Abstract
In the title compound, C14H13NO4S, the mean planes of the pyrrole and phenyl rings form a dihedral angle of 88.7 (1)°. The aldehyde groups are slightly twisted from the pyrrole plane. In the crystal structure, molecules are linked into a three-dimensional framework by C—H⋯O hydrogen bonds.
Related literature
For general background, see: Ali et al. (1989 ▶); Amal Raj et al. (2003 ▶). For bond-length data, see: Allen et al. (1987 ▶). For N-atom hybridization details, see: Beddoes et al. (1986 ▶).
Experimental
Crystal data
C14H13NO4S
M r = 291.31
Monoclinic,
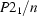
a = 9.0257 (3) Å
b = 12.6240 (5) Å
c = 11.9914 (5) Å
β = 97.700 (2)°
V = 1353.99 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.25 mm−1
T = 293 K
0.25 × 0.20 × 0.20 mm
Data collection
Bruker Kappa APEXII area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2001 ▶) T min = 0.940, T max = 0.952
19609 measured reflections
4736 independent reflections
3164 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.154
S = 1.00
4736 reflections
183 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.42 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: PARST (Nardelli, 1995 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809004425/wn2307sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004425/wn2307Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯O3 | 0.93 | 2.46 | 3.077 (2) | 124 |
| C7—H7A⋯O4 | 0.96 | 2.33 | 3.021 (3) | 128 |
| C11—H11⋯O4i | 0.93 | 2.53 | 3.456 (2) | 174 |
| C13—H13⋯O2 | 0.93 | 2.52 | 3.304 (2) | 143 |
| C14—H14⋯O3ii | 0.93 | 2.57 | 3.383 (2) | 146 |
Symmetry codes: (i)  ; (ii)
; (ii) 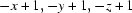 .
.
Acknowledgments
BB and RS thank Dr Babu Varghese, SAIF, IIT Madras, India, for his help with the data collection.
supplementary crystallographic information
Comment
Heterocyclic compounds, especially five-membered rings, have occupied an important place among organic compounds because of their biological activities. The fungicidal activity of novel heterocycles has been reported by Ali et al. (1989). These are crucial intermediates for various pyrrole natural products possessing antitumour properties. They are found to have antifungal activity against various pathogens (Amal Raj et al., 2003). Against this background and in order to obtain detailed information on molecular conformation in the solid state, an X-ray crystallographic study of the title compound has been carried out and the results are presented here.
The geometric parameters are normal (Allen et al., 1987). The mean planes of the pyrrole and phenyl rings form a dihedral angle of 88.7 (1)°. The aldehyde groups are slightly twisted from the pyrrole plane, with O3 towards C2 and O4 towards C1, as evidenced by the torsion angles C2—C3—C5—O3 = 2.1 (3)° and C1—C2—C6—O4 = 5.9 (3)° (Fig. 1). The sum of the angles at N is 360.0, which is an indication of sp2 hybridization (Beddoes et al., 1986). In the crystal structure, the molecules are linked into a three-dimensional framework by C—H···O hydrogen bonds (Fig. 2 and Table 1).
Experimental
To a suspension of 3°-butoxide (4.4 g, 39.7 mmol) in dry THF (20 ml), 18-crown-6 (catalyst) and 2,5-dimethyl-1H-pyrrole-3,4-dicarbaldehyde (4 g, 26.5 mmol) in dry THF were added slowly at room temperature and the reaction mixture was stirred for 15 min. To this, PhSO2Cl (6.1 g, 34.4 mmol) in dry THF (15 ml) was added and stirred for another 4 h. The mixture was then poured over ice–water (500 ml) and the solid obtained was filtered. The product, 2,5-dimethyl-1-(phenyl sulfonyl)-1H-pyrrole-3,4-dicarbaldehyde, was recrystallized from methanol. Yield 4.9 g (64%). M.p = 395 K.
Refinement
All H atoms were positioned geometrically and allowed to ride on their parent atoms, with C—H = 0.93–0.96 Å and Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2Ueq(C) for other H atoms.
Figures
Fig. 1.
Molecular structure of the title compound showing 30% probability displacement ellipsoids. Hydrogen atoms are represented by spheres of arbitrary radius.
Fig. 2.
Crystal packing of the title compound. Hydrogen bonds are shown as dashed lines.
Crystal data
| C14H13NO4S | F(000) = 608 |
| Mr = 291.31 | Dx = 1.429 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.0257 (3) Å | Cell parameters from 6814 reflections |
| b = 12.6240 (5) Å | θ = 2.4–32.2° |
| c = 11.9914 (5) Å | µ = 0.25 mm−1 |
| β = 97.700 (2)° | T = 293 K |
| V = 1353.99 (9) Å3 | Block, colourless |
| Z = 4 | 0.25 × 0.20 × 0.20 mm |
Data collection
| Bruker Kappa-APEXII area-detector diffractometer | 4736 independent reflections |
| Radiation source: fine-focus sealed tube | 3164 reflections with I > 2σ(I) |
| graphite | Rint = 0.032 |
| ω scans | θmax = 32.2°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2001) | h = −13→10 |
| Tmin = 0.940, Tmax = 0.952 | k = −18→18 |
| 19609 measured reflections | l = −16→17 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.154 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0787P)2 + 0.2246P] where P = (Fo2 + 2Fc2)/3 |
| 4736 reflections | (Δ/σ)max < 0.001 |
| 183 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.42 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.51190 (5) | 0.21083 (3) | 0.25629 (3) | 0.05026 (14) | |
| N | 0.56878 (14) | 0.29263 (9) | 0.36572 (10) | 0.0417 (3) | |
| O1 | 0.64275 (15) | 0.17150 (11) | 0.21659 (11) | 0.0704 (4) | |
| O3 | 0.82639 (16) | 0.54763 (12) | 0.58440 (12) | 0.0777 (4) | |
| O2 | 0.40939 (17) | 0.13956 (10) | 0.29555 (12) | 0.0743 (4) | |
| O4 | 0.49857 (19) | 0.36086 (17) | 0.70677 (12) | 0.1023 (6) | |
| C1 | 0.51247 (15) | 0.29778 (11) | 0.46937 (11) | 0.0422 (3) | |
| C2 | 0.59280 (15) | 0.37280 (11) | 0.53213 (11) | 0.0404 (3) | |
| C3 | 0.69918 (14) | 0.41747 (11) | 0.46621 (11) | 0.0383 (3) | |
| C4 | 0.68371 (15) | 0.36717 (11) | 0.36473 (11) | 0.0412 (3) | |
| C5 | 0.80621 (18) | 0.50177 (13) | 0.49571 (14) | 0.0524 (4) | |
| H5 | 0.8646 | 0.5223 | 0.4412 | 0.063* | |
| C6 | 0.57651 (19) | 0.40278 (16) | 0.64774 (13) | 0.0579 (4) | |
| H6 | 0.6327 | 0.4602 | 0.6779 | 0.069* | |
| C7 | 0.3863 (2) | 0.23246 (17) | 0.50008 (16) | 0.0661 (5) | |
| H7A | 0.3587 | 0.2571 | 0.5702 | 0.099* | |
| H7B | 0.4168 | 0.1597 | 0.5075 | 0.099* | |
| H7C | 0.3022 | 0.2386 | 0.4423 | 0.099* | |
| C8 | 0.7684 (2) | 0.38292 (17) | 0.26769 (14) | 0.0662 (5) | |
| H8A | 0.8349 | 0.4421 | 0.2825 | 0.099* | |
| H8B | 0.6997 | 0.3965 | 0.2009 | 0.099* | |
| H8C | 0.8253 | 0.3203 | 0.2571 | 0.099* | |
| C9 | 0.41822 (17) | 0.29562 (12) | 0.15557 (12) | 0.0455 (3) | |
| C10 | 0.4612 (2) | 0.29939 (16) | 0.04944 (14) | 0.0604 (4) | |
| H10 | 0.5403 | 0.2583 | 0.0315 | 0.072* | |
| C11 | 0.3837 (3) | 0.3659 (2) | −0.02973 (16) | 0.0767 (6) | |
| H11 | 0.4104 | 0.3696 | −0.1019 | 0.092* | |
| C12 | 0.2683 (3) | 0.42608 (17) | −0.00229 (17) | 0.0780 (6) | |
| H12 | 0.2176 | 0.4707 | −0.0561 | 0.094* | |
| C13 | 0.2260 (3) | 0.42188 (16) | 0.10310 (18) | 0.0744 (6) | |
| H13 | 0.1476 | 0.4638 | 0.1207 | 0.089* | |
| C14 | 0.3000 (2) | 0.35534 (13) | 0.18281 (14) | 0.0579 (4) | |
| H14 | 0.2708 | 0.3507 | 0.2541 | 0.069* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0664 (3) | 0.0385 (2) | 0.0462 (2) | −0.00334 (15) | 0.00855 (17) | −0.00739 (14) |
| N | 0.0513 (6) | 0.0408 (6) | 0.0347 (5) | −0.0058 (5) | 0.0120 (5) | −0.0013 (4) |
| O1 | 0.0800 (8) | 0.0655 (8) | 0.0655 (8) | 0.0209 (7) | 0.0084 (6) | −0.0213 (6) |
| O3 | 0.0858 (9) | 0.0823 (10) | 0.0652 (8) | −0.0240 (7) | 0.0105 (7) | −0.0272 (7) |
| O2 | 0.1039 (10) | 0.0500 (7) | 0.0681 (8) | −0.0305 (7) | 0.0080 (7) | 0.0000 (6) |
| O4 | 0.1016 (12) | 0.1637 (17) | 0.0496 (8) | −0.0353 (11) | 0.0398 (8) | −0.0136 (9) |
| C1 | 0.0459 (7) | 0.0463 (7) | 0.0362 (6) | −0.0003 (5) | 0.0121 (5) | 0.0061 (5) |
| C2 | 0.0429 (6) | 0.0467 (7) | 0.0333 (6) | 0.0049 (5) | 0.0115 (5) | 0.0032 (5) |
| C3 | 0.0418 (6) | 0.0389 (7) | 0.0347 (6) | 0.0024 (5) | 0.0074 (5) | 0.0024 (5) |
| C4 | 0.0465 (7) | 0.0430 (7) | 0.0358 (6) | −0.0019 (5) | 0.0123 (5) | 0.0015 (5) |
| C5 | 0.0560 (8) | 0.0535 (9) | 0.0480 (8) | −0.0081 (7) | 0.0078 (6) | −0.0012 (7) |
| C6 | 0.0595 (9) | 0.0796 (12) | 0.0366 (7) | 0.0025 (8) | 0.0143 (6) | −0.0055 (7) |
| C7 | 0.0662 (10) | 0.0772 (12) | 0.0595 (10) | −0.0208 (9) | 0.0248 (8) | 0.0053 (9) |
| C8 | 0.0784 (12) | 0.0817 (13) | 0.0445 (9) | −0.0255 (10) | 0.0295 (8) | −0.0068 (8) |
| C9 | 0.0551 (8) | 0.0435 (7) | 0.0379 (7) | −0.0089 (6) | 0.0067 (6) | −0.0098 (5) |
| C10 | 0.0643 (10) | 0.0774 (12) | 0.0411 (8) | −0.0095 (8) | 0.0134 (7) | −0.0091 (8) |
| C11 | 0.0942 (15) | 0.0952 (16) | 0.0402 (9) | −0.0202 (12) | 0.0072 (9) | 0.0005 (9) |
| C12 | 0.1054 (16) | 0.0665 (12) | 0.0567 (11) | −0.0007 (11) | −0.0086 (11) | 0.0022 (9) |
| C13 | 0.0900 (14) | 0.0586 (11) | 0.0710 (12) | 0.0162 (10) | −0.0029 (10) | −0.0134 (9) |
| C14 | 0.0742 (11) | 0.0527 (9) | 0.0472 (9) | 0.0036 (8) | 0.0099 (8) | −0.0136 (7) |
Geometric parameters (Å, °)
| S1—O2 | 1.4155 (13) | C7—H7A | 0.9600 |
| S1—O1 | 1.4209 (13) | C7—H7B | 0.9600 |
| S1—N | 1.6945 (12) | C7—H7C | 0.9600 |
| S1—C9 | 1.7452 (17) | C8—H8A | 0.9600 |
| N—C4 | 1.4018 (17) | C8—H8B | 0.9600 |
| N—C1 | 1.4060 (17) | C8—H8C | 0.9600 |
| O3—C5 | 1.203 (2) | C9—C10 | 1.380 (2) |
| O4—C6 | 1.187 (2) | C9—C14 | 1.381 (2) |
| C1—C2 | 1.358 (2) | C10—C11 | 1.385 (3) |
| C1—C7 | 1.492 (2) | C10—H10 | 0.9300 |
| C2—C3 | 1.4382 (18) | C11—C12 | 1.364 (3) |
| C2—C6 | 1.463 (2) | C11—H11 | 0.9300 |
| C3—C4 | 1.3631 (19) | C12—C13 | 1.370 (3) |
| C3—C5 | 1.449 (2) | C12—H12 | 0.9300 |
| C4—C8 | 1.4890 (19) | C13—C14 | 1.376 (3) |
| C5—H5 | 0.9300 | C13—H13 | 0.9300 |
| C6—H6 | 0.9300 | C14—H14 | 0.9300 |
| O2—S1—O1 | 119.94 (9) | H7A—C7—H7B | 109.5 |
| O2—S1—N | 105.88 (7) | C1—C7—H7C | 109.5 |
| O1—S1—N | 107.05 (7) | H7A—C7—H7C | 109.5 |
| O2—S1—C9 | 110.02 (8) | H7B—C7—H7C | 109.5 |
| O1—S1—C9 | 109.26 (8) | C4—C8—H8A | 109.5 |
| N—S1—C9 | 103.33 (7) | C4—C8—H8B | 109.5 |
| C4—N—C1 | 109.39 (11) | H8A—C8—H8B | 109.5 |
| C4—N—S1 | 123.39 (9) | C4—C8—H8C | 109.5 |
| C1—N—S1 | 127.22 (10) | H8A—C8—H8C | 109.5 |
| C2—C1—N | 107.03 (11) | H8B—C8—H8C | 109.5 |
| C2—C1—C7 | 128.14 (13) | C10—C9—C14 | 121.40 (16) |
| N—C1—C7 | 124.83 (14) | C10—C9—S1 | 119.29 (14) |
| C1—C2—C3 | 108.37 (12) | C14—C9—S1 | 119.29 (12) |
| C1—C2—C6 | 126.27 (13) | C9—C10—C11 | 118.31 (18) |
| C3—C2—C6 | 125.35 (14) | C9—C10—H10 | 120.8 |
| C4—C3—C2 | 108.18 (12) | C11—C10—H10 | 120.8 |
| C4—C3—C5 | 123.06 (13) | C12—C11—C10 | 120.27 (18) |
| C2—C3—C5 | 128.76 (13) | C12—C11—H11 | 119.9 |
| C3—C4—N | 107.02 (11) | C10—C11—H11 | 119.9 |
| C3—C4—C8 | 129.31 (14) | C11—C12—C13 | 121.2 (2) |
| N—C4—C8 | 123.67 (13) | C11—C12—H12 | 119.4 |
| O3—C5—C3 | 125.90 (15) | C13—C12—H12 | 119.4 |
| O3—C5—H5 | 117.1 | C12—C13—C14 | 119.66 (19) |
| C3—C5—H5 | 117.1 | C12—C13—H13 | 120.2 |
| O4—C6—C2 | 126.33 (18) | C14—C13—H13 | 120.2 |
| O4—C6—H6 | 116.8 | C13—C14—C9 | 119.18 (16) |
| C2—C6—H6 | 116.8 | C13—C14—H14 | 120.4 |
| C1—C7—H7A | 109.5 | C9—C14—H14 | 120.4 |
| C1—C7—H7B | 109.5 | ||
| O2—S1—N—C4 | 172.55 (12) | C1—N—C4—C3 | 0.29 (16) |
| O1—S1—N—C4 | 43.51 (14) | S1—N—C4—C3 | −179.53 (10) |
| C9—S1—N—C4 | −71.78 (13) | C1—N—C4—C8 | 179.88 (15) |
| O2—S1—N—C1 | −7.23 (15) | S1—N—C4—C8 | 0.1 (2) |
| O1—S1—N—C1 | −136.28 (13) | C4—C3—C5—O3 | −178.68 (17) |
| C9—S1—N—C1 | 108.43 (13) | C2—C3—C5—O3 | 2.1 (3) |
| C4—N—C1—C2 | −1.03 (16) | C1—C2—C6—O4 | 5.9 (3) |
| S1—N—C1—C2 | 178.78 (10) | C3—C2—C6—O4 | −173.12 (19) |
| C4—N—C1—C7 | 178.16 (15) | O2—S1—C9—C10 | −122.33 (14) |
| S1—N—C1—C7 | −2.0 (2) | O1—S1—C9—C10 | 11.30 (15) |
| N—C1—C2—C3 | 1.33 (16) | N—S1—C9—C10 | 125.00 (13) |
| C7—C1—C2—C3 | −177.83 (16) | O2—S1—C9—C14 | 55.92 (14) |
| N—C1—C2—C6 | −177.86 (14) | O1—S1—C9—C14 | −170.46 (12) |
| C7—C1—C2—C6 | 3.0 (3) | N—S1—C9—C14 | −56.75 (13) |
| C1—C2—C3—C4 | −1.18 (16) | C14—C9—C10—C11 | 0.7 (3) |
| C6—C2—C3—C4 | 178.02 (14) | S1—C9—C10—C11 | 178.93 (14) |
| C1—C2—C3—C5 | 178.14 (14) | C9—C10—C11—C12 | 0.3 (3) |
| C6—C2—C3—C5 | −2.7 (2) | C10—C11—C12—C13 | −0.4 (3) |
| C2—C3—C4—N | 0.53 (15) | C11—C12—C13—C14 | −0.5 (3) |
| C5—C3—C4—N | −178.84 (13) | C12—C13—C14—C9 | 1.4 (3) |
| C2—C3—C4—C8 | −179.04 (17) | C10—C9—C14—C13 | −1.6 (2) |
| C5—C3—C4—C8 | 1.6 (3) | S1—C9—C14—C13 | −179.77 (14) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O3 | 0.93 | 2.46 | 3.077 (2) | 124 |
| C7—H7A···O4 | 0.96 | 2.33 | 3.021 (3) | 128 |
| C11—H11···O4i | 0.93 | 2.53 | 3.456 (2) | 174 |
| C13—H13···O2i | 0.93 | 2.52 | 3.304 (2) | 143 |
| C14—H14···O3ii | 0.93 | 2.57 | 3.383 (2) | 146 |
Symmetry codes: (i) x, y, z−1; i; (ii) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2307).
References
- Ali, R., Misra, B. & Nizamuddin (1989). Indian J. Chem. Sect. B, 28, 526–528.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Amal Raj, A., Raghunathan, R., Sridevikumari, M. R. & Raman, N. (2003). Bioorg. Med. Chem.11, 407–419. [DOI] [PubMed]
- Beddoes, R. L., Dalton, L., Joule, J. A., Mills, O. S., Street, J. O. & Watt, C. I. F. (1986). J. Chem. Soc. Perkin Trans. 2, pp. 787–797.
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Nardelli, M. (1995). J. Appl. Cryst.28, 659.
- Sheldrick, G. M. (2001). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809004425/wn2307sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004425/wn2307Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




