Abstract
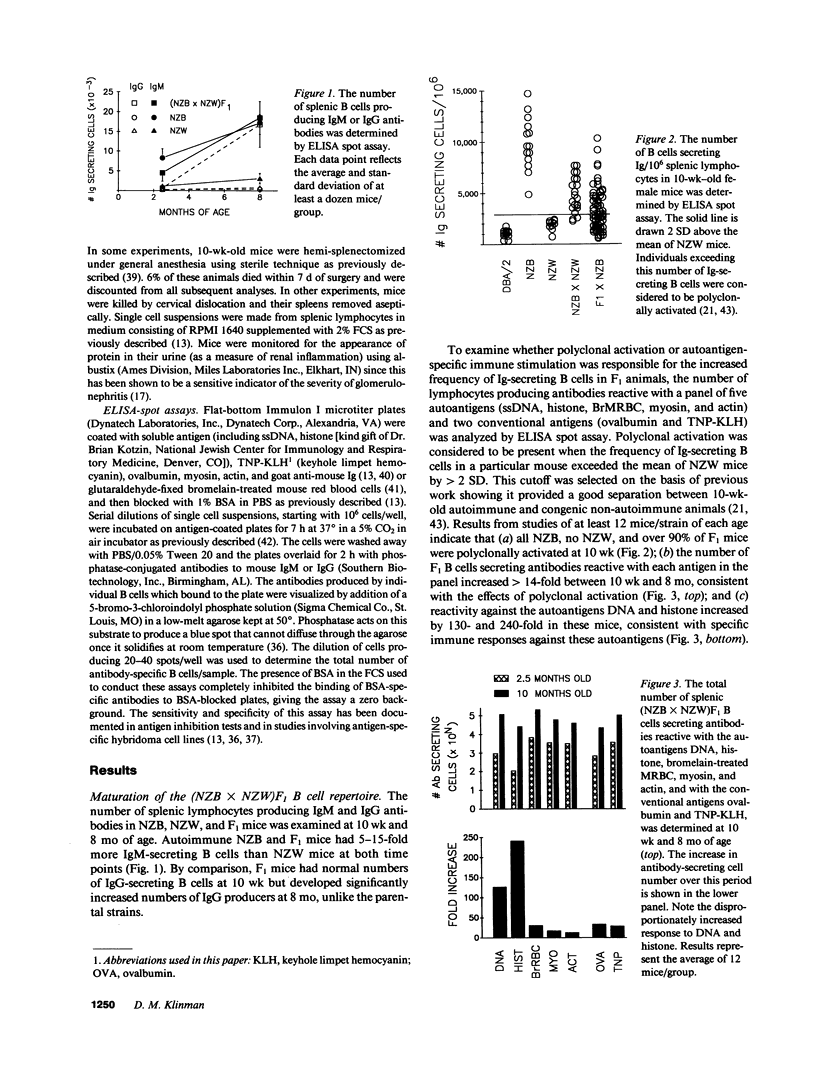
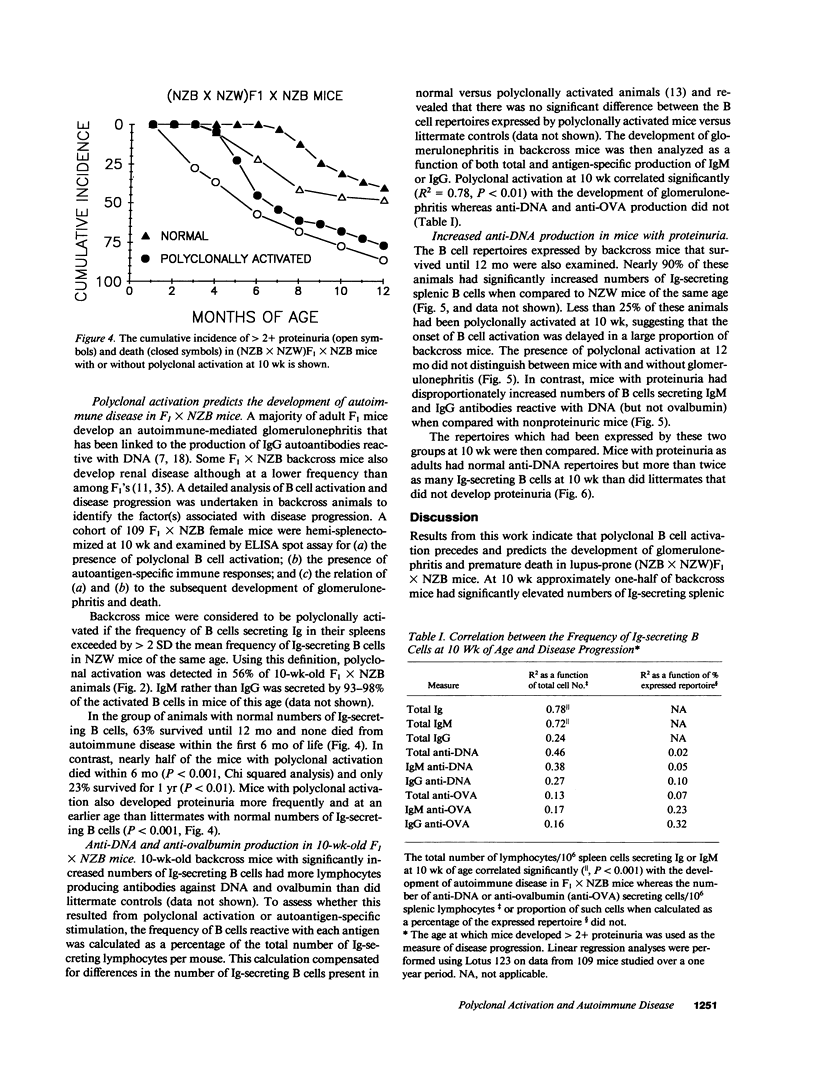
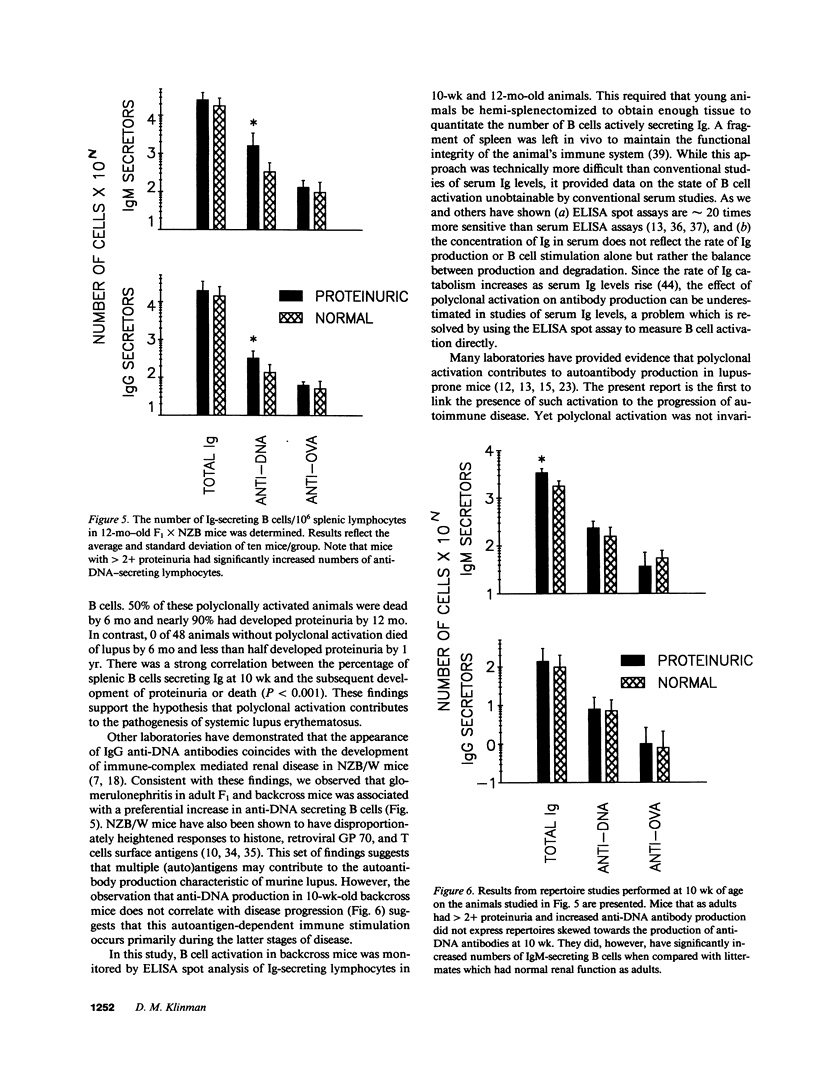
Polyclonal B cell activation is an early feature of autoimmune disease in humans and mice with systemic lupus erythematosus. The contribution of polyclonal activation to the progression of autoimmunity is unclear, however, since it precedes the development of end-organ damage by months or years. To examine this issue, 109 autoimmune-prone (NZB X NZW)F1 X NZB backcross mice were hemi-splenectomized at 10 wk and the number and antigenic specificity of their Ig-secreting B cells quantitated by ELISA spot assay. Of the 61 mice that had polyclonally increased numbers of Ig-secreting cells/spleen, 31 died by 6 mo. In contrast, 0/48 backcross mice with normal numbers of Ig-secreting B cells at 10 wk died over the same period (P less than 0.001). Polyclonally activated mice also developed proteinuria earlier and more frequently than littermates with normal numbers of Ig-secreting cells (P less than 0.001). As adults, backcross mice with proteinuria expressed repertoires skewed towards the production of anti-DNA antibodies. At 10 wk these same mice expressed repertoires marked by polyclonal activation rather than preferential anti-DNA production. These findings indicate that autoimmune disease in SLE is accompanied by the autoantigen-driven production of autoantibodies but is preceded and predicted by polyclonal B cell activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando D. G., Ebling F. M., Hahn B. H. Detection of native and denatured DNA antibody forming cells by the enzyme-linked immunospot assay. A clinical study of (New Zealand black x New Zealand white)F1 mice. Arthritis Rheum. 1986 Sep;29(9):1139–1146. doi: 10.1002/art.1780290912. [DOI] [PubMed] [Google Scholar]
- Blaese R. M., Grayson J., Steinberg A. D. Increased immunoglobulin-secreting cells in the blood of patients with active systemic lupus erythematosus. Am J Med. 1980 Sep;69(3):345–350. doi: 10.1016/0002-9343(80)90003-0. [DOI] [PubMed] [Google Scholar]
- Bocchieri M. H., Cooke A., Smith J. B., Weigert M., Riblet R. J. Independent segregation of NZB immune abnormalities in NZB x C58 recombinant inbred mice. Eur J Immunol. 1982 Apr;12(4):349–354. doi: 10.1002/eji.1830120417. [DOI] [PubMed] [Google Scholar]
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989 Nov;10(11):364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Datta S. K., Patel H., Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987 May 1;165(5):1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. A., Craven S. Y., Cohen P. L. Isotype progression and clonality of anti-Sm autoantibodies in MRL/Mp-lpr/lpr mice. J Immunol. 1987 Aug 1;139(3):728–733. [PubMed] [Google Scholar]
- Eisenberg R. A., Winfield J. B., Cohen P. L. Subclass restriction of anti-Sm antibodies in MRL mice. J Immunol. 1982 Nov;129(5):2146–2149. [PubMed] [Google Scholar]
- Feldman M. D., Huston D. P., Karsh J., Balow J. E., Klima E., Steinberg A. D. Correlation of serum IgG, IgM, and anti-native-DNA antibodies with renal and clinical indexes of activity in systemic lupus erythematosus. J Rheumatol. 1982 Jan-Feb;9(1):52–58. [PubMed] [Google Scholar]
- Gioud M., Kotzin B. L., Rubin R. L., Joslin F. G., Tan E. M. In vivo and in vitro production of anti-histone antibodies in NZB/NZW mice. J Immunol. 1983 Jul;131(1):269–274. [PubMed] [Google Scholar]
- Herron L. R., Coffman R. L., Bond M. W., Kotzin B. L. Increased autoantibody production by NZB/NZW B cells in response to IL-5. J Immunol. 1988 Aug 1;141(3):842–848. [PubMed] [Google Scholar]
- Hirose S., Nagasawa R., Sekikawa I., Hamaoki M., Ishida Y., Sato H., Shirai T. Enhancing effect of H-2-linked NZW gene(s) on the autoimmune traits of (NZB X NZW)F1 mice. J Exp Med. 1983 Jul 1;158(1):228–233. doi: 10.1084/jem.158.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigatsubo Y., Steinberg A. D., Klinman D. M. Autoantibody production is associated with polyclonal B cell activation in autoimmune mice which express the lpr or gld genes. Eur J Immunol. 1988 Jul;18(7):1089–1093. doi: 10.1002/eji.1830180718. [DOI] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Jyonouchi H., Kincade P. W., Good R. A., Gershwin M. E. B lymphocyte lineage cells in newborn and very young NZB mice: evidence for regulatory disorders affecting B cell formation. J Immunol. 1983 Nov;131(5):2219–2225. [PubMed] [Google Scholar]
- Klinman D. M., Eisenberg R. A., Steinberg A. D. Development of the autoimmune B cell repertoire in MRL-lpr/lpr mice. J Immunol. 1990 Jan 15;144(2):506–511. [PubMed] [Google Scholar]
- Klinman D. M., Ishigatsubo Y., Steinberg A. D. Acquisition and maturation of expressed B cell repertoires in normal and autoimmune mice. J Immunol. 1988 Aug 1;141(3):801–806. [PubMed] [Google Scholar]
- Klinman D. M. Regulation of B cell activation in autoimmune mice. Clin Immunol Immunopathol. 1989 Nov;53(2 Pt 2):S25–S34. doi: 10.1016/0090-1229(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Novel ELISA and ELISA-spot assays used to quantitate B cells and serum antibodies specific for T cell and bromelated mouse red blood cell autoantigens. J Immunol Methods. 1987 Sep 24;102(2):157–164. doi: 10.1016/0022-1759(87)90072-x. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Proliferation of anti-DNA-producing NZB B cells in a non-autoimmune environment. J Immunol. 1986 Jul 1;137(1):69–75. [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D. Three genes for lupus nephritis in NZB x NZW mice. J Exp Med. 1978 Jun 1;147(6):1653–1660. doi: 10.1084/jem.147.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno A., Yoshida H., Sekita K., Maruyama N., Ozaki S., Hirose S., Shirai T. Genetic regulation of the class conversion of dsDNA-specific antibodies in (NZB X NZW)F1 hybrid. Immunogenetics. 1983;18(5):513–524. doi: 10.1007/BF00364392. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Palmer E. The contribution of NZW genes to lupus-like disease in (NZB x NZW)F1 mice. J Exp Med. 1987 May 1;165(5):1237–1251. doi: 10.1084/jem.165.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manny N., Datta S. K., Schwartz R. S. Synthesis of IgM by cells of NZB and SWR mice and their crosses. J Immunol. 1979 Apr;122(4):1220–1227. [PubMed] [Google Scholar]
- Maruyama N., Furukawa F., Nakai Y., Sasaki Y., Ohta K., Ozaki S., Hirose S., Shirai T. Genetic studies of autoimmunity in New Zealand mice. IV. Contribution of NZB and NZW genes to the spontaneous occurrence of retroviral gp70 immune complexes in (NZB X NZW)F1 hybrid and the correlation to renal disease. J Immunol. 1983 Feb;130(2):740–746. [PubMed] [Google Scholar]
- Miller M. L., Raveche E. S., Laskin C. A., Klinman D. M., Steinberg A. D. Genetic studies in NZB mice. VI. Association of autoimmune traits in recombinant inbred lines. J Immunol. 1984 Sep;133(3):1325–1331. [PubMed] [Google Scholar]
- Monestier M., Manheimer-Lory A., Bellon B., Painter C., Dang H., Talal N., Zanetti M., Schwartz R., Pisetsky D., Kuppers R. Shared idiotypes and restricted immunoglobulin variable region heavy chain genes characterize murine autoantibodies of various specificities. J Clin Invest. 1986 Sep;78(3):753–759. doi: 10.1172/JCI112637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoian R., Pillarisetty R., Talal N. Immunological regulation of spontaneous antibodies to DNA and RNA. II. Sequential switch from IgM to IgG in NZB/NZW F1 mice. Immunology. 1977 Jan;32(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- Raveche E. S., Alabaster O., Tjio J. H., Taurog J., Steinberg A. D. Analysis of NZB hyperdiploid spleen cells. J Immunol. 1981 Jan;126(1):154–160. [PubMed] [Google Scholar]
- Raveche E. S., Novotny E. A., Hansen C. T., Tjio J. H., Steinberg A. D. Genetic studies in NZB mice. V. Recombinant inbred lines demonstrate that separate genes control autoimmune phenotype. J Exp Med. 1981 May 1;153(5):1187–1197. doi: 10.1084/jem.153.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveché E. S., Steinberg A. D., Klassen L. W., Tjio J. H. Genetic studies in NZB mice. I. Spontaneous autoantibody production. J Exp Med. 1978 May 1;147(5):1487–1502. doi: 10.1084/jem.147.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur P. H., Sandson J. Immunologic factors and clinical activity in systemic lupus erythematosus. N Engl J Med. 1968 Mar 7;278(10):533–538. doi: 10.1056/NEJM196803072781004. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shlomchik M., Nemazee D., van Snick J., Weigert M. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). II. Comparison of hybridomas derived by lipopolysaccharide stimulation and secondary protein immunization. J Exp Med. 1987 Apr 1;165(4):970–987. doi: 10.1084/jem.165.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A. D., Gelfand M. C., Hardin J. A., Lowenthal D. T. Therapeutic studies in NZB/W mice. III. Relationship between renal status and efficacy of immunosuppressive drug therapy. Arthritis Rheum. 1975 Jan-Feb;18(1):9–14. doi: 10.1002/art.1780180102. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Hay F. C. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976 Nov;26(2):363–370. [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Sakane T., Tsunematsu T. Hyperreactivity of activated B cells to B cell growth factor in patients with systemic lupus erythematosus. J Immunol. 1989 Dec 15;143(12):3988–3993. [PubMed] [Google Scholar]
- Umland S. P., Go N. F., Cupp J. E., Howard M. Responses of B cells from autoimmune mice to IL-5. J Immunol. 1989 Mar 1;142(5):1528–1535. [PubMed] [Google Scholar]
- Yoshida H., Kohno A., Ohta K., Hirose S., Maruyama N., Shirai T. Genetic studies of autoimmunity in New Zealand mice. III. Associations among anti-DNA antibodies, NTA, and renal disease in (NZB x NZW)F1 x NZW backcross mice. J Immunol. 1981 Aug;127(2):433–437. [PubMed] [Google Scholar]



