Abstract
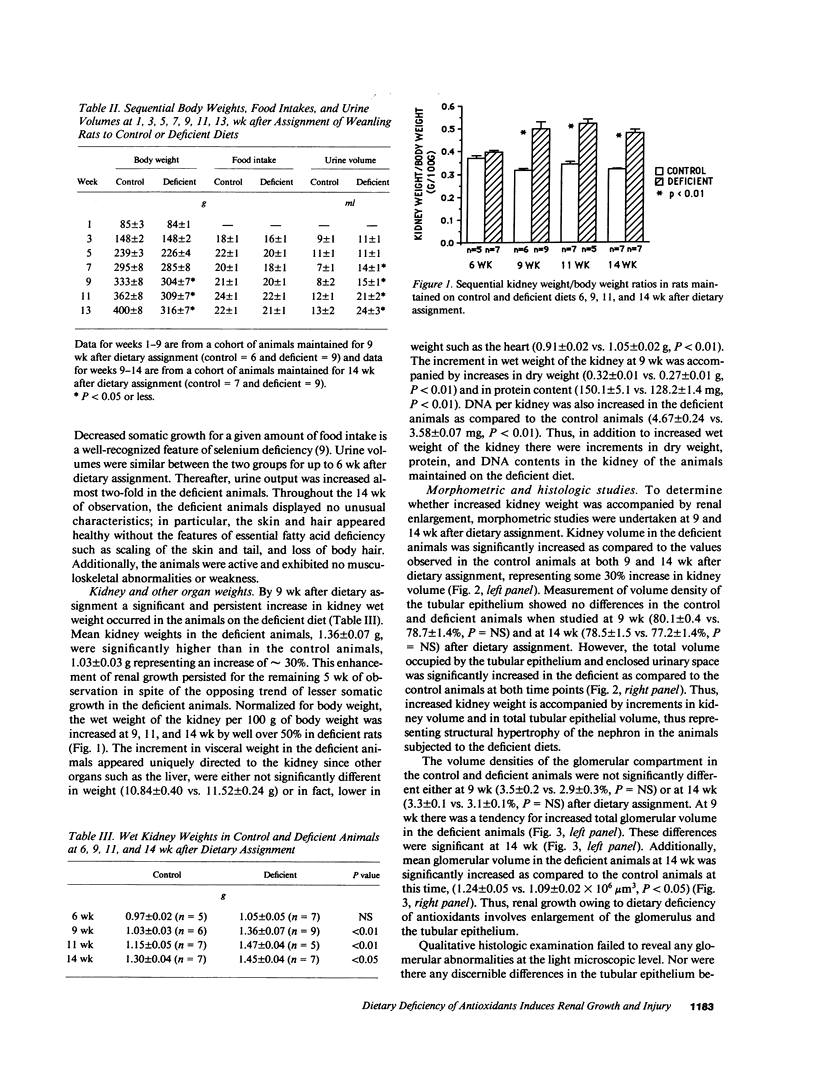
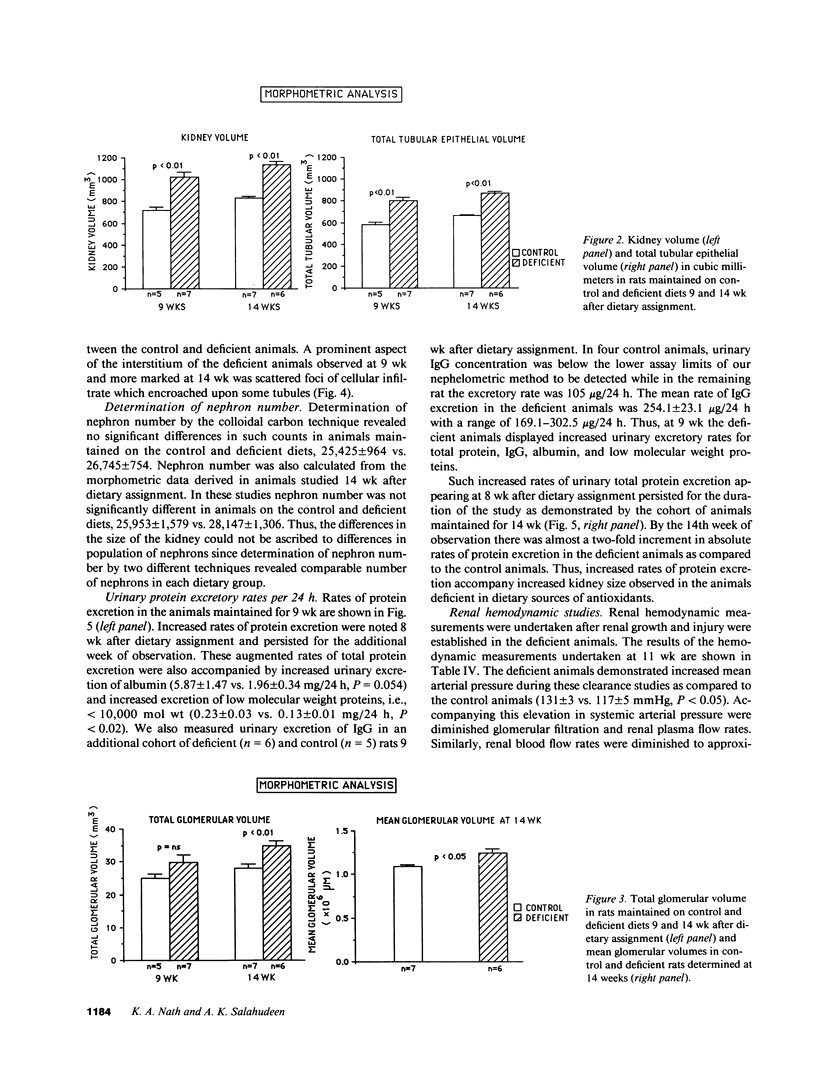
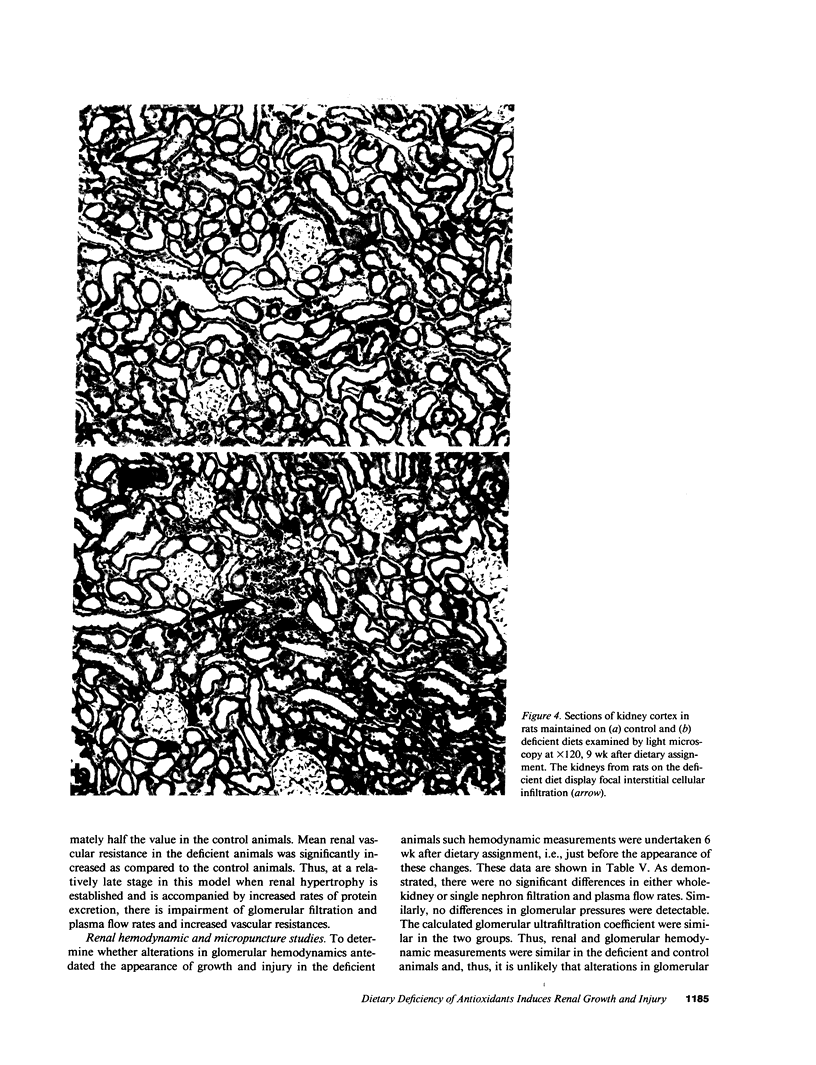
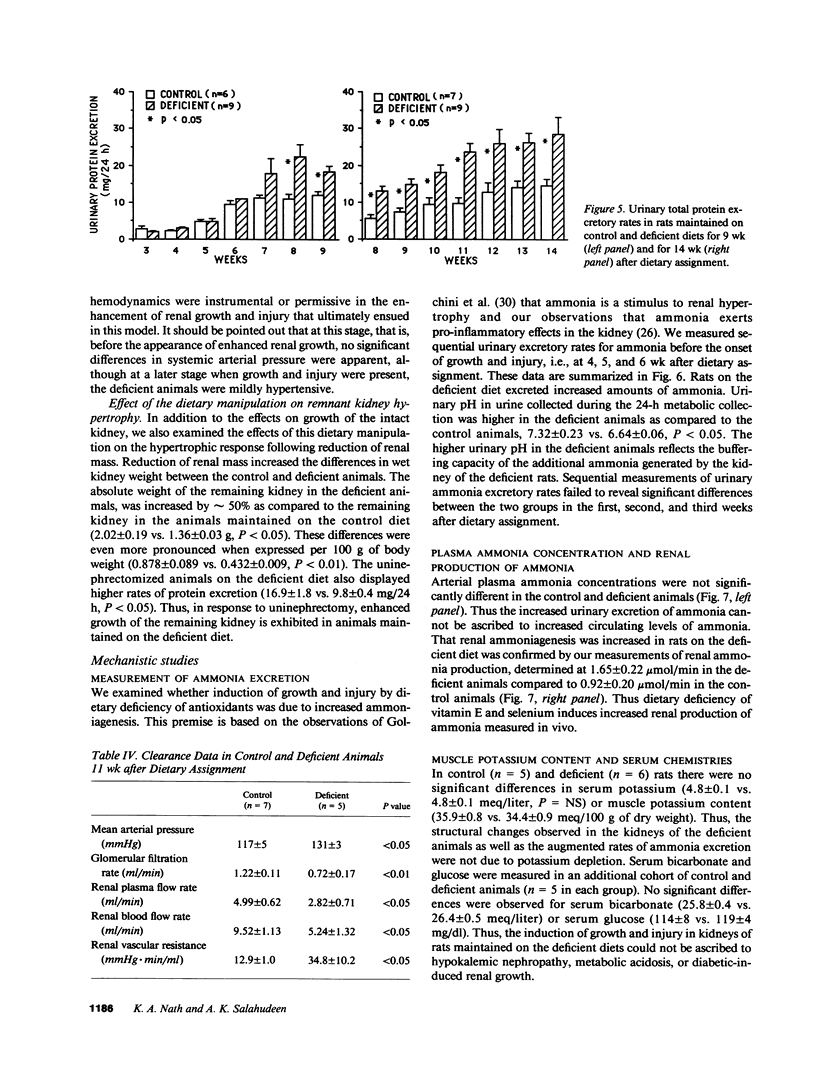
We report induction of renal growth and injury in the intact rat kidney using a diet deficient in vitamin E and selenium. This diet was imposed in 3-wk-old male weanling rats, and after 9 wk, enhancement of growth, characterized by increased wet weight, dry weight, protein content, and DNA content appeared. Morphometric analyses revealed increased kidney volume, tubular epithelial volume, and mean glomerular volume. There were no differences in nephron number. The animals on the deficient diet displayed increased urinary protein excretion at 9 wk. Renal injury was also characterized by an interstitial cellular infiltrate and diminutions in glomerular filtration rate. Enhanced growth and injury were antedated by increased renal ammoniagenesis. The deficient diet did not induce metabolic acidosis, potassium depletion, glucose intolerance, or elevated plasma amino acid concentration. Enhancement of renal growth and ammoniagenesis by the deficient diet was not suppressible by chronic alkali therapy. Stimulation of renal growth could not be ascribed to increased intrarenal iron, induction of ornithine decarboxylase, or alterations in glomerular hemodynamics. Stimulation of renal ammoniagenesis by dietary deficiency of antioxidants is a novel finding, as is induction of growth and injury. We suggest that increased renal ammoniagenesis contributes to induction of renal growth and injury.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Stahl R. A., Baker P. J., Chen Y. P., Pritzl P. M., Couser W. G. Biphasic effect of oxygen radicals on prostaglandin production by rat mesangial cells. Am J Physiol. 1987 Apr;252(4 Pt 2):F743–F749. doi: 10.1152/ajprenal.1987.252.4.F743. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986 Nov;251(5 Pt 2):F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- Berl T., Linas S. L., Aisenbrey G. A., Anderson R. J. On the mechanism of polyuria in potassium depletion. The role of polydipsia. J Clin Invest. 1977 Sep;60(3):620–625. doi: 10.1172/JCI108813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlett B. S., Chock P. B., Yim M. B., Stadtman E. R. Manganese(II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jan;87(1):389–393. doi: 10.1073/pnas.87.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., van Eijk H. G., Koster J. F. Superoxide dependent iron release from ferritin in inflammatory diseases. Free Radic Biol Med. 1988;4(3):185–198. doi: 10.1016/0891-5849(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Meyer T. W., Hostetter T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982 Sep 9;307(11):652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- Burk R. F. Biological activity of selenium. Annu Rev Nutr. 1983;3:53–70. doi: 10.1146/annurev.nu.03.070183.000413. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- DAMADIAN R. V., SHWAYRI E., BRICKER N. S. ON THE EXISTENCE OF NON-URINE FORMING NEPHRONS IN THE DISEASED KIDNEY OF THE DOG. J Lab Clin Med. 1965 Jan;65:26–39. [PubMed] [Google Scholar]
- Dubé F., Epel D. The relation between intracellular pH and rate of protein synthesis in sea urchin eggs and the existence of a pH-independent event triggered by ammonia. Exp Cell Res. 1986 Jan;162(1):191–204. doi: 10.1016/0014-4827(86)90438-6. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M. Influence of vitamin E on polyamine metabolism in ozone-exposed rat lungs. Arch Biochem Biophys. 1987 Jun;255(2):392–399. doi: 10.1016/0003-9861(87)90407-3. [DOI] [PubMed] [Google Scholar]
- Fine L. G. Preventing the progression of human renal disease: have rational therapeutic principles emerged? Kidney Int. 1988 Jan;33(1):116–128. doi: 10.1038/ki.1988.18. [DOI] [PubMed] [Google Scholar]
- Fine L. The biology of renal hypertrophy. Kidney Int. 1986 Mar;29(3):619–634. doi: 10.1038/ki.1986.45. [DOI] [PubMed] [Google Scholar]
- Fischer W. C., Whanger P. D. Effects of selenium deficiency on vitamin E metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 1977;23(4):273–280. doi: 10.3177/jnsv.23.273. [DOI] [PubMed] [Google Scholar]
- Friedman J., Cerutti P. The induction of ornithine decarboxylase by phorbol 12-myristate 13-acetate or by serum is inhibited by antioxidants. Carcinogenesis. 1983 Nov;4(11):1425–1427. doi: 10.1093/carcin/4.11.1425. [DOI] [PubMed] [Google Scholar]
- Golchini K., Norman J., Bohman R., Kurtz I. Induction of hypertrophy in cultured proximal tubule cells by extracellular NH4Cl. J Clin Invest. 1989 Dec;84(6):1767–1779. doi: 10.1172/JCI114361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. F. The colorimetric estimation of plasma amino nitrogen with DNFB. Clin Chem. 1968 Nov;14(11):1080–1090. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Guidet B. R., Shah S. V. In vivo generation of hydrogen peroxide by rat kidney cortex and glomeruli. Am J Physiol. 1989 Jan;256(1 Pt 2):F158–F164. doi: 10.1152/ajprenal.1989.256.1.F158. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987 Sep;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D. G., Hoekstra W. G. Lipid peroxidation in vivo during vitamin E and selenium deficiency in the rat as monitored by ethane evolution. J Nutr. 1977 Apr;107(4):666–672. doi: 10.1093/jn/107.4.666. [DOI] [PubMed] [Google Scholar]
- Hirose K., Osterby R., Nozawa M., Gundersen H. J. Development of glomerular lesions in experimental long-term diabetes in the rat. Kidney Int. 1982 May;21(5):689–695. doi: 10.1038/ki.1982.82. [DOI] [PubMed] [Google Scholar]
- Hoekstra W. G. Biochemical function of selenium and its relation to vitamin E. Fed Proc. 1975 Oct;34(11):2083–2089. [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter T. H., Meyer T. W., Rennke H. G., Brenner B. M. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int. 1986 Oct;30(4):509–517. doi: 10.1038/ki.1986.215. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. M., DiMeola H. J., Siegel N. J., Lytton B., Kashgarian M., Hayslett J. P. Compensatory adaptation of structure and function following progressive renal ablation. Kidney Int. 1974 Jul;6(1):10–17. doi: 10.1038/ki.1974.72. [DOI] [PubMed] [Google Scholar]
- Laskey J., Webb I., Schulman H. M., Ponka P. Evidence that transferrin supports cell proliferation by supplying iron for DNA synthesis. Exp Cell Res. 1988 May;176(1):87–95. doi: 10.1016/0014-4827(88)90123-1. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Machlin L. J., Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987 Dec;1(6):441–445. [PubMed] [Google Scholar]
- McCay P. B. Vitamin E: interactions with free radicals and ascorbate. Annu Rev Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- Mitch W. E. The influence of the diet on the progression of renal insufficiency. Annu Rev Med. 1984;35:249–264. doi: 10.1146/annurev.me.35.020184.001341. [DOI] [PubMed] [Google Scholar]
- Nagami G. T., Kurokawa K. Regulation of ammonia production by mouse proximal tubules perfused in vitro. Effect of luminal perfusion. J Clin Invest. 1985 Mar;75(3):844–849. doi: 10.1172/JCI111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K. A., Hostetter M. K., Hostetter T. H. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985 Aug;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K. A., Kren S. M., Hostetter T. H. Dietary protein restriction in established renal injury in the rat. Selective role of glomerular capillary pressure in progressive glomerular dysfunction. J Clin Invest. 1986 Nov;78(5):1199–1205. doi: 10.1172/JCI112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Reeds P. J., Atkinson T., Smith R. H. The influence of changes in tension on protein synthesis and prostaglandin release in isolated rabbit muscles. Biochem J. 1983 Sep 15;214(3):1011–1014. doi: 10.1042/bj2141011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasarma T. An iron-messenger system--a hypothesis. Free Radic Res Commun. 1986;2(3):153–162. doi: 10.3109/10715768609088067. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. Vascular effects of oxygen-derived free radicals. Free Radic Biol Med. 1988;4(2):107–120. doi: 10.1016/0891-5849(88)90071-8. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Ornithine decarboxylase: a key regulatory enzyme in normal and neoplastic growth. Drug Metab Rev. 1985;16(1-2):1–88. doi: 10.3109/03602538508991430. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Protein degradation in isolated rat hepatocytes is inhibited by ammonia. Biochem Biophys Res Commun. 1975 Sep 2;66(1):44–52. doi: 10.1016/s0006-291x(75)80292-0. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 1989 May;35(5):1093–1106. doi: 10.1038/ki.1989.96. [DOI] [PubMed] [Google Scholar]
- Sporn P. H., Peters-Golden M., Simon R. H. Hydrogen-peroxide-induced arachidonic acid metabolism in the rat alveolar macrophage. Am Rev Respir Dis. 1988 Jan;137(1):49–56. doi: 10.1164/ajrccm/137.1.49. [DOI] [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Tannen R. L. Effect of potassium on renal acidification and acid-base homeostasis. Semin Nephrol. 1987 Sep;7(3):263–273. [PubMed] [Google Scholar]
- Toback F. G., Ordónez N. G., Bortz S. L., Spargo B. H. Zonal changes in renal structure and phospholipid metabolism in potassium-deficient rats. Lab Invest. 1976 Feb;34(2):115–124. [PubMed] [Google Scholar]
- Tolins J. P., Hostetter M. K., Hostetter T. H. Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest. 1987 May;79(5):1447–1458. doi: 10.1172/JCI112973. [DOI] [PMC free article] [PubMed] [Google Scholar]




