocyte characteristics in rheumatic patients and the effect of azathioprine therapy. Arthritis Rheum. 1974 Jan-Feb;17(1):37–45. doi: 10.1002/art.1780170107. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Cohen I. Antigenic mimicry between mycobacteria and cartilage proteoglycans: the model of adjuvant arthritis. Concepts Immunopathol. 1987;4:144–170. [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van de Putte L. B., Meijer C. J., Lafeber G. J., Kleinjan R., Cats A. Lymphocytes in rheumatoid and nonrheumatoid synovial fluids. Nonspecificity of high T-cell and low B-cell percentages. Ann Rheum Dis. 1975 Oct;35(5):451–455. doi: 10.1136/ard.35.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]