Abstract
Two chiral centres exist in the title compound, C12H12N4O3·H2O. Molecules are linked into chains by series of intermolecular N—H⋯O and O—H⋯N hydrogen bonds, which causes supramolecular aggregation. Two chiral centres are formed in the title compound. The indole and creatinine moieties make a dihedral angle of 56.75 (4)°. The crystal structure of the compound indicates the presence of equimolar enantiomers (RR and SS) in the crystal structure.
Related literature
For 2-indol-3-yl-methylenequinuclidin-3-ols NADPH oxidase activity, see: Sekhar et al. (2003 ▶). For novel substituted (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-one and (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol derivatives as potent thermal sensitizing agents, see: Sonar et al. (2007 ▶). For the crystal and molecular structure of isatin, see: Frolova et al. (1988 ▶). For the structure of 1,1′-diacetyl-3-hydroxy-2,2′,3,3′-tetrahydro-3,3′-bi(1H-indole)-2,2′-dione, see: Usman et al. (2002 ▶). The aldol condensation enolate mechanism by six-membered transition states has been described by Zimmerman & Traxler (1957 ▶).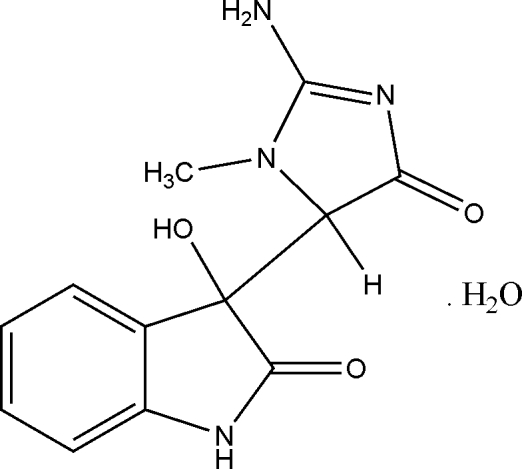
Experimental
Crystal data
C12H12N4O3·H2O
M r = 278.27
Monoclinic,
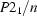
a = 8.3514 (1) Å
b = 10.7166 (2) Å
c = 13.9679 (2) Å
β = 104.755 (1)°
V = 1208.88 (3) Å3
Z = 4
Cu Kα radiation
μ = 0.99 mm−1
T = 90 K
0.20 × 0.15 × 0.06 mm
Data collection
Bruker X8 Proteum diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2006 ▶) T min = 0.780, T max = 0.943
17255 measured reflections
2181 independent reflections
2121 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.088
S = 1.04
2181 reflections
191 parameters
3 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.26 e Å−3
Δρmin = −0.27 e Å−3
Data collection: APEX2 (Bruker, 2006 ▶); cell refinement: APEX2 and SAINT (Bruker, 2006 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97 and local procedures.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004875/hg2475sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004875/hg2475Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1Wi | 0.88 | 1.96 | 2.8128 (15) | 163 |
| O8—H8⋯N12ii | 0.84 | 1.97 | 2.7984 (14) | 170 |
| N11—H11A⋯O13iii | 0.88 | 2.17 | 3.0371 (15) | 171 |
| N11—H11B⋯O1iv | 0.88 | 2.13 | 2.8678 (15) | 141 |
| O1W—H1W⋯O8 | 0.847 (18) | 2.049 (18) | 2.8812 (14) | 167.4 (19) |
| O1W—H2W⋯O13v | 0.861 (18) | 2.233 (18) | 3.0702 (14) | 164.1 (19) |
Symmetry codes: (i) 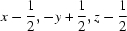 ; (ii)
; (ii) 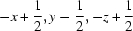 ; (iii)
; (iii) 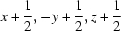 ; (iv)
; (iv) 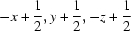 ; (v)
; (v) 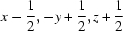 .
.
Acknowledgments
This investigation was supported by the NIH/National Cancer Institute [grant No. PO1CA104457 (to PAC)] and by the NSF [MRI grant No. CHE 0319176 (to SP)].
supplementary crystallographic information
Comment
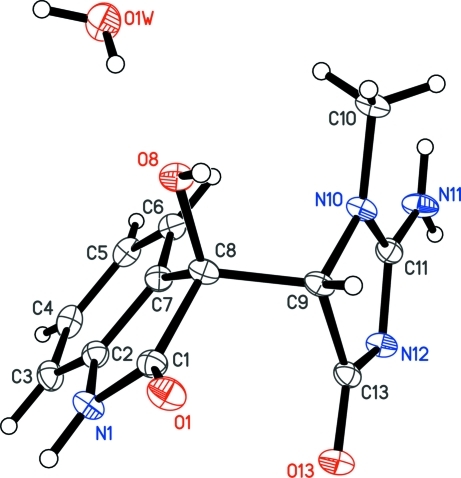
In our endeavor to design and synthesize novel radiosensitizers such as (Z)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-one and (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ol derivatives (Sekhar et al., 2003; Sonar et al., 2007), we have undertaken the design, synthesis and structural analysis of a series of 3-(2-amino-1-methyl-4-oxo-4,5-dihydro-1H-imidazol-5-yl)-3- hydroxyindolin-2-one analogs with different substituents on the indole moiety. The primary goal for X-ray analysis of the title compound is to confirm the stereochemistry of the molecule and to obtain detailed information on the structural conformation that may be useful in structure–activity relationship (SAR) analysis. The title compound was prepared by the aldol condensation of indol-2,3-dione (isatin) with 2-amino-1-methyl-1H-imidazol-4(5H)-one (creatinine) in the presence of sodium acetate in acetic acid under microwave irradiation. The compound was crystallized from 2% aqueous glycol. This aldol condensation reaction proceeds by the formation of the E-enolate, as per the Zimmerman–Traxler model (Zimmerman & Traxler, 1957), which favors anti products, and leads to the formation of equimolar RR and SS enantiomers. The molecular structure and the atom-numbering scheme are shown in Fig. 1. The isatin ring is planar (r.m.s. deviation = 0.0112 (10) Å) with bond distances and angles comparable with those previously reported for other isatin derivatives (Frolova et al., 1988; Usman et al., 2002). Atoms C8 and C9 are the two chiral centers of the title compound. The X-ray studies revealed that the obtained compound is racemic (having equimolar RR and SS enantiomers). The indole and creatinine moieties make a dihedral angle of 56.75 (4)°. Intermolecular N—H···O and O—H···N hydrogen bonds stabilize the crystal structure and form a supramolecular architecture.
Experimental
A mixture of isatin (1 mmol), creatinine (1.1 mmol) and sodium acetate (1.2 mmol) in acetic acid (1 ml) were irradiated in a domestic microwave oven for 40 sec with intermittent cooling every 10 sec. The reaction mixture was allowed to cool to room temperature, 10 ml of saturated sodium bicarbonate solution was added, and the mixture was stirred for 10 minutes. The precipitate thus obtained was collected by filtration, washed with cold water and dried, to afford the crude product. Crystallization from 2% aqueous glycol gave a light yellow crystalline product of 3-(2-amino-1-methyl-4-oxo-4,5-dihydro-1H- imidazol-5-yl)-3-hydroxyindolin-2-one monohydrate that was suitable for X-ray analysis. 1H NMR (DMSO-d6): δ 3.13 (s, 3H), 4.06 (s, 1H), 6.37 (s, 1H, OH), 6.73–6.75 (d, J = 7.5 Hz, 1H), 6.84–6.89 (t, J = 7.5 Hz, 1H), 7.04–7.06 (d, J = 7.5 Hz, 1H), 7.15–7.21 (t, J = 7.8 Hz, 1H), 7.51 (bs, 2H, NH2), 10.23 (s, 1H, NH) p.p.m.; 13C NMR (DMSO-d6): δ 32.59, 69.44, 76.28, 109.49, 121.1, 123.95, 127.98,129.34, 142.66, 171.76, 175.71, 182.26 p.p.m.
Refinement
Non-water H atoms were found in difference Fourier maps and subsequently placed in idealized positions with constrained distances of 0.98 Å (RCH3), 1.00 Å (R3CH), 0.95 Å (CArH), 0.84 Å (O—H) and 0.88 Å (N—H) distances. Uiso(H) values set to either 1.2Ueq or 1.5Ueq(RCH3, OH) of the attached atom. The water H atoms were refined subject to distance and angle restraints and assigned Uiso(H) values of 1.5Ueq of the water oxygen atom.
Figures
Fig. 1.
A view of the molecule with the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C12H12N4O3·H2O | F(000) = 584 |
| Mr = 278.27 | Dx = 1.529 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: -P 2yn | Cell parameters from 9952 reflections |
| a = 8.3514 (1) Å | θ = 5.3–68.0° |
| b = 10.7166 (2) Å | µ = 0.99 mm−1 |
| c = 13.9679 (2) Å | T = 90 K |
| β = 104.755 (1)° | Block, colourless |
| V = 1208.88 (3) Å3 | 0.20 × 0.15 × 0.06 mm |
| Z = 4 |
Data collection
| Bruker X8 Proteum diffractometer | 2181 independent reflections |
| Radiation source: fine-focus rotating anode | 2121 reflections with I > 2σ(I) |
| graded multilayer optics | Rint = 0.035 |
| Detector resolution: 5.6 pixels mm-1 | θmax = 68.0°, θmin = 5.3° |
| φ and ω scans | h = −10→10 |
| Absorption correction: multi-scan (SADABS in APEX2; Bruker, 2006) | k = −12→11 |
| Tmin = 0.780, Tmax = 0.943 | l = −16→16 |
| 17255 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.034 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.088 | w = 1/[σ2(Fo2) + (0.0431P)2 + 0.777P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 2181 reflections | Δρmax = 0.26 e Å−3 |
| 191 parameters | Δρmin = −0.27 e Å−3 |
| 3 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0029 (7) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.09392 (12) | 0.10429 (9) | 0.10391 (7) | 0.0184 (2) | |
| N1 | −0.15262 (14) | 0.29911 (10) | 0.15528 (8) | 0.0158 (3) | |
| H1 | −0.2381 | 0.3179 | 0.1060 | 0.019* | |
| C1 | −0.06677 (16) | 0.19095 (12) | 0.16253 (9) | 0.0146 (3) | |
| C2 | −0.08770 (16) | 0.37782 (12) | 0.23678 (9) | 0.0150 (3) | |
| C3 | −0.14037 (16) | 0.49625 (13) | 0.25289 (10) | 0.0183 (3) | |
| H3 | −0.2307 | 0.5349 | 0.2072 | 0.022* | |
| C4 | −0.05487 (17) | 0.55663 (13) | 0.33926 (10) | 0.0192 (3) | |
| H4 | −0.0875 | 0.6383 | 0.3527 | 0.023* | |
| C5 | 0.07679 (16) | 0.49981 (13) | 0.40589 (10) | 0.0179 (3) | |
| H5 | 0.1332 | 0.5433 | 0.4640 | 0.021* | |
| C6 | 0.12728 (16) | 0.37988 (12) | 0.38869 (9) | 0.0161 (3) | |
| H6 | 0.2163 | 0.3405 | 0.4348 | 0.019* | |
| C7 | 0.04456 (16) | 0.31943 (12) | 0.30268 (9) | 0.0143 (3) | |
| O8 | 0.03982 (11) | 0.09668 (8) | 0.32447 (6) | 0.0154 (2) | |
| H8 | 0.0481 | 0.0270 | 0.2986 | 0.023* | |
| C8 | 0.07032 (16) | 0.19298 (12) | 0.26163 (9) | 0.0142 (3) | |
| C9 | 0.24325 (16) | 0.17641 (12) | 0.24175 (9) | 0.0134 (3) | |
| H9 | 0.2513 | 0.0945 | 0.2090 | 0.016* | |
| C10 | 0.41472 (17) | 0.10072 (12) | 0.41347 (9) | 0.0171 (3) | |
| H10A | 0.5352 | 0.0920 | 0.4366 | 0.026* | |
| H10B | 0.3655 | 0.0195 | 0.3909 | 0.026* | |
| H10C | 0.3705 | 0.1309 | 0.4678 | 0.026* | |
| N10 | 0.37459 (13) | 0.18921 (10) | 0.33208 (8) | 0.0138 (3) | |
| C11 | 0.45660 (16) | 0.29528 (12) | 0.32705 (9) | 0.0148 (3) | |
| N11 | 0.57702 (14) | 0.33716 (11) | 0.40073 (8) | 0.0195 (3) | |
| H11A | 0.6067 | 0.2948 | 0.4564 | 0.023* | |
| H11B | 0.6276 | 0.4075 | 0.3942 | 0.023* | |
| N12 | 0.40787 (14) | 0.35577 (10) | 0.23870 (8) | 0.0156 (3) | |
| O13 | 0.21974 (12) | 0.30271 (8) | 0.09262 (7) | 0.0174 (2) | |
| C13 | 0.28724 (16) | 0.28445 (12) | 0.18112 (9) | 0.0140 (3) | |
| O1W | 0.03730 (13) | 0.14500 (11) | 0.52700 (8) | 0.0256 (3) | |
| H1W | 0.023 (2) | 0.1260 (19) | 0.4666 (13) | 0.038* | |
| H2W | −0.058 (2) | 0.1453 (19) | 0.5402 (14) | 0.038* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0209 (5) | 0.0161 (5) | 0.0156 (5) | −0.0002 (4) | −0.0002 (4) | −0.0028 (4) |
| N1 | 0.0151 (5) | 0.0148 (6) | 0.0149 (5) | 0.0014 (4) | −0.0010 (4) | 0.0007 (4) |
| C1 | 0.0150 (6) | 0.0141 (6) | 0.0140 (6) | −0.0015 (5) | 0.0025 (5) | 0.0010 (5) |
| C2 | 0.0149 (6) | 0.0147 (6) | 0.0155 (6) | −0.0017 (5) | 0.0043 (5) | 0.0001 (5) |
| C3 | 0.0161 (6) | 0.0153 (7) | 0.0238 (7) | 0.0021 (5) | 0.0058 (5) | 0.0026 (5) |
| C4 | 0.0205 (7) | 0.0123 (6) | 0.0278 (7) | −0.0005 (5) | 0.0117 (6) | −0.0025 (5) |
| C5 | 0.0201 (7) | 0.0169 (7) | 0.0182 (7) | −0.0046 (5) | 0.0079 (5) | −0.0044 (5) |
| C6 | 0.0170 (6) | 0.0160 (7) | 0.0153 (6) | −0.0013 (5) | 0.0040 (5) | −0.0002 (5) |
| C7 | 0.0157 (6) | 0.0120 (6) | 0.0151 (6) | −0.0003 (5) | 0.0038 (5) | 0.0003 (5) |
| O8 | 0.0198 (5) | 0.0106 (5) | 0.0151 (5) | −0.0008 (4) | 0.0032 (4) | 0.0000 (3) |
| C8 | 0.0164 (7) | 0.0116 (6) | 0.0128 (6) | 0.0004 (5) | 0.0007 (5) | 0.0007 (5) |
| C9 | 0.0155 (6) | 0.0111 (6) | 0.0115 (6) | 0.0006 (5) | −0.0004 (5) | −0.0004 (5) |
| C10 | 0.0201 (7) | 0.0139 (6) | 0.0146 (6) | 0.0003 (5) | −0.0006 (5) | 0.0034 (5) |
| N10 | 0.0153 (5) | 0.0113 (5) | 0.0124 (5) | −0.0002 (4) | −0.0009 (4) | 0.0013 (4) |
| C11 | 0.0156 (6) | 0.0132 (6) | 0.0147 (6) | 0.0008 (5) | 0.0026 (5) | −0.0001 (5) |
| N11 | 0.0228 (6) | 0.0159 (6) | 0.0156 (6) | −0.0059 (5) | −0.0029 (5) | 0.0029 (4) |
| N12 | 0.0186 (6) | 0.0129 (5) | 0.0134 (5) | −0.0008 (4) | 0.0006 (4) | 0.0011 (4) |
| O13 | 0.0215 (5) | 0.0154 (5) | 0.0128 (5) | 0.0004 (4) | −0.0003 (4) | 0.0010 (3) |
| C13 | 0.0157 (6) | 0.0116 (6) | 0.0140 (6) | 0.0030 (5) | 0.0024 (5) | 0.0000 (5) |
| O1W | 0.0214 (5) | 0.0353 (6) | 0.0184 (5) | −0.0051 (4) | 0.0019 (4) | −0.0057 (4) |
Geometric parameters (Å, °)
| O1—C1 | 1.2206 (16) | C8—C9 | 1.5488 (18) |
| N1—C1 | 1.3531 (17) | C9—N10 | 1.4522 (16) |
| N1—C2 | 1.4096 (17) | C9—C13 | 1.5334 (17) |
| N1—H1 | 0.8800 | C9—H9 | 1.0000 |
| C1—C8 | 1.5557 (17) | C10—N10 | 1.4525 (16) |
| C2—C3 | 1.3803 (19) | C10—H10A | 0.9800 |
| C2—C7 | 1.3932 (18) | C10—H10B | 0.9800 |
| C3—C4 | 1.395 (2) | C10—H10C | 0.9800 |
| C3—H3 | 0.9500 | N10—C11 | 1.3382 (17) |
| C4—C5 | 1.387 (2) | C11—N11 | 1.3209 (17) |
| C4—H4 | 0.9500 | C11—N12 | 1.3612 (17) |
| C5—C6 | 1.3925 (19) | N11—H11A | 0.8800 |
| C5—H5 | 0.9500 | N11—H11B | 0.8800 |
| C6—C7 | 1.3847 (18) | N12—C13 | 1.3540 (17) |
| C6—H6 | 0.9500 | O13—C13 | 1.2366 (16) |
| C7—C8 | 1.5080 (17) | O1W—H1W | 0.847 (18) |
| O8—C8 | 1.4192 (15) | O1W—H2W | 0.861 (18) |
| O8—H8 | 0.8400 | ||
| C1—N1—C2 | 111.39 (11) | C7—C8—C1 | 101.94 (10) |
| C1—N1—H1 | 124.3 | C9—C8—C1 | 110.33 (10) |
| C2—N1—H1 | 124.3 | N10—C9—C13 | 100.04 (10) |
| O1—C1—N1 | 126.67 (12) | N10—C9—C8 | 111.46 (10) |
| O1—C1—C8 | 125.20 (11) | C13—C9—C8 | 112.21 (10) |
| N1—C1—C8 | 108.08 (10) | N10—C9—H9 | 110.9 |
| C3—C2—C7 | 122.50 (12) | C13—C9—H9 | 110.9 |
| C3—C2—N1 | 127.51 (12) | C8—C9—H9 | 110.9 |
| C7—C2—N1 | 109.99 (11) | N10—C10—H10A | 109.5 |
| C2—C3—C4 | 116.96 (12) | N10—C10—H10B | 109.5 |
| C2—C3—H3 | 121.5 | H10A—C10—H10B | 109.5 |
| C4—C3—H3 | 121.5 | N10—C10—H10C | 109.5 |
| C5—C4—C3 | 121.36 (12) | H10A—C10—H10C | 109.5 |
| C5—C4—H4 | 119.3 | H10B—C10—H10C | 109.5 |
| C3—C4—H4 | 119.3 | C11—N10—C9 | 108.54 (10) |
| C4—C5—C6 | 120.82 (12) | C11—N10—C10 | 125.20 (11) |
| C4—C5—H5 | 119.6 | C9—N10—C10 | 126.23 (10) |
| C6—C5—H5 | 119.6 | N11—C11—N10 | 123.09 (12) |
| C7—C6—C5 | 118.39 (12) | N11—C11—N12 | 122.48 (12) |
| C7—C6—H6 | 120.8 | N10—C11—N12 | 114.41 (11) |
| C5—C6—H6 | 120.8 | C11—N11—H11A | 120.0 |
| C6—C7—C2 | 119.96 (12) | C11—N11—H11B | 120.0 |
| C6—C7—C8 | 131.47 (12) | H11A—N11—H11B | 120.0 |
| C2—C7—C8 | 108.56 (11) | C13—N12—C11 | 105.97 (11) |
| C8—O8—H8 | 109.5 | O13—C13—N12 | 125.82 (12) |
| O8—C8—C7 | 110.67 (10) | O13—C13—C9 | 124.01 (11) |
| O8—C8—C9 | 110.48 (10) | N12—C13—C9 | 110.17 (10) |
| C7—C8—C9 | 113.64 (10) | H1W—O1W—H2W | 108.3 (17) |
| O8—C8—C1 | 109.44 (10) | ||
| C2—N1—C1—O1 | 179.49 (12) | O1—C1—C8—C9 | 59.80 (16) |
| C2—N1—C1—C8 | 1.73 (14) | N1—C1—C8—C9 | −122.39 (11) |
| C1—N1—C2—C3 | 177.98 (13) | O8—C8—C9—N10 | −64.03 (13) |
| C1—N1—C2—C7 | −1.42 (15) | C7—C8—C9—N10 | 61.06 (14) |
| C7—C2—C3—C4 | −0.05 (19) | C1—C8—C9—N10 | 174.83 (10) |
| N1—C2—C3—C4 | −179.38 (12) | O8—C8—C9—C13 | −175.30 (10) |
| C2—C3—C4—C5 | −0.16 (19) | C7—C8—C9—C13 | −50.22 (14) |
| C3—C4—C5—C6 | −0.3 (2) | C1—C8—C9—C13 | 63.55 (13) |
| C4—C5—C6—C7 | 1.00 (19) | C13—C9—N10—C11 | 8.33 (13) |
| C5—C6—C7—C2 | −1.19 (19) | C8—C9—N10—C11 | −110.49 (12) |
| C5—C6—C7—C8 | 178.47 (13) | C13—C9—N10—C10 | −169.97 (11) |
| C3—C2—C7—C6 | 0.7 (2) | C8—C9—N10—C10 | 71.21 (15) |
| N1—C2—C7—C6 | −179.82 (11) | C9—N10—C11—N11 | 175.73 (12) |
| C3—C2—C7—C8 | −179.00 (12) | C10—N10—C11—N11 | −5.9 (2) |
| N1—C2—C7—C8 | 0.44 (14) | C9—N10—C11—N12 | −5.46 (15) |
| C6—C7—C8—O8 | 64.51 (18) | C10—N10—C11—N12 | 172.86 (11) |
| C2—C7—C8—O8 | −115.79 (12) | N11—C11—N12—C13 | 178.05 (12) |
| C6—C7—C8—C9 | −60.47 (18) | N10—C11—N12—C13 | −0.77 (15) |
| C2—C7—C8—C9 | 119.23 (12) | C11—N12—C13—O13 | −174.16 (12) |
| C6—C7—C8—C1 | −179.17 (13) | C11—N12—C13—C9 | 6.41 (14) |
| C2—C7—C8—C1 | 0.53 (13) | N10—C9—C13—O13 | 171.44 (12) |
| O1—C1—C8—O8 | −61.96 (16) | C8—C9—C13—O13 | −70.29 (16) |
| N1—C1—C8—O8 | 115.85 (11) | N10—C9—C13—N12 | −9.12 (13) |
| O1—C1—C8—C7 | −179.17 (12) | C8—C9—C13—N12 | 109.15 (12) |
| N1—C1—C8—C7 | −1.36 (13) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1Wi | 0.88 | 1.96 | 2.8128 (15) | 163 |
| O8—H8···N12ii | 0.84 | 1.97 | 2.7984 (14) | 170 |
| N11—H11A···O13iii | 0.88 | 2.17 | 3.0371 (15) | 171 |
| N11—H11B···O1iv | 0.88 | 2.13 | 2.8678 (15) | 141 |
| O1W—H1W···O8 | 0.85 (2) | 2.05 (2) | 2.8812 (14) | 167 (2) |
| O1W—H2W···O13v | 0.86 (2) | 2.23 (2) | 3.0702 (14) | 164 (2) |
Symmetry codes: (i) x−1/2, −y+1/2, z−1/2; (ii) −x+1/2, y−1/2, −z+1/2; (iii) x+1/2, −y+1/2, z+1/2; (iv) −x+1/2, y+1/2, −z+1/2; (v) x−1/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2475).
References
- Bruker (2006). APEX2, SAINT and SADABS Bruker AXS Inc, Madison, Wisconsin, USA.
- Frolova, N. A., Kravtsov, V. K., Biyushkin, V. N., Chumakov, Yu. M., Bel’kova, O. N. & Malinovskii, T. I. (1988). J. Struct. Chem.29, 491–493.
- Sekhar, K. R., Crooks, P. A., Sonar, V. N., Friedman, D. B., Chan, J. Y., Meredith, M. J., Stames, J. H., Kelton, K. R., Summar, S. R., Sasi, S. & Freeman, M. L. (2003). Cancer Res.63, 5636–5645. [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sonar, V. N., Reddy, Y. T., Sekhar, K. R., Sowmya, S., Freeman, M. L. & Crooks, P. A. (2007). Bioorg. Med. Chem. Lett.17, 6821–6824. [DOI] [PMC free article] [PubMed]
- Usman, A., Razak, I. A., Fun, H.-K., Chantrapromma, S., Zhao, B.-G. & Xu, J.-H. (2002). Acta Cryst. C58, o24–o25. [DOI] [PubMed]
- Zimmerman, H. E. & Traxler, M. D. (1957). J. Am. Chem. Soc.79, 1920–1923.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004875/hg2475sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004875/hg2475Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report