Abstract
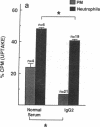
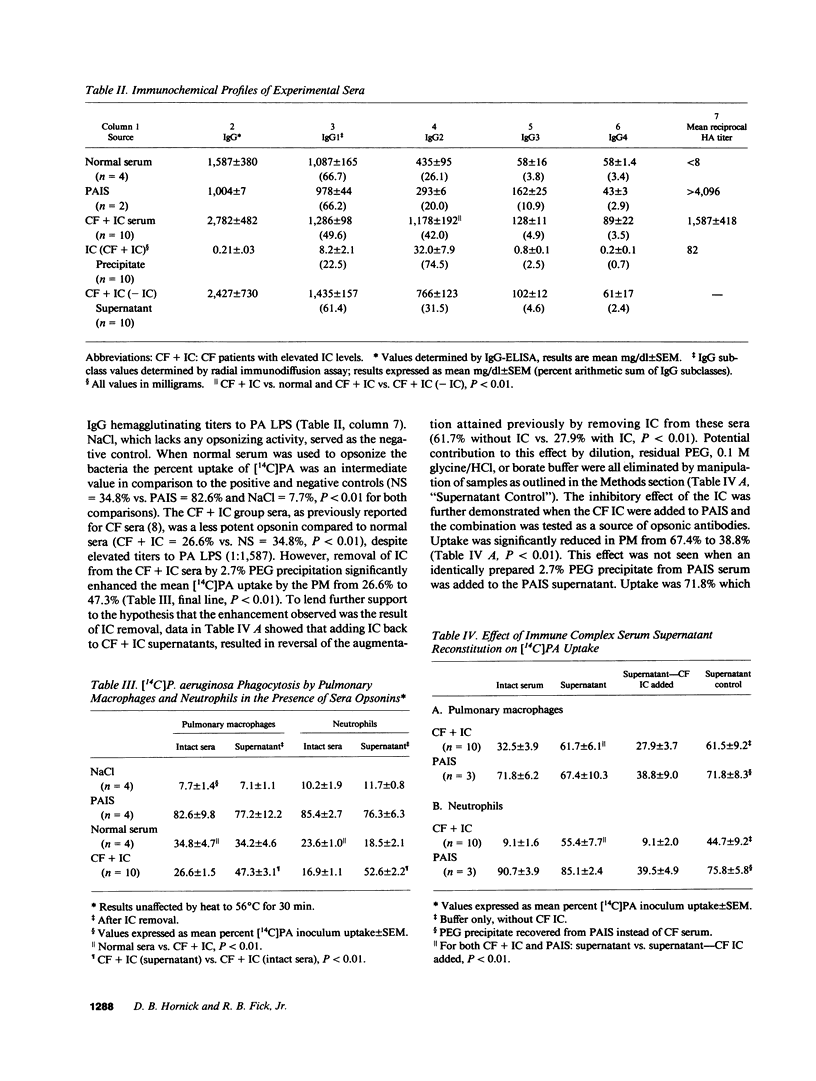
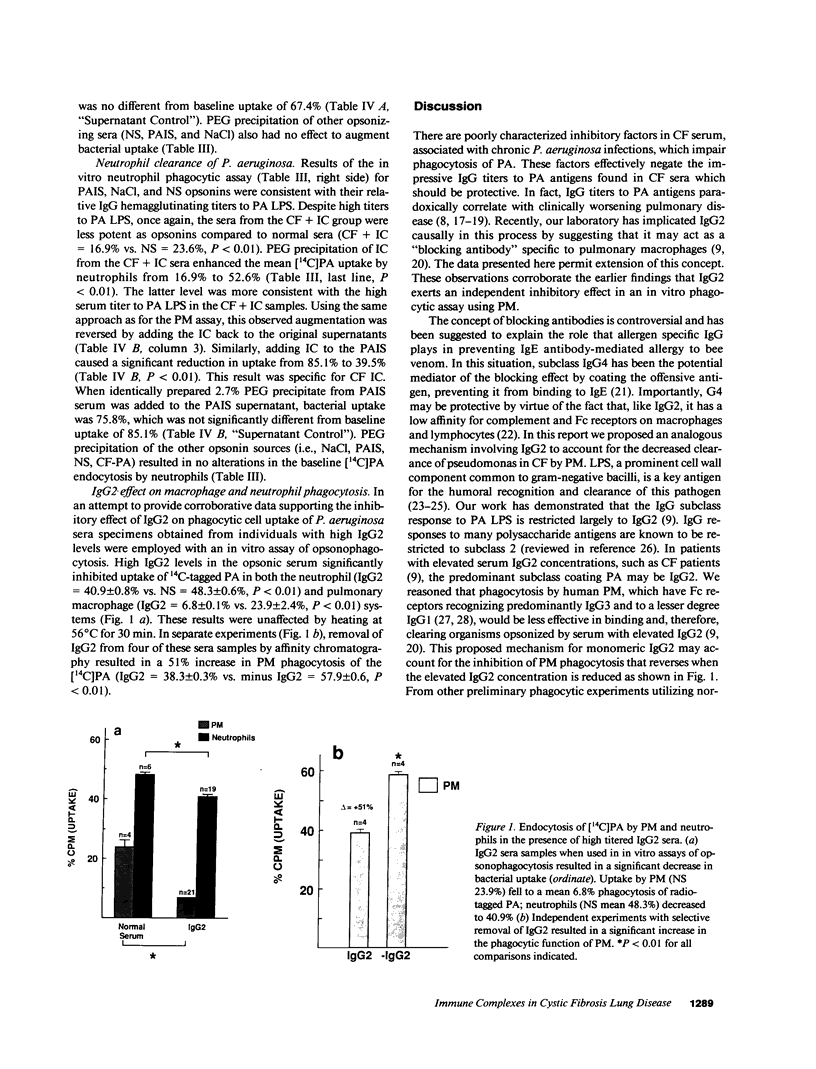
It has been shown that pulmonary macrophage (PM) phagocytosis of Pseudomonas aeruginosa (PA) is inhibited in the presence of serum from cystic fibrosis (CF) patients colonized by Pseudomonas, and that these sera contain high concentrations of IgG2 antibodies. The goal of these studies was to investigate the role that IgG2-containing immune complexes (IC) play in this inhibition of both PM and neutrophil phagocytosis. We found that serum IgG2 concentrations were elevated significantly in CF patients with chronic PA colonization and that in selected sera from CF patients with chronic PA colonization (CF + IC, n = 10), the mean IC level was significantly elevated (2.90 +/- 0.22 mg/dl [SEM]). IgG2 comprised 74.5% of IgG precipitated in IC from CF + IC sera. An invitro phagocytic assay of [14C]PA uptake using CF + IC whole-sera opsonins confirmed that endocytosis by normal PM and neutrophils was significantly depressed. Removal of IC from CF + IC sera resulted in significantly decreased serum IgG2 concentrations without a significant change in the other subclass concentrations, and enhanced [14C]PA uptake by PM (26.6% uptake increased to 47.3%) and neutrophils (16.9% increased to 52.6%). Return of the soluble IgG2 IC to the original CF sera supernatants and the positive control sera resulted in return of the inhibitory capacity of the CF + IC sera. We conclude that immune sera from patients with chronic Pseudomonas infections characterized by elevated IgG2 subclass level functions poorly as an opsonin. In these individuals, IgG2 contributes significantly to circulating IC and removal of IC, matched by a simultaneous fall in IgG2, improves bacterial uptake by neutrophil and mononuclear phagocytes. IgG2 antibodies exert antiphagocytic effects by both direct inhibition and the formation of IC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton A. D., Ryder K., Lourenço R. V., Dralle W., Weiss S. G. Inflammatory reaction and airway damage in cystic fibrosis. J Lab Clin Med. 1976 Sep;88(3):423–426. [PubMed] [Google Scholar]
- Berdischewsky M., Pollack M., Young L. S., Chia D., Osher A. B., Barnett E. V. Circulating immune complexes in cystic fibrosis. Pediatr Res. 1980 Jun;14(6):830–833. doi: 10.1203/00006450-198006000-00011. [DOI] [PubMed] [Google Scholar]
- Boxerbaum B., Kagumba A., Matthews L. W. Selective inhibition of phagocytic activity of rabbit alveolar macrophages by cystic fibrosis serum. Am Rev Respir Dis. 1973 Oct;108(4):777–783. doi: 10.1164/arrd.1973.108.4.777. [DOI] [PubMed] [Google Scholar]
- Creighton W. D., Lambert P. H., Miescher P. A. Detection of antibodies and soluble antigen-antibody complexes by precipitation with polyethylene glycol. J Immunol. 1973 Oct;111(4):1219–1227. [PubMed] [Google Scholar]
- Dasgupta M. K., Zuberbuhler P., Abbi A., Harley F. L., Brown N. E., Lam K., Dossetor J. B., Costerton J. W. Combined evaluation of circulating immune complexes and antibodies to Pseudomonas aeruginosa as an immunologic profile in relation to pulmonary function in cystic fibrosis. J Clin Immunol. 1987 Jan;7(1):51–58. doi: 10.1007/BF00915425. [DOI] [PubMed] [Google Scholar]
- Djurup R. The subclass nature and clinical significance of the IgG antibody response in patients undergoing allergen-specific immunotherapy. Allergy. 1985 Oct;40(7):469–486. doi: 10.1111/j.1398-9995.1985.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Engels W., Endert J., Kamps M. A., van Boven C. P. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1985 Jul;49(1):182–189. doi: 10.1128/iai.49.1.182-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Olchowski J., Squier S. U., Merrill W. W., Reynolds H. Y. Immunoglobulin-G subclasses in cystic fibrosis. IgG2 response to Pseudomonas aeruginosa lipopolysaccharide. Am Rev Respir Dis. 1986 Mar;133(3):418–422. doi: 10.1164/arrd.1986.133.3.418. [DOI] [PubMed] [Google Scholar]
- Fomsgaard A., Høiby N., Shand G. H., Conrad R. S., Galanos C. Longitudinal study of antibody response to lipopolysaccharides during chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. Infect Immun. 1988 Sep;56(9):2270–2278. doi: 10.1128/iai.56.9.2270-2278.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graft D. F., Mischler E., Farrell P. M., Busse W. W. Granulocyte chemiluminescence in adolescent patients with cystic fibrosis. Am Rev Respir Dis. 1982 May;125(5):540–543. doi: 10.1164/arrd.1982.125.5.540. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Walker L. C., Steere A. C., Trumble T. C., Tung K. S., Williams R. C., Jr, Ruddy S., Malawista S. E. Circulating immune complexes in Lyme arthritis. Detection by the 125I-C1q binding, C1q solid phase, and Raji cell assays. J Clin Invest. 1979 Mar;63(3):468–477. doi: 10.1172/JCI109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Warwick W. J., Dettloff J., Quie P. G. Neutrophil granulocyte function in patients with pulmonary infection. J Pediatr. 1974 Jan;84(1):55–58. doi: 10.1016/s0022-3476(74)80553-6. [DOI] [PubMed] [Google Scholar]
- Hodson M. E., Beldon I., Batten J. C. Circulating immune complexes in patients with cystic fibrosis in relation to clinical features. Clin Allergy. 1985 Jul;15(4):363–370. doi: 10.1111/j.1365-2222.1985.tb03004.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Karas S. P., Rosse W. F., Kurlander R. J. Characterization of the IgG-Fc receptor on human platelets. Blood. 1982 Dec;60(6):1277–1282. [PubMed] [Google Scholar]
- Kulczycki A., Jr Human neutrophils and eosinophils have structurally distinct Fc gamma receptors. J Immunol. 1984 Aug;133(2):849–854. [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane H., Holzel A., Brenchley P., Allan J. D., Wallwork J. C., Singer B. E., Worsley B. Immune complexes in cystic fibrosis. Br Med J. 1975 Feb 22;1(5955):423–428. doi: 10.1136/bmj.1.5955.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Lewiston N. J. 125I-Clq-binding and specific antibodies as indicators of pulmonary disease activity in cystic fibrosis. J Pediatr. 1981 Aug;99(2):215–222. doi: 10.1016/s0022-3476(81)80453-2. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Lewiston N. J., Curd J. G., Milgrom H., Hart S., Dyer B., Larrick J. W. Association of systemic immune complexes, complement activation, and antibodies to Pseudomonas aeruginosa lipopolysaccharide and exotoxin A with mortality in cystic fibrosis. Am Rev Respir Dis. 1986 Apr;133(4):648–652. doi: 10.1164/arrd.1986.133.4.648. [DOI] [PubMed] [Google Scholar]
- Moss R. B. Hypergammaglobulinemia in cystic fibrosis. Role of Pseudomonas endobronchial infection. Chest. 1987 Apr;91(4):522–526. doi: 10.1378/chest.91.4.522. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Lewiston N. J. Immune complexes and humoral response to Pseudomonas aeruginosa in cystic fibrosis. Am Rev Respir Dis. 1980 Jan;121(1):23–29. doi: 10.1164/arrd.1980.121.1.23. [DOI] [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- Naegel G. P., Young K. R., Jr, Reynolds H. Y. Receptors for human IgG subclasses on human alveolar macrophages. Am Rev Respir Dis. 1984 Mar;129(3):413–418. doi: 10.1164/arrd.1984.129.3.413. [DOI] [PubMed] [Google Scholar]
- Nydegger U. E., Lambert P. H., Gerber H., Miescher P. A. Circulating immune complexes in the serum in systemic lupus erythematosus and in carriers of hepatitis B antigen. Quantitation by binding to radiolabeled C1q. J Clin Invest. 1974 Aug;54(2):297–309. doi: 10.1172/JCI107765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Scharmann W., Jacob F., Porstendörfer J. The cytotoxic action of leucocidan from Pseudomonas aeruginosa on human polymorphonuclear leucocytes. J Gen Microbiol. 1976 Apr;93(2):303–308. doi: 10.1099/00221287-93-2-303. [DOI] [PubMed] [Google Scholar]
- Schiotz P. O., Hoiby N., Juhl F., Permin H., Nielsen H., Svehag S. E. Immune complexes in cystic fibrosis. Acta Pathol Microbiol Scand C. 1977 Feb;85(1):57–64. doi: 10.1111/j.1699-0463.1977.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Schiøtz P. O., Nielsen H., Høiby N., Glikmann G., Svehag S. E. Immune complexes in the sputum of patients with cystic fibrosis suffering from chronic Pseudomonas aeruginosa lung infection. Acta Pathol Microbiol Scand C. 1978 Feb;86(1):37–40. doi: 10.1111/j.1699-0463.1978.tb02555.x. [DOI] [PubMed] [Google Scholar]
- Schur P. H. Human gamma-g subclasses. Prog Clin Immunol. 1972;1:71–104. [PubMed] [Google Scholar]
- Shryock T. R., Mollé J. S., Klinger J. D., Thomassen M. J. Association with phagocytic inhibition of anti-Pseudomonas aeruginosa immunoglobulin G antibody subclass levels in serum from patients with cystic fibrosis. J Clin Microbiol. 1986 Mar;23(3):513–516. doi: 10.1128/jcm.23.3.513-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P. Host defenses in patients with cystic fibrosis: modulation by Pseudomonas aeruginosa. Surv Synth Pathol Res. 1985;4(1):14–33. doi: 10.1159/000156962. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Boxerbaum B., Demko C. A., Kuchenbrod P. J., Dearborn D. G., Wood R. E. Inhibitory effect of cystic fibrosis serum on pseudomonas phagocytosis by rabbit and human alveolar macrophages. Pediatr Res. 1979 Sep;13(9):1085–1088. doi: 10.1203/00006450-197909000-00030. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R., Guay C. M., DesJardins D., Pier G. B. Respiratory-mucin inhibition of the opsonophagocytic killing of Pseudomonas aeruginosa. Infect Immun. 1988 Sep;56(9):2218–2222. doi: 10.1128/iai.56.9.2218-2222.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski J. J., Todd E. W., Fuller R. K., Jones P. K., Dearborn D. G., Boat T. F., Naff G. B. Immune complexes and complement abnormalities in patients with cystic fibrosis. Increased mortality associated with circulating immune complexes and decreased function of the alternative complement pathway. Am Rev Respir Dis. 1985 Oct;132(4):770–776. doi: 10.1164/arrd.1985.132.4.770. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lambert P. H. Detection of immune complexes in human diseases. Prog Allergy. 1978;24:1–48. [PubMed] [Google Scholar]
- di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (third of three parts). N Engl J Med. 1976 Sep 9;295(11):597–602. doi: 10.1056/NEJM197609092951105. [DOI] [PubMed] [Google Scholar]