Abstract
In the molecule of the title compound, C16H19O4P, the coordination around the P atom is distorted tetrahedral. The aromatic rings are oriented at a dihedral angle of 72.28 (11)°. Intramolecular C—H⋯O hydrogen bonding result in the formation of five- and six-membered rings. In the crystal structure, intermolecular C—H⋯O hydrogen bonds link the molecules. There is also a weak C—H⋯π interaction.
Related literature
For related structures, see: Hudson et al. (1993 ▶); Tahir et al. (2007 ▶); Wroblewski et al. (2000 ▶).
Experimental
Crystal data
C16H19O4P
M r = 306.28
Monoclinic,

a = 6.0671 (12) Å
b = 17.0962 (11) Å
c = 15.0502 (12) Å
β = 95.0021 (11)°
V = 1555.1 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.19 mm−1
T = 296 K
0.28 × 0.12 × 0.10 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.968, T max = 0.982
2800 measured reflections
2794 independent reflections
1655 reflections with I > 2σ(I)
R int = 0.059
3 standard reflections frequency: 120 min intensity decay: −1.3%
Refinement
R[F 2 > 2σ(F 2)] = 0.064
wR(F 2) = 0.159
S = 1.01
2794 reflections
191 parameters
H-atom parameters constrained
Δρmax = 0.40 e Å−3
Δρmin = −0.35 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1994 ▶); cell refinement: CAD-4 EXPRESS; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SHELXS86 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and PLATON (Spek, 2003 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004036/hk2619sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004036/hk2619Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O2i | 0.8200 | 1.9000 | 2.710 (3) | 172.00 |
| C2—H2⋯O4 | 0.9300 | 2.5200 | 2.956 (4) | 109.00 |
| C6—H6⋯O1 | 0.9300 | 2.3000 | 2.671 (4) | 103.00 |
| C14—H14⋯O1ii | 0.9300 | 2.5700 | 3.432 (5) | 154.00 |
| C16—H16A⋯O3ii | 0.9600 | 2.4700 | 3.336 (5) | 149.00 |
| C15—H15B⋯CgA | 0.9600 | 2.7200 | 3.608 (5) | 154.00 |
Symmetry codes: (i)  ; (ii)
; (ii)  . CgA is centroid of the C1–C6 ring.
. CgA is centroid of the C1–C6 ring.
supplementary crystallographic information
Comment
The crystal structure of (R)-dimethyl [(2-chlorophenyl)hydroxymethyl]- phosphonate (Tahir et al., 2007), which is a member of α-hydroxy phosphonates, has been reported, previously. In continuation to the study of such organic compounds, we report herein the synthesis and crystal structure of the title compound, (I).
In the molecule of (I), (Fig. 1) the coordination around the P atom is a distorted tetrahedral. The crystal structure of dimethyl α-chloromethyl-α -hydroxybenzylphosphonate, (II) (Hudson et al., 1993) and C24H26NO4P, (III) (Wroblewski et al., 2000) have also been reported, in which both of them have similar coordinations around the C-atom having α-hydroxy group. In (I), the benzene rings A (C1-C6) and B (C9-C14) are oriented at a dihedral angle of 72.28 (11)°. The intramolecular C-H···O hydrogen bonds (Table 1) result in the formations of five- and six-membered rings C (O1/C1/C6/C7/H6) and D (P1/O4/C1/C2/C7/H2), respectively, having planar and boat conformations.
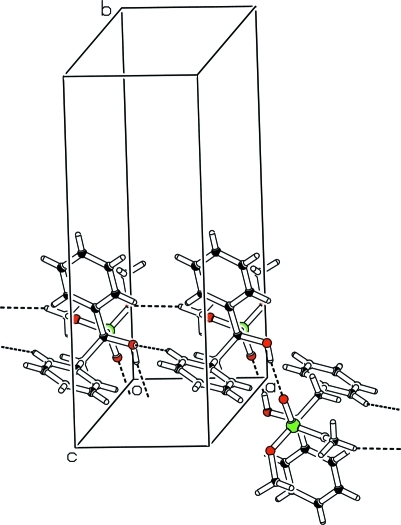
In the crystal structure, intermolecular O-H···O and C-H···O hydrogen bonds (Table 1) link the molecules (Fig. 2), in which they may be effective in the stabilization of the structure. There is also a weak C—H···π interaction (Table 1).
Experimental
For the preparation of the title compound, 2-phenylacetophenone (1.96 g, 10 mmol) was disolved in dimethylphosphonate (1.10 g, 10 mmol) at room temperature. Then, KF (2.5 g) and ψ-Al2O3 (2.5 g) were added partwise and the mixture was kept at room temperature for 72 h. The product was extracted twice with 50 ml portions of a dichloromethane/methanol mixture (1:1). After the evaporation of the solvent on a rotary evaporator, the residue was crystallized from a mixture of diethyl ether/acetone (3:1) (m.p. 414 K).
Refinement
H atoms were positioned geometrically, with O-H = 0.82 Å (for OH), C-H = 0.93, 0.96 and 0.97 Å for aromatic, methyl and methylene H, and constrained to ride on their parent atoms with Uiso(H) = xUeq(C), where x = 1.5 for methyl H, and x = 1.2 for all other H atoms.
Figures
Fig. 1.
The molecular structure of the title molecule, with the atom-numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
A partial packing diagram of the title compound. Hydrogen bonds are shown as dashed lines.
Crystal data
| C16H19O4P | F(000) = 648 |
| Mr = 306.28 | Dx = 1.308 Mg m−3 |
| Monoclinic, P21/c | Melting point = 83–86 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.0671 (12) Å | Cell parameters from 15 reflections |
| b = 17.0962 (11) Å | θ = 10.0–11.3° |
| c = 15.0502 (12) Å | µ = 0.19 mm−1 |
| β = 95.0021 (11)° | T = 296 K |
| V = 1555.1 (3) Å3 | Needle, colorless |
| Z = 4 | 0.28 × 0.12 × 0.10 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.059 |
| ω/2θ scans | θmax = 25.2°, θmin = 2.4° |
| Absorption correction: ψ scan (North et al., 1968) | h = 0→7 |
| Tmin = 0.968, Tmax = 0.982 | k = 0→20 |
| 2800 measured reflections | l = −18→17 |
| 2794 independent reflections | 3 standard reflections every 120 min |
| 1655 reflections with I > 2σ(I) | intensity decay: −1.3% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.064 | H-atom parameters constrained |
| wR(F2) = 0.159 | w = 1/[σ2(Fo2) + (0.088P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 2794 reflections | Δρmax = 0.40 e Å−3 |
| 191 parameters | Δρmin = −0.35 e Å−3 |
| 0 restraints |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| P1 | 0.79483 (14) | 0.12098 (5) | −0.05193 (6) | 0.0416 (3) | |
| O1 | 1.0479 (3) | 0.10996 (12) | 0.09544 (16) | 0.0460 (8) | |
| O2 | 0.8463 (4) | 0.04357 (13) | −0.08487 (15) | 0.0576 (9) | |
| O3 | 0.9546 (5) | 0.18125 (15) | −0.08944 (18) | 0.0659 (10) | |
| O4 | 0.5517 (4) | 0.14911 (15) | −0.07722 (15) | 0.0582 (9) | |
| C1 | 0.7793 (5) | 0.21108 (17) | 0.1007 (2) | 0.0368 (10) | |
| C2 | 0.5716 (5) | 0.24481 (19) | 0.0869 (2) | 0.0444 (11) | |
| C3 | 0.5409 (6) | 0.3227 (2) | 0.1076 (2) | 0.0533 (12) | |
| C4 | 0.7133 (7) | 0.3671 (2) | 0.1449 (3) | 0.0567 (14) | |
| C5 | 0.9175 (7) | 0.3333 (2) | 0.1623 (3) | 0.0555 (14) | |
| C6 | 0.9516 (6) | 0.25648 (18) | 0.1401 (2) | 0.0470 (11) | |
| C7 | 0.8226 (5) | 0.12778 (17) | 0.0709 (2) | 0.0387 (10) | |
| C8 | 0.6654 (5) | 0.06725 (18) | 0.1090 (2) | 0.0435 (11) | |
| C9 | 0.6703 (6) | 0.06607 (17) | 0.2091 (2) | 0.0411 (10) | |
| C10 | 0.8471 (6) | 0.03455 (19) | 0.2618 (2) | 0.0522 (12) | |
| C11 | 0.8457 (7) | 0.0313 (2) | 0.3538 (2) | 0.0573 (14) | |
| C12 | 0.6657 (8) | 0.0583 (2) | 0.3943 (3) | 0.0628 (14) | |
| C13 | 0.4908 (8) | 0.0895 (2) | 0.3432 (3) | 0.0635 (16) | |
| C14 | 0.4917 (6) | 0.0931 (2) | 0.2520 (3) | 0.0537 (12) | |
| C15 | 0.9185 (9) | 0.2641 (2) | −0.0979 (3) | 0.0863 (19) | |
| C16 | 0.4445 (7) | 0.1432 (3) | −0.1647 (3) | 0.0733 (16) | |
| H1 | 1.06744 | 0.06272 | 0.09092 | 0.0552* | |
| H2 | 0.45212 | 0.21495 | 0.06361 | 0.0534* | |
| H3 | 0.40178 | 0.34518 | 0.09602 | 0.0636* | |
| H4 | 0.69192 | 0.41953 | 0.15824 | 0.0679* | |
| H5 | 1.03397 | 0.36254 | 0.18933 | 0.0666* | |
| H6 | 1.09151 | 0.23458 | 0.15143 | 0.0565* | |
| H8A | 0.51529 | 0.07819 | 0.08461 | 0.0520* | |
| H8B | 0.70402 | 0.01557 | 0.08879 | 0.0520* | |
| H10 | 0.96854 | 0.01529 | 0.23501 | 0.0623* | |
| H11 | 0.96685 | 0.01083 | 0.38820 | 0.0688* | |
| H12 | 0.66330 | 0.05520 | 0.45591 | 0.0750* | |
| H13 | 0.36975 | 0.10860 | 0.37031 | 0.0764* | |
| H14 | 0.37005 | 0.11401 | 0.21825 | 0.0644* | |
| H15A | 1.04258 | 0.28789 | −0.12280 | 0.1294* | |
| H15B | 0.90211 | 0.28613 | −0.04020 | 0.1294* | |
| H15C | 0.78662 | 0.27362 | −0.13644 | 0.1294* | |
| H16A | 0.29675 | 0.16336 | −0.16523 | 0.1095* | |
| H16B | 0.43893 | 0.08928 | −0.18277 | 0.1095* | |
| H16C | 0.52532 | 0.17277 | −0.20525 | 0.1095* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P1 | 0.0398 (5) | 0.0398 (5) | 0.0458 (5) | 0.0101 (4) | 0.0066 (4) | −0.0001 (4) |
| O1 | 0.0323 (13) | 0.0371 (12) | 0.0669 (15) | 0.0059 (10) | −0.0053 (11) | −0.0040 (11) |
| O2 | 0.0693 (17) | 0.0495 (15) | 0.0533 (14) | 0.0195 (13) | 0.0007 (12) | −0.0048 (11) |
| O3 | 0.0681 (18) | 0.0571 (17) | 0.0766 (17) | 0.0082 (13) | 0.0295 (14) | 0.0097 (13) |
| O4 | 0.0503 (15) | 0.0781 (17) | 0.0450 (14) | 0.0233 (13) | −0.0034 (11) | −0.0082 (12) |
| C1 | 0.0363 (18) | 0.0357 (17) | 0.0389 (17) | 0.0016 (14) | 0.0064 (14) | 0.0009 (13) |
| C2 | 0.0339 (19) | 0.047 (2) | 0.053 (2) | 0.0006 (15) | 0.0075 (14) | −0.0066 (15) |
| C3 | 0.051 (2) | 0.051 (2) | 0.060 (2) | 0.0171 (19) | 0.0163 (18) | −0.0002 (17) |
| C4 | 0.071 (3) | 0.0351 (19) | 0.067 (2) | 0.0015 (19) | 0.024 (2) | −0.0090 (17) |
| C5 | 0.057 (3) | 0.041 (2) | 0.069 (2) | −0.0050 (18) | 0.0085 (19) | −0.0118 (17) |
| C6 | 0.0391 (19) | 0.042 (2) | 0.059 (2) | −0.0002 (15) | 0.0000 (16) | 0.0002 (16) |
| C7 | 0.0306 (18) | 0.0371 (17) | 0.0476 (18) | 0.0025 (14) | −0.0013 (14) | −0.0015 (14) |
| C8 | 0.0370 (19) | 0.0395 (18) | 0.053 (2) | −0.0038 (14) | −0.0009 (15) | 0.0002 (15) |
| C9 | 0.0426 (19) | 0.0294 (16) | 0.0501 (19) | −0.0046 (14) | −0.0022 (16) | −0.0009 (14) |
| C10 | 0.054 (2) | 0.048 (2) | 0.054 (2) | 0.0105 (17) | 0.0010 (17) | 0.0012 (16) |
| C11 | 0.063 (3) | 0.053 (2) | 0.054 (2) | 0.0051 (19) | −0.0051 (19) | 0.0080 (18) |
| C12 | 0.085 (3) | 0.059 (2) | 0.045 (2) | −0.010 (2) | 0.009 (2) | −0.0025 (18) |
| C13 | 0.062 (3) | 0.068 (2) | 0.063 (3) | 0.000 (2) | 0.019 (2) | −0.004 (2) |
| C14 | 0.041 (2) | 0.055 (2) | 0.065 (2) | −0.0028 (16) | 0.0034 (18) | 0.0010 (17) |
| C15 | 0.131 (4) | 0.050 (3) | 0.082 (3) | −0.005 (3) | 0.032 (3) | 0.010 (2) |
| C16 | 0.061 (3) | 0.098 (3) | 0.059 (2) | 0.012 (2) | −0.006 (2) | −0.001 (2) |
Geometric parameters (Å, °)
| P1—O2 | 1.457 (2) | C12—C13 | 1.364 (6) |
| P1—O3 | 1.554 (3) | C13—C14 | 1.374 (6) |
| P1—O4 | 1.567 (3) | C2—H2 | 0.9300 |
| P1—C7 | 1.845 (3) | C3—H3 | 0.9300 |
| O1—C7 | 1.418 (4) | C4—H4 | 0.9300 |
| O3—C15 | 1.437 (4) | C5—H5 | 0.9300 |
| O4—C16 | 1.420 (5) | C6—H6 | 0.9300 |
| O1—H1 | 0.8200 | C8—H8A | 0.9700 |
| C1—C6 | 1.392 (5) | C8—H8B | 0.9700 |
| C1—C7 | 1.523 (4) | C10—H10 | 0.9300 |
| C1—C2 | 1.385 (4) | C11—H11 | 0.9300 |
| C2—C3 | 1.384 (5) | C12—H12 | 0.9300 |
| C3—C4 | 1.372 (5) | C13—H13 | 0.9300 |
| C4—C5 | 1.371 (6) | C14—H14 | 0.9300 |
| C5—C6 | 1.375 (5) | C15—H15A | 0.9600 |
| C7—C8 | 1.551 (4) | C15—H15B | 0.9600 |
| C8—C9 | 1.504 (4) | C15—H15C | 0.9600 |
| C9—C14 | 1.388 (5) | C16—H16A | 0.9600 |
| C9—C10 | 1.386 (5) | C16—H16B | 0.9600 |
| C10—C11 | 1.387 (4) | C16—H16C | 0.9600 |
| C11—C12 | 1.376 (6) | ||
| O1···O2 | 3.094 (3) | C9···H16Bvi | 2.7600 |
| O1···O3 | 3.045 (4) | C10···H16Bvi | 2.9200 |
| O1···C10 | 3.154 (4) | C10···H1 | 3.0400 |
| O1···O2i | 2.710 (3) | C11···H5vii | 3.0600 |
| O2···O1i | 2.710 (3) | C12···H15Ciii | 3.0100 |
| O2···O1 | 3.094 (3) | C13···H15Ciii | 2.9500 |
| O3···C16ii | 3.336 (5) | C16···H15C | 3.0500 |
| O3···O1 | 3.045 (4) | H1···O2 | 2.8800 |
| O4···C2 | 2.956 (4) | H1···C10 | 3.0400 |
| O1···H14ii | 2.5700 | H1···H8B | 2.3500 |
| O1···H6 | 2.3000 | H1···H10 | 2.4400 |
| O1···H10 | 2.7300 | H1···O2i | 1.9000 |
| O1···H8Aii | 2.9100 | H2···O4 | 2.5200 |
| O2···H16B | 2.8700 | H2···C8 | 2.8900 |
| O2···H8Bi | 2.9100 | H2···H8A | 2.3900 |
| O2···H10i | 2.8000 | H5···C11viii | 3.0600 |
| O2···H1i | 1.9000 | H6···O1 | 2.3000 |
| O2···H8B | 2.8600 | H8A···O1v | 2.9100 |
| O2···H1 | 2.8800 | H8A···O4 | 2.7500 |
| O3···H16Aii | 2.4700 | H8A···C2 | 2.8700 |
| O4···H2 | 2.5200 | H8A···H2 | 2.3900 |
| O4···H8A | 2.7500 | H8A···H14 | 2.3500 |
| O4···H15C | 2.7500 | H8B···O2 | 2.8600 |
| C1···C15 | 3.303 (5) | H8B···H1 | 2.3500 |
| C2···O4 | 2.956 (4) | H8B···O2i | 2.9100 |
| C2···C9 | 3.590 (4) | H8B···H16Bvi | 2.4900 |
| C3···C16iii | 3.574 (5) | H10···O1 | 2.7300 |
| C4···C16iii | 3.423 (6) | H10···H1 | 2.4400 |
| C6···C15 | 3.572 (5) | H10···O2i | 2.8000 |
| C9···C2 | 3.590 (4) | H14···O1v | 2.5700 |
| C10···O1 | 3.154 (4) | H14···H8A | 2.3500 |
| C11···C15iii | 3.592 (5) | H15B···C1 | 2.6400 |
| C12···C15iii | 3.399 (5) | H15B···C2 | 2.9700 |
| C15···C1 | 3.303 (5) | H15B···C6 | 2.7500 |
| C15···C11iv | 3.592 (5) | H15C···O4 | 2.7500 |
| C15···C12iv | 3.399 (5) | H15C···C16 | 3.0500 |
| C15···C6 | 3.572 (5) | H15C···H16C | 2.5000 |
| C16···O3v | 3.336 (5) | H15C···C12iv | 3.0100 |
| C16···C3iv | 3.574 (5) | H15C···C13iv | 2.9500 |
| C16···C4iv | 3.423 (6) | H16A···O3v | 2.4700 |
| C1···H15B | 2.6400 | H16B···O2 | 2.8700 |
| C2···H8A | 2.8700 | H16B···C8vi | 2.9900 |
| C2···H15B | 2.9700 | H16B···C9vi | 2.7600 |
| C3···H16Ciii | 2.8300 | H16B···C10vi | 2.9200 |
| C4···H16Ciii | 2.7000 | H16B···H8Bvi | 2.4900 |
| C6···H15B | 2.7500 | H16C···H15C | 2.5000 |
| C8···H16Bvi | 2.9900 | H16C···C3iv | 2.8300 |
| C8···H2 | 2.8900 | H16C···C4iv | 2.7000 |
| O2—P1—O3 | 108.65 (15) | C4—C3—H3 | 120.00 |
| O2—P1—O4 | 114.93 (14) | C3—C4—H4 | 120.00 |
| O2—P1—C7 | 113.24 (14) | C5—C4—H4 | 120.00 |
| O3—P1—O4 | 108.19 (15) | C4—C5—H5 | 120.00 |
| O3—P1—C7 | 108.47 (14) | C6—C5—H5 | 120.00 |
| O4—P1—C7 | 103.05 (13) | C1—C6—H6 | 120.00 |
| P1—O3—C15 | 126.2 (3) | C5—C6—H6 | 120.00 |
| P1—O4—C16 | 123.3 (2) | C7—C8—H8A | 109.00 |
| C7—O1—H1 | 109.00 | C7—C8—H8B | 109.00 |
| C2—C1—C6 | 118.1 (3) | C9—C8—H8A | 109.00 |
| C6—C1—C7 | 120.3 (3) | C9—C8—H8B | 109.00 |
| C2—C1—C7 | 121.6 (3) | H8A—C8—H8B | 108.00 |
| C1—C2—C3 | 120.4 (3) | C9—C10—H10 | 119.00 |
| C2—C3—C4 | 120.8 (3) | C11—C10—H10 | 120.00 |
| C3—C4—C5 | 119.3 (3) | C10—C11—H11 | 120.00 |
| C4—C5—C6 | 120.6 (4) | C12—C11—H11 | 120.00 |
| C1—C6—C5 | 120.8 (3) | C11—C12—H12 | 120.00 |
| P1—C7—C1 | 110.6 (2) | C13—C12—H12 | 120.00 |
| P1—C7—C8 | 108.9 (2) | C12—C13—H13 | 120.00 |
| O1—C7—C1 | 108.2 (2) | C14—C13—H13 | 120.00 |
| O1—C7—C8 | 111.7 (2) | C9—C14—H14 | 119.00 |
| C1—C7—C8 | 112.8 (2) | C13—C14—H14 | 119.00 |
| P1—C7—O1 | 104.4 (2) | O3—C15—H15A | 109.00 |
| C7—C8—C9 | 114.9 (3) | O3—C15—H15B | 109.00 |
| C8—C9—C10 | 121.7 (3) | O3—C15—H15C | 109.00 |
| C10—C9—C14 | 117.4 (3) | H15A—C15—H15B | 109.00 |
| C8—C9—C14 | 120.9 (3) | H15A—C15—H15C | 109.00 |
| C9—C10—C11 | 121.0 (3) | H15B—C15—H15C | 109.00 |
| C10—C11—C12 | 120.2 (4) | O4—C16—H16A | 110.00 |
| C11—C12—C13 | 119.3 (4) | O4—C16—H16B | 109.00 |
| C12—C13—C14 | 120.7 (4) | O4—C16—H16C | 110.00 |
| C9—C14—C13 | 121.4 (4) | H16A—C16—H16B | 109.00 |
| C1—C2—H2 | 120.00 | H16A—C16—H16C | 110.00 |
| C3—C2—H2 | 120.00 | H16B—C16—H16C | 109.00 |
| C2—C3—H3 | 120.00 | ||
| O2—P1—O3—C15 | 158.6 (3) | C2—C1—C7—C8 | −55.0 (4) |
| O4—P1—O3—C15 | 33.2 (3) | C6—C1—C7—P1 | −110.6 (3) |
| C7—P1—O3—C15 | −77.9 (3) | C6—C1—C7—O1 | 3.2 (4) |
| O2—P1—O4—C16 | −47.0 (3) | C6—C1—C7—C8 | 127.3 (3) |
| O3—P1—O4—C16 | 74.6 (3) | C1—C2—C3—C4 | −2.3 (5) |
| C7—P1—O4—C16 | −170.7 (3) | C2—C3—C4—C5 | −0.5 (6) |
| O2—P1—C7—O1 | 60.7 (2) | C3—C4—C5—C6 | 2.1 (6) |
| O2—P1—C7—C1 | 176.8 (2) | C4—C5—C6—C1 | −0.9 (6) |
| O2—P1—C7—C8 | −58.8 (2) | P1—C7—C8—C9 | 180.0 (2) |
| O3—P1—C7—O1 | −60.0 (2) | O1—C7—C8—C9 | 65.2 (3) |
| O3—P1—C7—C1 | 56.1 (2) | C1—C7—C8—C9 | −56.9 (3) |
| O3—P1—C7—C8 | −179.5 (2) | C7—C8—C9—C10 | −73.5 (4) |
| O4—P1—C7—O1 | −174.51 (18) | C7—C8—C9—C14 | 109.9 (3) |
| O4—P1—C7—C1 | −58.4 (2) | C8—C9—C10—C11 | −177.6 (3) |
| O4—P1—C7—C8 | 66.0 (2) | C14—C9—C10—C11 | −0.8 (5) |
| C6—C1—C2—C3 | 3.4 (4) | C8—C9—C14—C13 | 177.4 (3) |
| C7—C1—C2—C3 | −174.4 (3) | C10—C9—C14—C13 | 0.6 (5) |
| C2—C1—C6—C5 | −1.8 (5) | C9—C10—C11—C12 | 1.1 (5) |
| C7—C1—C6—C5 | 176.0 (3) | C10—C11—C12—C13 | −1.2 (5) |
| C2—C1—C7—P1 | 67.1 (3) | C11—C12—C13—C14 | 1.0 (6) |
| C2—C1—C7—O1 | −179.1 (3) | C12—C13—C14—C9 | −0.7 (6) |
Symmetry codes: (i) −x+2, −y, −z; (ii) x+1, y, z; (iii) x, −y+1/2, z+1/2; (iv) x, −y+1/2, z−1/2; (v) x−1, y, z; (vi) −x+1, −y, −z; (vii) −x+2, y−1/2, −z+1/2; (viii) −x+2, y+1/2, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O2i | 0.8200 | 1.9000 | 2.710 (3) | 172.00 |
| C2—H2···O4 | 0.9300 | 2.5200 | 2.956 (4) | 109.00 |
| C6—H6···O1 | 0.9300 | 2.3000 | 2.671 (4) | 103.00 |
| C14—H14···O1v | 0.9300 | 2.5700 | 3.432 (5) | 154.00 |
| C16—H16A···O3v | 0.9600 | 2.4700 | 3.336 (5) | 149.00 |
| C15—H15B···CgA | 0.9600 | 2.7200 | 3.608 (5) | 154.00 |
Symmetry codes: (i) −x+2, −y, −z; (v) x−1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HK2619).
References
- Enraf–Nonius (1994). CAD-4 EXPRESS Enraf–Nonius, Delft, The Netherlands.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- Hudson, H. R., McPartlin, M., Matthews, R. W., Powell, H. R., Yusuf, R. O., Jaszay, Z. M., Keglevich, G., Petnehazy, I. & Toke, L. (1993). Phosphorus Sulfur Silicon Relat. Elem 79, 239–243.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Tahir, M. N., Acar, N., Yilmaz, H., Danish, M. & Ülkü, D. (2007). Acta Cryst. E63, o3817–o3818.
- Wroblewski, A. E., Maniukiewicz, W. & Karolczak, W. (2000). J. Chem. Soc. Perkin Trans. 1, pp. 1433–1435.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004036/hk2619sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004036/hk2619Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report