Abstract
The β-lactam ring of the title compound, C35H23NO2, is nearly planar with a maximum deviation of 0.003 (3) Å from the mean plane. It makes dihedral angles of 17.4 (2), 85.22 (17) and 65.39 (16)°, respectively, with the phenyl, xanthene and anthracene ring systems. In the crystal structure, there are intramolecular C—H⋯O and C—H⋯N contacts and molecules are also linked by C—H⋯π interactions.
Related literature
For general background on β-lactam antibiotics, see: Banik et al. (2003 ▶); Jarrahpour & Khalili (2007 ▶); Miller (2000 ▶); Palomo et al. (2004 ▶). For the crystal structures of related compounds, see: Akkurt, Jarrahpour et al. (2008 ▶); Akkurt, Karaca et al. (2008 ▶); Pınar et al. (2006 ▶). For geometric analysis, see: Cremer & Pople (1975 ▶).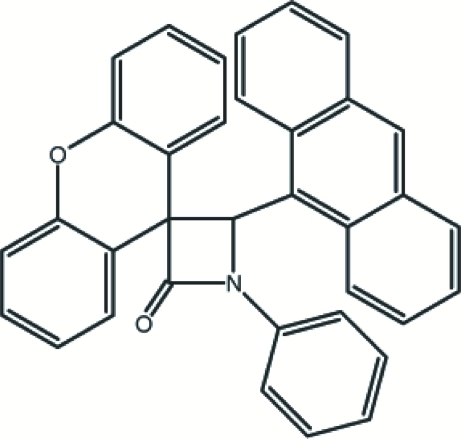
Experimental
Crystal data
C35H23NO2
M r = 489.54
Monoclinic,

a = 13.6906 (8) Å
b = 13.3085 (7) Å
c = 17.3527 (10) Å
β = 127.548 (4)°
V = 2506.7 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 295 (2) K
0.24 × 0.18 × 0.14 mm
Data collection
STOE IPDS 2 diffractometer
Absorption correction: integration (X-RED32; Stoe & Cie, 2002 ▶) T min = 0.981, T max = 0.989
19631 measured reflections
5191 independent reflections
2442 reflections with I > 2σ(I)
R int = 0.067
Refinement
R[F 2 > 2σ(F 2)] = 0.051
wR(F 2) = 0.110
S = 0.90
5191 reflections
343 parameters
H-atom parameters constrained
Δρmax = 0.23 e Å−3
Δρmin = −0.13 e Å−3
Data collection: X-AREA (Stoe & Cie, 2002 ▶); cell refinement: X-AREA; data reduction: X-RED32 (Stoe & Cie, 2002 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004255/is2388sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004255/is2388Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯N1 | 0.93 | 2.28 | 2.964 (4) | 130 |
| C31—H31⋯O2 | 0.93 | 2.48 | 3.092 (3) | 124 |
| C3—H3⋯Cg1i | 0.93 | 2.86 | 3.601 (3) | 138 |
| C11—H11⋯Cg2ii | 0.93 | 2.63 | 3.543 (3) | 166 |
Symmetry codes: (i)  ; (ii)
; (ii)  . Cg1 and Cg2 are the centroids of the benzene ring (C8–C13) and phenyl ring (C30–C35), respectively.
. Cg1 and Cg2 are the centroids of the benzene ring (C8–C13) and phenyl ring (C30–C35), respectively.
Acknowledgments
The authors acknowledge the Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey, for the use of the Stoe IPDS 2 diffractometer (purchased under grant F.279 of the University Research Fund).
supplementary crystallographic information
Comment
The stereo selective synthesis of β-lactams has received considerable attention over recent years because of their wide variety of biological activities (Miller, 2000), in particular, asymmetric synthesis by means of a Staudinger ketene–imine reaction has been extensively studied (Palomo et al., 2004). Several syntheses of spiro-β-lactams are available in the literature (Jarrahpour & Khalili, 2007; Pınar et al., 2006; Akkurt, Jarrahpour et al., 2008; Akkurt, Karaca et al., 2008). The synthesis of polycyclic aromatic β-lactams from polyaromatic imines have been reported in literature (Banik et al., 2003).
The β-lactam unit in (I) (Fig. 1) is essentially planar, with a maximum deviation of 0.003 (3) Å from the mean plane. The β-lactam ring makes a dihedral angle of 17.4 (2)° with the phenyl ring C30—C35. In the xanthene ring system, attached at C16, the benzene rings (C17–C22) and (C23–C28) are almost planar, the dihedral angle between them 17.20 (14)°. Its central ring, C16/C17/C22/O1/C23/C28, is not planar, with puckering parameters: QT = 0.247 (3) Å, θ = 98.9 (7)° and φ = 355.1 (7)° (Cremer & Pople, 1975). The mean plane of the xanthene ring system forms the dihedral angles of 85.22 (17) and 85.62 (13)°, with the β-lactam ring and the phenyl ring, respectively. The anthracene ring system, attached at C15, is almost planar, with maximum deviations of 0.041 (2) Å for C1, -0.049 (3) Å for C4 and, -0.067 (2) Å for C13, makes dihedral angle of 65.39 (16), 80.60 (13) and 56.63 (8)°, with the β-lactam, the phenyl and the mean plane of the xanthene ring system, respectively.
In the crystal structure, there are intramolecular C—H···O and C—H···N contacts and molecules are linked to each other by C—H···π interactions between the adjacent molecules (Table 1 and Fig. 2) [Cg1 and Cg2 are the centroids of the benzene ring (C8–C13) and phenyl ring (C30–C35), respectively].
Experimental
A mixture of (E)—N-(antheracen-9-ylmethylene)aniline (0.30 g, 1.07 mmol) and triethylamine (0.73 g, 7.21 mmol), 9H-xanthen-9-carboxylic acid (0.49 g, 2.17 mmol) and tosyl chloride (0.42 g, 2.20 mmol) in CH2Cl2 (15 ml) was stirred at room temperature for 24 h. Then it was washed with HCl 1 N (20 ml) and saturated sodiumbicarbonate solution (20 ml), brine (20 ml), dried (Na2SO4) and the solvent was evaporated to give the crude product as a light yellow crystal which was then purified by recrystallization from ethyl acetate (Yield 63%). dec: 511–513 K. IR (KBr, cm-1): 1758 (CO β-lactam). 1H-NMR δ (p.p.m.): 6.34 (s, 1H, 4), 6.51–8.83 (m, ArH, 22H).13C-NMR δ (p.p.m.): 65.6 (C-3), 75.4 (C-4), 115.9–152.0 (aromatic carbon), 167.5 (CO β-lactam). Analysis calculated for C35H23NO2: C 85.87, H 4.74, N 2.86%. Found: C 85.87, H 4.74, N 2.86%.
Refinement
The H atoms were positioned geometrically and refined a riding model, with the C—H = 0.93 and 0.98 Å and with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The title molecular structure, with the atom-numbering scheme and 30% probability displacement ellipsoids
Fig. 2.
A view down the b axis of the packing of (I).
Crystal data
| C35H23NO2 | F(000) = 1024 |
| Mr = 489.54 | Dx = 1.297 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 12888 reflections |
| a = 13.6906 (8) Å | θ = 1.5–28.1° |
| b = 13.3085 (7) Å | µ = 0.08 mm−1 |
| c = 17.3527 (10) Å | T = 295 K |
| β = 127.548 (4)° | Block, colourless |
| V = 2506.7 (3) Å3 | 0.24 × 0.18 × 0.14 mm |
| Z = 4 |
Data collection
| STOE IPDS 2 diffractometer | 5191 independent reflections |
| Radiation source: sealed X-ray tube, 12 x 0.4 mm long-fine focus | 2442 reflections with I > 2σ(I) |
| plane graphite | Rint = 0.067 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 26.5°, θmin = 1.9° |
| ω scans | h = −17→17 |
| Absorption correction: integration (X-RED32; Stoe & Cie, 2002) | k = −16→16 |
| Tmin = 0.981, Tmax = 0.989 | l = −21→21 |
| 19631 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.051 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.110 | H-atom parameters constrained |
| S = 0.90 | w = 1/[σ2(Fo2) + (0.0406P)2] where P = (Fo2 + 2Fc2)/3 |
| 5191 reflections | (Δ/σ)max < 0.001 |
| 343 parameters | Δρmax = 0.23 e Å−3 |
| 0 restraints | Δρmin = −0.13 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.26666 (17) | 0.35613 (13) | 0.18443 (13) | 0.0741 (7) | |
| O2 | 0.23966 (18) | 0.72931 (13) | 0.19798 (13) | 0.0792 (7) | |
| N1 | 0.26629 (17) | 0.67174 (14) | 0.33789 (13) | 0.0537 (7) | |
| C1 | 0.0819 (2) | 0.54484 (18) | 0.34789 (15) | 0.0537 (8) | |
| C2 | 0.0287 (2) | 0.6401 (2) | 0.30421 (17) | 0.0654 (9) | |
| C3 | −0.0826 (3) | 0.6684 (2) | 0.27919 (19) | 0.0782 (11) | |
| C4 | −0.1483 (3) | 0.6065 (3) | 0.2989 (2) | 0.0925 (13) | |
| C5 | −0.1006 (3) | 0.5181 (3) | 0.3433 (2) | 0.0852 (11) | |
| C6 | 0.0134 (2) | 0.4829 (2) | 0.36802 (18) | 0.0630 (9) | |
| C7 | 0.0576 (2) | 0.3892 (2) | 0.40911 (19) | 0.0727 (11) | |
| C8 | 0.1641 (2) | 0.34938 (19) | 0.42885 (16) | 0.0587 (9) | |
| C9 | 0.2069 (3) | 0.2516 (2) | 0.46931 (19) | 0.0748 (11) | |
| C10 | 0.3073 (3) | 0.2113 (2) | 0.4844 (2) | 0.0771 (11) | |
| C11 | 0.3703 (2) | 0.2655 (2) | 0.45823 (18) | 0.0697 (10) | |
| C12 | 0.3355 (2) | 0.35957 (19) | 0.42191 (16) | 0.0577 (9) | |
| C13 | 0.23202 (19) | 0.40766 (18) | 0.40696 (15) | 0.0509 (8) | |
| C14 | 0.19341 (19) | 0.50727 (18) | 0.37026 (14) | 0.0495 (8) | |
| C15 | 0.2778 (2) | 0.56287 (16) | 0.35636 (15) | 0.0500 (8) | |
| C16 | 0.2588 (2) | 0.54946 (17) | 0.25569 (15) | 0.0521 (8) | |
| C17 | 0.1450 (2) | 0.49372 (19) | 0.17648 (15) | 0.0547 (8) | |
| C18 | 0.0275 (2) | 0.5320 (2) | 0.13170 (17) | 0.0684 (10) | |
| C19 | −0.0758 (2) | 0.4769 (3) | 0.06386 (19) | 0.0788 (13) | |
| C20 | −0.0634 (3) | 0.3830 (3) | 0.03877 (19) | 0.0809 (13) | |
| C21 | 0.0515 (3) | 0.3438 (2) | 0.08025 (18) | 0.0739 (11) | |
| C22 | 0.1546 (2) | 0.3999 (2) | 0.14835 (17) | 0.0590 (9) | |
| C23 | 0.3694 (2) | 0.4171 (2) | 0.23285 (17) | 0.0615 (9) | |
| C24 | 0.4717 (3) | 0.3782 (2) | 0.24527 (19) | 0.0751 (11) | |
| C25 | 0.5788 (3) | 0.4324 (3) | 0.2946 (2) | 0.0833 (13) | |
| C26 | 0.5831 (3) | 0.5262 (3) | 0.3300 (2) | 0.0841 (13) | |
| C27 | 0.4806 (2) | 0.5649 (2) | 0.31657 (18) | 0.0710 (10) | |
| C28 | 0.3711 (2) | 0.51099 (19) | 0.26791 (16) | 0.0556 (9) | |
| C29 | 0.2507 (2) | 0.66467 (19) | 0.25206 (17) | 0.0577 (9) | |
| C30 | 0.3017 (2) | 0.75205 (18) | 0.40329 (16) | 0.0544 (8) | |
| C31 | 0.3090 (2) | 0.8482 (2) | 0.3781 (2) | 0.0703 (10) | |
| C32 | 0.3526 (3) | 0.9250 (2) | 0.4456 (2) | 0.0847 (11) | |
| C33 | 0.3860 (2) | 0.9052 (2) | 0.5366 (2) | 0.0820 (11) | |
| C34 | 0.3757 (2) | 0.8104 (2) | 0.56045 (19) | 0.0736 (10) | |
| C35 | 0.3336 (2) | 0.7321 (2) | 0.49401 (17) | 0.0630 (9) | |
| H2 | 0.07130 | 0.68380 | 0.29260 | 0.0780* | |
| H3 | −0.11600 | 0.72990 | 0.24850 | 0.0940* | |
| H4 | −0.22450 | 0.62690 | 0.28120 | 0.1110* | |
| H5 | −0.14300 | 0.47870 | 0.35840 | 0.1020* | |
| H7 | 0.01420 | 0.35130 | 0.42410 | 0.0870* | |
| H9 | 0.16430 | 0.21470 | 0.48570 | 0.0900* | |
| H10 | 0.33450 | 0.14780 | 0.51200 | 0.0920* | |
| H11 | 0.43750 | 0.23640 | 0.46600 | 0.0840* | |
| H12 | 0.38030 | 0.39400 | 0.40620 | 0.0690* | |
| H15 | 0.36340 | 0.54710 | 0.41060 | 0.0600* | |
| H18 | 0.01840 | 0.59610 | 0.14790 | 0.0820* | |
| H19 | −0.15380 | 0.50350 | 0.03510 | 0.0950* | |
| H20 | −0.13320 | 0.34550 | −0.00660 | 0.0970* | |
| H21 | 0.05990 | 0.28020 | 0.06270 | 0.0890* | |
| H24 | 0.46790 | 0.31520 | 0.22020 | 0.0900* | |
| H25 | 0.64830 | 0.40610 | 0.30420 | 0.1000* | |
| H26 | 0.65550 | 0.56350 | 0.36310 | 0.1010* | |
| H27 | 0.48460 | 0.62850 | 0.34060 | 0.0850* | |
| H31 | 0.28480 | 0.86160 | 0.31600 | 0.0840* | |
| H32 | 0.35930 | 0.98990 | 0.42940 | 0.1020* | |
| H33 | 0.41570 | 0.95670 | 0.58200 | 0.0980* | |
| H34 | 0.39700 | 0.79780 | 0.62170 | 0.0880* | |
| H35 | 0.32710 | 0.66740 | 0.51050 | 0.0760* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0753 (12) | 0.0664 (12) | 0.0832 (12) | −0.0113 (10) | 0.0496 (10) | −0.0175 (10) |
| O2 | 0.1112 (14) | 0.0678 (12) | 0.0720 (11) | −0.0093 (11) | 0.0628 (11) | 0.0030 (10) |
| N1 | 0.0632 (12) | 0.0525 (13) | 0.0528 (11) | −0.0055 (10) | 0.0392 (10) | −0.0030 (9) |
| C1 | 0.0527 (13) | 0.0623 (16) | 0.0490 (13) | −0.0030 (12) | 0.0325 (11) | −0.0062 (11) |
| C2 | 0.0601 (15) | 0.0730 (19) | 0.0648 (15) | 0.0042 (14) | 0.0390 (13) | −0.0026 (14) |
| C3 | 0.0683 (18) | 0.091 (2) | 0.0712 (17) | 0.0159 (16) | 0.0404 (15) | −0.0006 (15) |
| C4 | 0.0645 (18) | 0.115 (3) | 0.109 (2) | 0.009 (2) | 0.0585 (19) | −0.008 (2) |
| C5 | 0.0673 (18) | 0.097 (2) | 0.114 (2) | −0.0050 (17) | 0.0669 (18) | −0.009 (2) |
| C6 | 0.0554 (14) | 0.0742 (19) | 0.0702 (15) | −0.0046 (13) | 0.0438 (13) | −0.0050 (14) |
| C7 | 0.0760 (18) | 0.080 (2) | 0.0831 (18) | −0.0110 (16) | 0.0593 (16) | −0.0021 (16) |
| C8 | 0.0619 (15) | 0.0625 (17) | 0.0603 (15) | −0.0054 (13) | 0.0417 (13) | −0.0004 (12) |
| C9 | 0.090 (2) | 0.0675 (19) | 0.0786 (18) | −0.0088 (16) | 0.0574 (17) | 0.0034 (15) |
| C10 | 0.0818 (19) | 0.0644 (18) | 0.0797 (19) | 0.0027 (16) | 0.0465 (17) | 0.0099 (14) |
| C11 | 0.0612 (16) | 0.0695 (19) | 0.0721 (17) | 0.0044 (14) | 0.0373 (14) | 0.0034 (15) |
| C12 | 0.0494 (13) | 0.0633 (17) | 0.0566 (14) | −0.0037 (12) | 0.0303 (12) | 0.0012 (12) |
| C13 | 0.0504 (13) | 0.0579 (15) | 0.0438 (12) | −0.0076 (11) | 0.0284 (11) | −0.0050 (10) |
| C14 | 0.0453 (12) | 0.0620 (16) | 0.0435 (11) | −0.0075 (11) | 0.0283 (10) | −0.0055 (11) |
| C15 | 0.0499 (12) | 0.0560 (15) | 0.0490 (12) | −0.0044 (11) | 0.0326 (11) | −0.0024 (11) |
| C16 | 0.0563 (14) | 0.0564 (15) | 0.0499 (12) | −0.0076 (12) | 0.0356 (11) | −0.0034 (11) |
| C17 | 0.0561 (14) | 0.0660 (17) | 0.0474 (12) | −0.0059 (12) | 0.0343 (12) | −0.0012 (12) |
| C18 | 0.0636 (17) | 0.0830 (19) | 0.0585 (15) | −0.0028 (15) | 0.0371 (14) | −0.0002 (14) |
| C19 | 0.0594 (17) | 0.108 (3) | 0.0619 (16) | −0.0105 (17) | 0.0333 (14) | −0.0050 (16) |
| C20 | 0.0683 (19) | 0.111 (3) | 0.0595 (16) | −0.0346 (19) | 0.0369 (15) | −0.0147 (17) |
| C21 | 0.087 (2) | 0.079 (2) | 0.0657 (17) | −0.0253 (17) | 0.0517 (16) | −0.0161 (14) |
| C22 | 0.0625 (15) | 0.0657 (18) | 0.0586 (14) | −0.0086 (14) | 0.0420 (13) | −0.0053 (13) |
| C23 | 0.0639 (16) | 0.0690 (18) | 0.0574 (14) | −0.0034 (14) | 0.0400 (13) | −0.0035 (13) |
| C24 | 0.0736 (18) | 0.089 (2) | 0.0699 (17) | 0.0089 (17) | 0.0474 (16) | −0.0057 (15) |
| C25 | 0.0657 (18) | 0.121 (3) | 0.0718 (18) | 0.0061 (18) | 0.0463 (16) | −0.0052 (18) |
| C26 | 0.0598 (17) | 0.125 (3) | 0.0759 (18) | −0.0160 (18) | 0.0457 (15) | −0.0142 (18) |
| C27 | 0.0675 (17) | 0.089 (2) | 0.0688 (16) | −0.0162 (15) | 0.0479 (14) | −0.0145 (14) |
| C28 | 0.0586 (15) | 0.0649 (17) | 0.0520 (13) | −0.0072 (13) | 0.0382 (12) | −0.0052 (12) |
| C29 | 0.0640 (15) | 0.0623 (17) | 0.0534 (14) | −0.0100 (13) | 0.0392 (12) | −0.0027 (12) |
| C30 | 0.0539 (14) | 0.0546 (16) | 0.0584 (14) | −0.0032 (12) | 0.0361 (12) | −0.0080 (12) |
| C31 | 0.0794 (18) | 0.0630 (18) | 0.0679 (16) | −0.0004 (14) | 0.0446 (15) | −0.0034 (14) |
| C32 | 0.093 (2) | 0.0632 (19) | 0.095 (2) | −0.0005 (16) | 0.0558 (18) | −0.0113 (17) |
| C33 | 0.0748 (19) | 0.075 (2) | 0.081 (2) | −0.0019 (16) | 0.0397 (17) | −0.0241 (17) |
| C34 | 0.0698 (17) | 0.081 (2) | 0.0609 (16) | 0.0058 (16) | 0.0352 (14) | −0.0108 (15) |
| C35 | 0.0648 (15) | 0.0661 (17) | 0.0597 (15) | −0.0032 (13) | 0.0387 (13) | −0.0061 (13) |
Geometric parameters (Å, °)
| O1—C22 | 1.384 (4) | C23—C28 | 1.384 (4) |
| O1—C23 | 1.379 (4) | C24—C25 | 1.369 (5) |
| O2—C29 | 1.213 (3) | C25—C26 | 1.377 (6) |
| N1—C15 | 1.472 (3) | C26—C27 | 1.375 (6) |
| N1—C29 | 1.373 (3) | C27—C28 | 1.390 (4) |
| N1—C30 | 1.412 (3) | C30—C31 | 1.375 (4) |
| C1—C2 | 1.428 (4) | C30—C35 | 1.379 (3) |
| C1—C6 | 1.442 (4) | C31—C32 | 1.386 (4) |
| C1—C14 | 1.417 (4) | C32—C33 | 1.376 (4) |
| C2—C3 | 1.359 (5) | C33—C34 | 1.362 (4) |
| C3—C4 | 1.407 (6) | C34—C35 | 1.392 (4) |
| C4—C5 | 1.338 (5) | C2—H2 | 0.9300 |
| C5—C6 | 1.422 (6) | C3—H3 | 0.9300 |
| C6—C7 | 1.380 (4) | C4—H4 | 0.9300 |
| C7—C8 | 1.384 (5) | C5—H5 | 0.9300 |
| C8—C9 | 1.424 (4) | C7—H7 | 0.9300 |
| C8—C13 | 1.429 (4) | C9—H9 | 0.9300 |
| C9—C10 | 1.344 (6) | C10—H10 | 0.9300 |
| C10—C11 | 1.396 (5) | C11—H11 | 0.9300 |
| C11—C12 | 1.351 (4) | C12—H12 | 0.9300 |
| C12—C13 | 1.427 (4) | C15—H15 | 0.9800 |
| C13—C14 | 1.426 (3) | C18—H18 | 0.9300 |
| C14—C15 | 1.510 (4) | C19—H19 | 0.9300 |
| C15—C16 | 1.612 (3) | C20—H20 | 0.9300 |
| C16—C17 | 1.502 (3) | C21—H21 | 0.9300 |
| C16—C28 | 1.508 (4) | C24—H24 | 0.9300 |
| C16—C29 | 1.536 (3) | C25—H25 | 0.9300 |
| C17—C18 | 1.388 (4) | C26—H26 | 0.9300 |
| C17—C22 | 1.376 (4) | C27—H27 | 0.9300 |
| C18—C19 | 1.376 (4) | C31—H31 | 0.9300 |
| C19—C20 | 1.368 (6) | C32—H32 | 0.9300 |
| C20—C21 | 1.373 (6) | C33—H33 | 0.9300 |
| C21—C22 | 1.383 (4) | C34—H34 | 0.9300 |
| C23—C24 | 1.381 (5) | C35—H35 | 0.9300 |
| O2···C27 | 3.410 (4) | C29···H31 | 2.7700 |
| O2···C31 | 3.092 (3) | C29···H2 | 2.9500 |
| O2···H31 | 2.4800 | C30···H2 | 2.6600 |
| O2···H25i | 2.8200 | C30···H11iv | 2.8400 |
| O2···H20ii | 2.8700 | C31···H11iv | 3.0300 |
| N1···C2 | 2.964 (4) | C33···H19iii | 2.9200 |
| N1···H2 | 2.2800 | C33···H11iv | 3.0900 |
| C1···C18 | 3.354 (3) | C34···H20iii | 2.8900 |
| C2···C30 | 3.363 (4) | C34···H11iv | 2.9300 |
| C2···N1 | 2.964 (4) | C35···H15 | 3.0100 |
| C2···C18 | 3.312 (4) | C35···H11iv | 2.8000 |
| C3···C21iii | 3.212 (4) | H2···N1 | 2.2800 |
| C4···C21iii | 3.569 (5) | H2···C15 | 2.8300 |
| C11···C30iv | 3.584 (4) | H2···C29 | 2.9500 |
| C12···C16 | 3.481 (3) | H2···C30 | 2.6600 |
| C13···C17 | 3.594 (3) | H2···H18 | 2.4400 |
| C14···C35 | 3.496 (3) | H3···C21iii | 2.9500 |
| C14···C18 | 3.308 (3) | H5···H7 | 2.4100 |
| C16···C12 | 3.481 (3) | H5···H25vii | 2.5900 |
| C17···C20ii | 3.579 (4) | H7···H5 | 2.4100 |
| C17···C13 | 3.594 (3) | H7···H9 | 2.4500 |
| C18···C20ii | 3.470 (5) | H9···H7 | 2.4500 |
| C18···C2 | 3.312 (4) | H9···C21viii | 2.9600 |
| C18···C1 | 3.354 (3) | H9···H21viii | 2.4800 |
| C18···C21ii | 3.547 (4) | H11···C30iv | 2.8400 |
| C18···C14 | 3.308 (3) | H11···C31iv | 3.0300 |
| C19···C22ii | 3.546 (4) | H11···C33iv | 3.0900 |
| C20···C17ii | 3.579 (4) | H11···C34iv | 2.9300 |
| C20···C18ii | 3.470 (5) | H11···C35iv | 2.8000 |
| C21···C18ii | 3.547 (4) | H12···C15 | 2.5100 |
| C21···C3v | 3.212 (4) | H12···C16 | 2.9300 |
| C21···C4v | 3.569 (5) | H12···C23 | 2.9400 |
| C22···C19ii | 3.546 (4) | H12···C28 | 2.8000 |
| C27···O2 | 3.410 (4) | H12···H15 | 2.0600 |
| C30···C2 | 3.363 (4) | H15···C12 | 2.5500 |
| C30···C11iv | 3.584 (4) | H15···C27 | 2.9100 |
| C31···O2 | 3.092 (3) | H15···C35 | 3.0100 |
| C35···C14 | 3.496 (3) | H15···H12 | 2.0600 |
| C1···H18 | 3.1000 | H18···C1 | 3.1000 |
| C2···H18 | 2.6900 | H18···C2 | 2.6900 |
| C3···H21iii | 2.9700 | H18···C29 | 2.6800 |
| C4···H21iii | 3.0100 | H18···H2 | 2.4400 |
| C12···H15 | 2.5500 | H19···C33v | 2.9200 |
| C14···H35 | 2.9000 | H20···C34v | 2.8900 |
| C15···H12 | 2.5100 | H20···O2ii | 2.8700 |
| C15···H2 | 2.8300 | H21···C3v | 2.9700 |
| C15···H35 | 2.7100 | H21···C4v | 3.0100 |
| C16···H12 | 2.9300 | H21···H9vi | 2.4800 |
| C21···H3v | 2.9500 | H25···H5ix | 2.5900 |
| C21···H9vi | 2.9600 | H25···O2x | 2.8200 |
| C23···H12 | 2.9400 | H27···C29 | 2.6200 |
| C24···H34iv | 3.0000 | H31···O2 | 2.4800 |
| C25···H35iv | 3.0900 | H31···C29 | 2.7700 |
| C27···H15 | 2.9100 | H34···C24iv | 3.0000 |
| C28···H12 | 2.8000 | H35···C14 | 2.9000 |
| C29···H27 | 2.6200 | H35···C15 | 2.7100 |
| C29···H18 | 2.6800 | H35···C25iv | 3.0900 |
| C22—O1—C23 | 117.8 (2) | O2—C29—C16 | 135.4 (2) |
| C15—N1—C29 | 95.39 (18) | N1—C29—C16 | 93.69 (19) |
| C15—N1—C30 | 129.15 (18) | N1—C30—C31 | 120.3 (2) |
| C29—N1—C30 | 132.4 (2) | N1—C30—C35 | 119.1 (2) |
| C2—C1—C6 | 116.1 (3) | C31—C30—C35 | 120.5 (2) |
| C2—C1—C14 | 125.1 (3) | C30—C31—C32 | 119.7 (3) |
| C6—C1—C14 | 118.9 (2) | C31—C32—C33 | 120.0 (3) |
| C1—C2—C3 | 121.7 (3) | C32—C33—C34 | 120.2 (3) |
| C2—C3—C4 | 121.2 (3) | C33—C34—C35 | 120.6 (3) |
| C3—C4—C5 | 119.7 (4) | C30—C35—C34 | 119.0 (2) |
| C4—C5—C6 | 121.6 (4) | C1—C2—H2 | 119.00 |
| C1—C6—C5 | 119.6 (3) | C3—C2—H2 | 119.00 |
| C1—C6—C7 | 119.8 (3) | C2—C3—H3 | 119.00 |
| C5—C6—C7 | 120.6 (3) | C4—C3—H3 | 119.00 |
| C6—C7—C8 | 122.6 (3) | C3—C4—H4 | 120.00 |
| C7—C8—C9 | 121.8 (3) | C5—C4—H4 | 120.00 |
| C7—C8—C13 | 119.0 (2) | C4—C5—H5 | 119.00 |
| C9—C8—C13 | 119.2 (3) | C6—C5—H5 | 119.00 |
| C8—C9—C10 | 121.7 (3) | C6—C7—H7 | 119.00 |
| C9—C10—C11 | 119.5 (3) | C8—C7—H7 | 119.00 |
| C10—C11—C12 | 121.2 (3) | C8—C9—H9 | 119.00 |
| C11—C12—C13 | 122.0 (3) | C10—C9—H9 | 119.00 |
| C8—C13—C12 | 116.3 (2) | C9—C10—H10 | 120.00 |
| C8—C13—C14 | 120.0 (3) | C11—C10—H10 | 120.00 |
| C12—C13—C14 | 123.7 (3) | C10—C11—H11 | 119.00 |
| C1—C14—C13 | 119.7 (3) | C12—C11—H11 | 119.00 |
| C1—C14—C15 | 125.8 (2) | C11—C12—H12 | 119.00 |
| C13—C14—C15 | 114.6 (2) | C13—C12—H12 | 119.00 |
| N1—C15—C14 | 121.8 (2) | N1—C15—H15 | 109.00 |
| N1—C15—C16 | 87.01 (16) | C14—C15—H15 | 109.00 |
| C14—C15—C16 | 119.02 (19) | C16—C15—H15 | 109.00 |
| C15—C16—C17 | 116.0 (2) | C17—C18—H18 | 119.00 |
| C15—C16—C28 | 114.08 (19) | C19—C18—H18 | 119.00 |
| C15—C16—C29 | 83.91 (17) | C18—C19—H19 | 120.00 |
| C17—C16—C28 | 111.2 (2) | C20—C19—H19 | 120.00 |
| C17—C16—C29 | 116.5 (2) | C19—C20—H20 | 120.00 |
| C28—C16—C29 | 112.8 (2) | C21—C20—H20 | 120.00 |
| C16—C17—C18 | 122.2 (2) | C20—C21—H21 | 120.00 |
| C16—C17—C22 | 120.2 (3) | C22—C21—H21 | 120.00 |
| C18—C17—C22 | 117.6 (2) | C23—C24—H24 | 120.00 |
| C17—C18—C19 | 121.3 (3) | C25—C24—H24 | 120.00 |
| C18—C19—C20 | 119.8 (3) | C24—C25—H25 | 120.00 |
| C19—C20—C21 | 120.4 (3) | C26—C25—H25 | 120.00 |
| C20—C21—C22 | 119.3 (3) | C25—C26—H26 | 120.00 |
| O1—C22—C17 | 122.8 (2) | C27—C26—H26 | 120.00 |
| O1—C22—C21 | 115.6 (3) | C26—C27—H27 | 119.00 |
| C17—C22—C21 | 121.6 (3) | C28—C27—H27 | 119.00 |
| O1—C23—C24 | 115.8 (2) | C30—C31—H31 | 120.00 |
| O1—C23—C28 | 122.5 (3) | C32—C31—H31 | 120.00 |
| C24—C23—C28 | 121.7 (3) | C31—C32—H32 | 120.00 |
| C23—C24—C25 | 119.9 (3) | C33—C32—H32 | 120.00 |
| C24—C25—C26 | 119.7 (4) | C32—C33—H33 | 120.00 |
| C25—C26—C27 | 120.2 (4) | C34—C33—H33 | 120.00 |
| C26—C27—C28 | 121.4 (3) | C33—C34—H34 | 120.00 |
| C16—C28—C23 | 120.2 (3) | C35—C34—H34 | 120.00 |
| C16—C28—C27 | 122.7 (2) | C30—C35—H35 | 120.00 |
| C23—C28—C27 | 117.1 (3) | C34—C35—H35 | 121.00 |
| O2—C29—N1 | 130.9 (2) | ||
| C23—O1—C22—C21 | 164.1 (2) | N1—C15—C16—C29 | 0.4 (2) |
| C22—O1—C23—C24 | −163.8 (2) | C14—C15—C16—C17 | 8.8 (3) |
| C22—O1—C23—C28 | 16.9 (3) | N1—C15—C16—C17 | −116.2 (2) |
| C23—O1—C22—C17 | −14.8 (4) | N1—C15—C16—C28 | 112.6 (2) |
| C29—N1—C30—C31 | 10.4 (5) | C14—C15—C16—C29 | 125.4 (2) |
| C29—N1—C30—C35 | −167.0 (3) | C14—C15—C16—C28 | −122.3 (2) |
| C29—N1—C15—C16 | −0.5 (2) | C29—C16—C28—C27 | 29.6 (3) |
| C15—N1—C30—C35 | −12.0 (5) | C29—C16—C28—C23 | −152.6 (2) |
| C30—N1—C15—C14 | 75.2 (4) | C15—C16—C28—C27 | −63.9 (3) |
| C15—N1—C29—C16 | 0.5 (2) | C15—C16—C29—O2 | 177.0 (4) |
| C30—N1—C15—C16 | −162.2 (3) | C15—C16—C28—C23 | 113.9 (2) |
| C29—N1—C15—C14 | −123.1 (2) | C28—C16—C17—C18 | −160.8 (2) |
| C30—N1—C29—C16 | 161.3 (3) | C28—C16—C17—C22 | 21.6 (3) |
| C15—N1—C30—C31 | 165.4 (3) | C29—C16—C17—C18 | −29.7 (4) |
| C15—N1—C29—O2 | −177.1 (4) | C29—C16—C17—C22 | 152.7 (3) |
| C30—N1—C29—O2 | −16.3 (6) | C15—C16—C17—C18 | 66.7 (3) |
| C2—C1—C14—C13 | −175.2 (2) | C17—C16—C28—C23 | −19.6 (3) |
| C2—C1—C6—C7 | 179.2 (2) | C17—C16—C28—C27 | 162.6 (2) |
| C6—C1—C14—C15 | −178.4 (2) | C15—C16—C29—N1 | −0.4 (2) |
| C14—C1—C6—C5 | −178.1 (2) | C15—C16—C17—C22 | −110.9 (3) |
| C2—C1—C6—C5 | 0.6 (3) | C28—C16—C29—N1 | −114.0 (2) |
| C14—C1—C6—C7 | 0.5 (3) | C28—C16—C29—O2 | 63.4 (4) |
| C14—C1—C2—C3 | 175.7 (2) | C17—C16—C29—O2 | −66.9 (5) |
| C6—C1—C14—C13 | 3.4 (3) | C17—C16—C29—N1 | 115.7 (3) |
| C6—C1—C2—C3 | −2.9 (3) | C16—C17—C22—C21 | 175.7 (3) |
| C2—C1—C14—C15 | 3.1 (3) | C22—C17—C18—C19 | 2.0 (4) |
| C1—C2—C3—C4 | 2.6 (4) | C18—C17—C22—O1 | 176.8 (2) |
| C2—C3—C4—C5 | 0.2 (4) | C16—C17—C18—C19 | −175.6 (3) |
| C3—C4—C5—C6 | −2.5 (5) | C18—C17—C22—C21 | −2.0 (4) |
| C4—C5—C6—C1 | 2.0 (4) | C16—C17—C22—O1 | −5.5 (4) |
| C4—C5—C6—C7 | −176.6 (3) | C17—C18—C19—C20 | −0.8 (5) |
| C1—C6—C7—C8 | −2.6 (4) | C18—C19—C20—C21 | −0.6 (5) |
| C5—C6—C7—C8 | 176.0 (3) | C19—C20—C21—C22 | 0.6 (5) |
| C6—C7—C8—C13 | 0.7 (4) | C20—C21—C22—C17 | 0.8 (5) |
| C6—C7—C8—C9 | −178.6 (2) | C20—C21—C22—O1 | −178.2 (3) |
| C7—C8—C13—C14 | 3.3 (3) | O1—C23—C24—C25 | −178.4 (3) |
| C7—C8—C9—C10 | 177.0 (3) | C28—C23—C24—C25 | 1.0 (4) |
| C13—C8—C9—C10 | −2.3 (4) | O1—C23—C28—C16 | 1.3 (4) |
| C9—C8—C13—C12 | 4.0 (3) | C24—C23—C28—C16 | −178.1 (2) |
| C7—C8—C13—C12 | −175.3 (2) | C24—C23—C28—C27 | −0.1 (4) |
| C9—C8—C13—C14 | −177.4 (2) | O1—C23—C28—C27 | 179.2 (2) |
| C8—C9—C10—C11 | −1.2 (4) | C23—C24—C25—C26 | −1.2 (5) |
| C9—C10—C11—C12 | 2.9 (4) | C24—C25—C26—C27 | 0.5 (5) |
| C10—C11—C12—C13 | −1.0 (4) | C25—C26—C27—C28 | 0.3 (4) |
| C11—C12—C13—C8 | −2.4 (3) | C26—C27—C28—C16 | 177.4 (2) |
| C11—C12—C13—C14 | 179.0 (2) | C26—C27—C28—C23 | −0.6 (4) |
| C12—C13—C14—C15 | −5.3 (3) | N1—C30—C31—C32 | −175.2 (3) |
| C8—C13—C14—C15 | 176.22 (19) | C35—C30—C31—C32 | 2.1 (5) |
| C12—C13—C14—C1 | 173.2 (2) | N1—C30—C35—C34 | 176.1 (3) |
| C8—C13—C14—C1 | −5.4 (3) | C31—C30—C35—C34 | −1.3 (5) |
| C1—C14—C15—N1 | 14.0 (3) | C30—C31—C32—C33 | −1.3 (6) |
| C1—C14—C15—C16 | −91.9 (3) | C31—C32—C33—C34 | −0.4 (6) |
| C13—C14—C15—C16 | 86.4 (2) | C32—C33—C34—C35 | 1.2 (5) |
| C13—C14—C15—N1 | −167.69 (18) | C33—C34—C35—C30 | −0.4 (5) |
Symmetry codes: (i) −x+1, y+1/2, −z+1/2; (ii) −x, −y+1, −z; (iii) −x, y+1/2, −z+1/2; (iv) −x+1, −y+1, −z+1; (v) −x, y−1/2, −z+1/2; (vi) x, −y+1/2, z−1/2; (vii) x−1, y, z; (viii) x, −y+1/2, z+1/2; (ix) x+1, y, z; (x) −x+1, y−1/2, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···N1 | 0.93 | 2.28 | 2.964 (4) | 130 |
| C31—H31···O2 | 0.93 | 2.48 | 3.092 (3) | 124 |
| C3—H3···Cg1iii | 0.93 | 2.86 | 3.601 (3) | 138 |
| C11—H11···Cg2iv | 0.93 | 2.63 | 3.543 (3) | 166 |
Symmetry codes: (iii) −x, y+1/2, −z+1/2; (iv) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2388).
References
- Akkurt, M., Jarrahpour, A., Ebrahimi, E., Gençaslan, M. & Büyükgüngör, O. (2008). Acta Cryst. E64, o2466–o2467. [DOI] [PMC free article] [PubMed]
- Akkurt, M., Karaca, S., Jarrahpour, A., Ebrahimi, E. & Büyükgüngör, O. (2008). Acta Cryst. E64, o902–o903. [DOI] [PMC free article] [PubMed]
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Banik, I., Becker, F. F. & Banik, B. K. (2003). J. Med. Chem.46, 12–15. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Jarrahpour, A. & Khalili, D. (2007). Tetrahedron Lett.48, 7140–7143.
- Miller, M. J. (2000). Tetrahedron, 56, 5553–5742.
- Palomo, C., Aizpurua, J. M., Ganboa, I. & Oiarbide, M. (2004). Curr. Med. Chem.11, 1837–1872. [DOI] [PubMed]
- Pınar, S., Akkurt, M., Jarrahpour, A. A., Khalili, D. & Büyükgüngör, O. (2006). Acta Cryst. E62, o804–o806.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stoe & Cie (2002). X-AREA and X-RED32 Stoe & Cie, Darmstadt, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004255/is2388sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004255/is2388Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




